J. Cent. South Univ. Technol. (2007)05-0643-04
DOI: 10.1007/s11771-007-0123-z ![]()
Electrical conductivity of Cu/(10NiO-NiFe2O4) cermet inert anode for aluminum electrolysis
TIAN Zhong-liang(田忠良), LAI Yan-qing(赖延清), LI Jie(李 劼), LIU Ye-xiang(刘业翔)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
Cu/(10NiO-NiFe2O4) cermets containing mass fractions of Cu of 5%, 10%, 15% and 20% were prepared, and their electrical conductivities were measured at different temperatures. The effects of temperature and content of metal Cu on the electrical conductivity were investigated especially. The results indicate that the metallic phase Cu distributes evenly in 10NiO-NiFe2O4 ceramic matrix. The mechanism of electrical conductivity of Cu/(10NiO-NiFe2O4) cermets obeys the rule of electrical mechanism of semiconductor, the electrical conductivity for cermet containing 5% Cu increases from 2.70 to 20.41 S/cm with temperature increasing from 200 to 900 ℃. The change trend of electrical conductivity with temperature is similar with each other and it increases with increasing temperature and content of metal Cu. At 960 ℃, the electrical conductivity of cermet increases from 2.88 to 82.65 S/cm with the content of metal Cu increasing from 0 to 20%.
Key words:
NiFe2O4; NiO; cermet; inert anode; aluminum electrolysis; electrical conductivity; ;
1 Introduction
The application of inert anode will eliminate anode carbon consumption and reduce environmental problems such as the emission of carbon dioxide, perfluorocarbon and evolve oxygen[1-2], which makes it commercially attractive. As an inert anode material, the anodic ohmic voltage drop caused by its resistance should be comparable with that of current carbon anode used in aluminum electrolysis, or the purpose for decreasing cell voltage and saving energy cannot be reached. So the inert anode materials need not only good corrosion resistance to the molten salts and high stability with respect to oxidizing gases such as oxygen, but also good electrical conductivity[3-4].
Recently, the studies of materials used as inert anode were mainly concentrated on the alloy[5] and the cermet[6]. Nickel ferrite spinel was found to have very low solubility at high temperature in the molten cryolite and was regarded as one of the most promising matrix of cermet inert anodes with respect to the corrosion resistance to melts[6-8]. But generally, the ceramic oxides have very low electrical conductivity even at high temperature[9-11]. To improve the electrical conductivity, metal was added and dispersed in ceramic oxide matrix. The intention is to make the inert anode material possess the desirable properties of metallic material as well as those of the ceramic.
LAI et al[12] studied the effect of content of NiO on the corrosion resistance and electrical conductivity of NiFe2O4 based cermets, and the content of NiO in ceramic phase was determined to be 10%. Though NiFe2O4-based cermet anode containing 17% Cu was chosen and tested by Alcoa, the reason for the determination of the content of metal Cu was not explained[13]. In this study, 10NiO-NiFe2O4 based cermet inert anodes with Cu content of 0, 5%, 10%, 15% and 20%(mass fraction) were prepared by cold pressing- sintering based on previous works[14], and their electrical conductivities were measured at different temperatures.
2 Experimental
2.1 Preparation of ceramic and cermets
The raw materials, Cu powder, NiO and Fe2O3 were all of reagent grade. 10Ni-NiFe2O4 based cermet samples were prepared by conventional cold isostatic pressing-sintering process[14]. A proper amount of NiO and Fe2O3 were mixed using a ball mill and then calcined to form the 10NiO-NiFe2O4 ceramic powder. The calcined ceramic powder was then mixed with Cu powder (mass fraction of Cu is 0, 5%, 10%, 15% and 20%, respectively) by ball milling in the medium containing organic dispersant and adhesive to avoid the metal oxide. Finally, the mixed ceramic-metal powder was dried and cold isostatically pressed into some cylindrical blocks (d20 mm×40 mm) at 200 MPa, and was sintered at certain temperature in the range from 1 150 to 1 350 ℃ for 2 h under atmosphere of efficaciously controlled oxygen partial pressure to get the desired cermet samples. High density, low porosity and uniform dispersion of metal phase among ceramic phase were desirable.
2.2 Measurement methods
Phase compositions of 10NiO-NiFe2O4 ceramic and cermets were identified by X-ray diffraction analysis using Rigaku3014 X-ray diffractometer with Cu Kα radiation. The microstructure was analyzed with JSM- 6360LV scanning electron microscope.
