- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions ▲
- References
- Figure
- Fig.1 Sketch of microwave sensor system for measuring microwave absorbing properties
- Fig.2 Schematic diagram of microwave reactor
- Fig.3 Temperature rise curve of high titanium slag by microwave irradiation
- Fig.4 XRD patterns of samples: (a) Untreated by microwave irradiation; (b) Treated by microwave irradiation at 1 050 ℃ for 20 min
- Fig.5 Microwave spectra of different particle sizes of high titanium slag
- Fig.6 Relationship between frequency shift and particle size of high titanium slag
- Fig.7 Relationship between amplitude of voltage of microwave sensor and particle size of high titanium slag
- Fig.8 Microwave spectra of mixtures of high titanium slag with different mass fractions of V2O5
- Fig.9 Relationship between frequency shift and mass fraction of V2O5
- Fig.10 Relationship between amplitude of voltage and mass fraction of V2O5
J. Cent. South Univ. Technol. (2009) 16: 0588-0593
DOI: 10.1007/s11771-009-0098-z
![]()
Microwave absorbing properties of high titanium slag
ZHANG Li-bo(张利波), CHEN Guo(陈菓), PENG Jin-hui(彭金辉), CHEN Jin(陈晋),
GUO Sheng-hui(郭胜惠), DUAN Xin-hui(段昕辉)
School of Materials and Metallurgical Engineering, Kunming University of Science and Technology,Kunming 650093, China
Abstract:
Microwave absorbing properties of high titanium slag were investigated by using microwave cavity perturbation technique. High titanium slag containing more than 90% TiO2 was prepared by carbothermal reduction of ilmenite. The temperature rise curve of high titanium slag in microwave heating process was obtained. Crystalline compounds of high titanium slag before and after microwave irradiation were obtained and characterized by X-ray diffractometry (XRD). Effects of particle size of high titanium slag and mixtures of high titanium slag with different mass fractions of V2O5 on microwave absorbing properties were investigated systematically. The results show that high titanium slag has good microwave absorption property; untreated high titanium slag mainly consists of crystalline compounds of anatase and iron titanium oxide, while the microwave-irradiation treated one is mainly composed of crystalline compounds of rutile and iron titanium oxide. Synthetic anatase is transformed completely into rutile at about 1 050 ℃ for 20 min under microwave irradiation. High frequency shift and low amplitude of voltage make high titanium slag an ideal microwave absorbent. 180 ?m of particle size and 10% mass fraction of V2O5 are found to be the optimum conditions for microwave absorption.
Key words:
high titanium slag; microwave absorbing; microwave cavity perturbation; microwave irradiation;
1 Introduction
It is known that titanium dioxide (TiO2) exists in three main polymorphic phases: rutile, anatase and brookite [1]. Anatase and brookite are metastable phases, and their exothermic and irreversible conversions to rutile at high temperatures have been widely investigated. The transformation of anatase to rutile in TiO2 is influenced by several experimental conditions such as temperature, particle size and synthetic method of dioxide [2-4].
Titanium dioxide pigments are manufactured by the chloride or sulfate processes, in which natural or synthetic rutile is used as raw materials. However, these processes pollute the environment [5]. Since available resources of high grade natural rutile are diminishing, the shortage of natural rutile has encouraged researchers to find an efficient method to convert anatase in high titanium slag to synthetic rutile. To explore new method to produce synthetic rutile with low energy consumption and less environment pollution is necessary [6-8]. Microwave can eliminate the environmental pollution from production source and realize sustainable development of titanium resources, and produce synthetic rutile from high titanium slag.
The microwave absorbing property of materials is an important physical indicator in the fields of microwave chemistry, microwave detection, and microwave processing [9-11]. Microwave cavity perturbation technique is widely adopted for microwave dielectric properties measurements [12]. A microwave experiment is based on the cavity perturbation method, employing the single resonant mode [13]. The change in the cavity characteristics in the presence of a sample is measured. In addition, the complex conductivity is directly evaluated. HUANG et al [14] invented a novel microwave sensor for measuring the properties of a liquid drop, and the technique can easily be extended to microwave processing materials. Its analytical theory is established and a working prototype has been constructed and tested. It is also found that the theory based on the microwave sensor is in a good agreement with the experimental results. HUANG et al [15] investigated the technique of microwave cavity perturbation in measuring the characteristics of microwave absorbing of the mixtures of carbonaceous reducer and ilmenite. HUANG et al [16] also used the microwave cavity perturbation technique to measure the moisture content of a sulphide mineral concentrate.
In this work, a new method, based on microwave cavity perturbation technique and digital signal processing technique, was applied to detecting the microwave absorbing properties of high