High-temperature electrical conductivity of the ceramic and cermet samples were determined with a direct current four-end electrodes measuring apparatus[15]. In this study, test was carried out in atmosphere of efficaciously controlled oxygen partial pressure to avoid the oxidization of metal. The electrical conductivity σ was determined by the following equation:
![]() (1)
(1)
where r is the radius of cylinder sample, l and U are the interval and voltage drop between two electrodes, respectively, I is the current through the sample.
3 Results and discussion
3.1 Phase analysis and microstructure of Cu/(10NiO-NiFe2O4) cermet
The X-ray diffraction patterns of 10NiO-NiFe2O4 ceramic and 5%Cu/(10NiO-NiFe2O4) cermet are shown in Fig.1. From Fig.1, it can be seen that there are no impurity diffract peaks for the ceramic matrix and cermet, which shows that the inert anode material with the desired phase composition can be obtained by controlling the partial oxygen pressure.
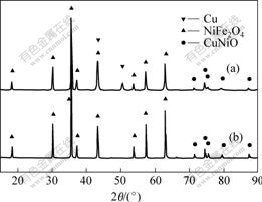
Fig.1 XRD patterns of 10NiO-NiFe2O4 ceramic and Cu/(10NiO-NiFe2O4) cermet
(a) 10NiO-NiFe2O4; (b) 5%Cu/(10NiO-NiFe2O4)
Fig.2 shows the microstructures of Cu/(10NiO- NiFe2O4) cermets with Cu content of 10% and 20%(mass fraction). From Fig.2, it can be seen that the metallic phase Cu is distributed evenly in 10NiO-NiFe2O4 ceramic matrix. However, there are conglomerations for the cermet with 20%Cu, and the grain diameter of metallic phase Cu is larger than that with 10%Cu.
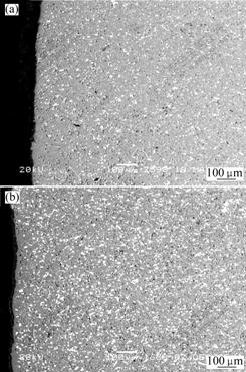
Fig.2 Microstructures of Cu/(10NiO-NiFe2O4) cermets
(a)10%Cu/(10NiO-NiFe2O4); (b)20%Cu/(10NiO-NiFe2O4)
3.2 Effect of Cu content on electrical conductivity of Cu/(10NiO-NiFe2O4) cermets
The electrical conductivities of 10NiO-NiFe2O4 ceramic and Cu/(10NiO-NiFe2O4) cermets were measured at different temperatures, and the results are listed in Table 1.
Table 1 Electrical conductivities of 10NiO-NiFe2O4 ceramic and Cu/(10NiO-NiFe2O4) cermets
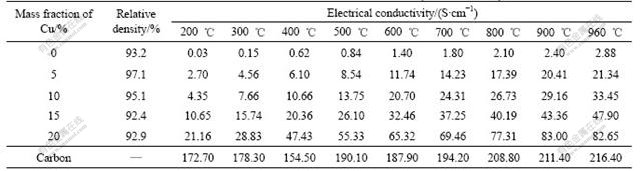
As illustrated in Table 1, although the content of metal Cu added to 10NiO-NiFe2O4 ceramic is only 5%, the electrical conductivity at the same temperature is much higher than that of 10NiO-NiFe2O4 ceramic. For example, the electrical conductivities of 10NiO-NiFe2O4 ceramic and 5%Cu/(10NiO-NiFe2O4) cermet at 900 ℃ are 2.40 and 20.41 S/cm, respectively, there exists difference of about 8 times between them. And the difference becomes more obvious at low temperature than at high temperature. From Table 1, the electrical conductivity of 5%Cu/(10NiO-NiFe2O4) cermet is 2.70 S/cm at 200 ℃, which is 100 times as high as that of 10NiO-NiFe2O4 ceramic.
The data listed in Table 1 also show that the electrical conductivity of Cu/(10NiO-NiFe2O4) cermet increases with increasing content Cu at the same temperature. But there is no liner correlation between the electrical conductivity and Cu content. For example, at 960 ℃, the electrical conductivity increases from 2.88 to 21.34 S/cm when 5%Cu is added to the 10NiO-NiFe2O4 ceramic, the ascend value is one level of magnitude. And when Cu content is added up to 20%, the electrical conductivity of cermet reaches 82.65 S/cm. Though their electrical conductivities are low compared with that of the current carbon anode used in aluminum electrolysis, which is 216.40 S/cm at 960 ℃, the anodic voltage drop caused by its resistance can be decreased by optimizing the configuration of the cermet inert anode. Thus, the voltage drop should be comparable with that of current carbon anode. So, it is acceptable as inert anode material in aluminum electrolysis.
From Fig.2, Cu/(10NiO-NiFe2O4) cermets prepared include metallic phase Cu and oxide ceramic phase 10NiO-NiFe2O4. So, the cermets may conduct electricity by semiconductors and metallic conductivity. WANG et al[9] found that the electrical conductivity of cermet(σ), with metal content less than 30%, obeys the following equation:
![]() (2)
(2)
where σ0 is the electrical conductivity of ceramic, x1 is the fraction of the effective transfer distance of electric charges of metal phase in the cermet and it is related to: 1) the content of the metal; 2) the dispersion of the metal phase among ceramic phases. Eqn.(2) shows that σ is only affected by σ0 and x1, not by the electrical conductivity of metal phase. When the content of the metal added in the cermet increases, x1 increases, therefore the electrical conductivity of Cu/(10NiO- NiFe2O4) cermet increases.
3.3 Effect of temperature on electrical conductivity of Cu/(10NiO-NiFe2O4) cermets
According to the theory of energy band of the solid[16], the electrical conductivity of semiconductor can be given by
![]() (3)
(3)
where e is the electronic charge, u is the mobility of the electron, which does not change obviously with temperature, nc is the charge carrier density, which is specified as
![]() (4)
(4)
where E is the energy gap that is the difference between the conduction band and the valence band in oxide crystal, N0 is the density of energy state, k is the Boltzmann constant, T is absolute temperature.
Therefore,
![]()
![]() (5)
(5)
where σ0 is the electrical conductivity at infinitely high temperatures.
Fig.3 shows the relation between electrical conductivity and temperature, which indicates that the electrical conductivity of Cu/(10NiO-NiFe2O4) cermet changes with the temperature, plotted as ln σ vs 1/T. It is obvious that ln σ is linear with 1/T, and this is fit to this theory. And the curves also show that there is the Curie temperature, where a change in the slope of the straight line appears. There are similar slopes of straight lines for Cu/(10NiO-NiFe2O4) cermets.
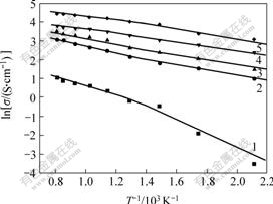
Fig.3 Relation between ln σ and 1/T of different samples
1—10NiO-NiFe2O4; 2—5%Cu/(10NiO-NiFe2O4);3—10%Cu/(10NiO-NiFe2O4); 4—15%Cu/(10NiO-NiFe2O4);
5—20%Cu/(10NiO-NiFe2O4)
In fact, for 10NiO-NiFe2O4 ceramic, the presence of Ni ions on octahedral-(B) sites, Fe ions on tetrahedral-(A) sites and B-sites favor the ion exchange interactions expressed as
Ni2++Fe3+=Ni3++Fe2+ (6)
Thus the conductive mechanism for the N-type semiconductor is predominant due to the hopping of electrons from Fe2+ to Fe3+ ions, while that for the P-type semiconductor is due to the holes transfer from Ni3+ to Ni2+ ions. It seems that the present sample has both types of charge carriers, which participate in the conduction process, and the conductivity is the sum of conductivity for both types. When temperature increases, the charge carrier density increases and therefore the electrical conductivity is improved.
4 Conclusions
1) The metallic phase Cu distributes evenly in 10NiO-NiFe2O4 ceramic matrix. But conglomeration phenomenon appears when Cu content increases up to 20%, the grain diameter of metallic phase Cu becomes larger.
2) The mechanism of electrical conductivity of 10NiO-NiFe2O4 ceramic and Cu/(10NiO-NiFe2O4) cermets obeys the rule of electrical mechanism of semiconductor. Their electrical conductivities mainly depend on temperature, electrical conductivity of ceramic matrix, content and dispersion of the metal phase Cu among ceramic matrix. The change trend of the electrical conductivity with temperature is similar with each other and increases with increasing temperature and content of metal Cu. The electrical conductivity of 5%Cu/(10NiO-NiFe2O4) cermet increases from 2.70 to 20.41 S/cm when temperature increases from 200 to 900 ℃. At 960 ℃, the electrical conductivity of the sample increases from 2.88 to 82.65 S/cm when the content of metal Cu increases from 0 to 20%.
references
[1] PAWLEK R P. Inert anodes: An update[C]// Schneider W. Light Metals 2002. Warrendale, PA: TMS, 2002: 449-456.
[2] SADOWY D R. Inert anode for the Hall-Héroult cell: The ultimate materials challenge[J]. JOM, 2001, 53(5): 34-35.
[3] OLSEN E, THONSTAD J. The behaviour of nickel ferrite cermet materials as inert anodes[C]// HALE W. Light Metals 1996. Warrendale, PA: TMS, 1996: 249-257.
[4] OLSEN E, THONSTAD J. Nickel ferrite as inert anodes in aluminium electrolysis: PartⅠ. Material fabrication and preliminary testing[J]. Journal of Applied Electrochemistry, 1999, 29(3): 293-299.
[5] MARK G, MARGARET H. Laboratory-scale performance of a binary Cu-Al alloy as an anode for aluminium electrowinning[J]. Corrosion Science, 2006, 48: 2457-2469.
[6] JENTOFTSEN T E, LORENTSEN O A, DEWING E W, et al. Solubility of iron and nickel oxides in cryolite-alumina melts[C]// ANJIER J L. Light Melts 2001. Warrendale, PA: TMS, 2001: 455-460.
[7] LAI Yan-qing, TIAN Zhong-liang, QIN Qing-wei, et al. Solubility of composite oxide ceramics in the melt of Na3AlF6-Al2O3[J]. Journal of Central South University of Technology: Natural Science, 2003, 34(3): 245-248.(in Chinese)
[8] MCLEOD A D, LIHRMAN J M, HAGGERTY J S, et al. Selection and testing of inert anode materials for Hall cells[C]// ZABREZNIK R D. Light Metals 1987. Warrendale, PA: TMS, 1987: 357-365.
[9] WANG Chuan-fu, LI Guo-xun. Influence of metal additives on the electrical conductivities of the oxide ceramics as an electrode material[J]. Rare Metals, 1993, 12(2): 126-130.
[10] YU Ya-xin, YANG Bao-gang, YU Xian-jin, et al. Electrical conductivities of zinc ferrite and nickel ferrite spinels material at high temperature[J]. The Chinese Journal of Nonferrous Metals, 1998, 8(S2): 336-337. (in Chinese)
[11] ELSHORA A I, ELHITI M A, ELNIMR M K, et al. Semiconductivity in Ni1+xMnxFe2-2xO4 ferrites[J]. Journal of Magnetism and Magnetic Materials, 1999, 204(1): 20-28.
[12] LAI Yan-qing, DUAN Hua-nan, LI Jie, et al. On the corrosion behaviour of Ni-NiO-NiFe2O4 cermets as inert anodes in aluminium electrolysis[C]// KVANDE H. Light Metals 2005. Warrendale, PA: TMS, 2005: 529-534.
[13] WEYAND J D, DEYOUNG D H, RAY S P, et al. Inert anodes for aluminium smelting[R]. PA 15069, Washington D C: Aluminum Company of America, 1986.
[14] ZHANG Gang, LAI Yan-qing, TIAN Zhong-liang, et al. Preparation of nickel ferrite based cermets for aluminum electrolysis[J]. Journal of Material Science and Engineering, 2003, 21(4): 44-47. (in Chinese)
[15] QIN Qing-wei, LAI Yan-qing, XIAO Jin, et al. Preliminary testing of NiFe2O4-NiO as ceramic matrix of cermet inert anode in aluminum electrolysis[J]. Transactions of Nonferrous Metals Society of China, 2003, 13(5): 1208-1212.
[16] SU Mian-zeng. Solid State Chemistry[M]. Beijing: Peking University Press, 1987: 92-97. (in Chinese)
Foundation item: Project(2005CB623703) supported by the National Basic Research and Development Program of China
Received date: 2007-03-12; Accepted date: 2007-05-15
Corresponding author: TIAN Zhong-liang, PhD; Tel: +86-731-8876454; E-mail:tianzhongliang@126.com
(Edited by CHEN Wei-ping)
Abstract: Cu/(10NiO-NiFe2O4) cermets containing mass fractions of Cu of 5%, 10%, 15% and 20% were prepared, and their electrical conductivities were measured at different temperatures. The effects of temperature and content of metal Cu on the electrical conductivity were investigated especially. The results indicate that the metallic phase Cu distributes evenly in 10NiO-NiFe2O4 ceramic matrix. The mechanism of electrical conductivity of Cu/(10NiO-NiFe2O4) cermets obeys the rule of electrical mechanism of semiconductor, the electrical conductivity for cermet containing 5% Cu increases from 2.70 to 20.41 S/cm with temperature increasing from 200 to 900 ℃. The change trend of electrical conductivity with temperature is similar with each other and it increases with increasing temperature and content of metal Cu. At 960 ℃, the electrical conductivity of cermet increases from 2.88 to 82.65 S/cm with the content of metal Cu increasing from 0 to 20%.


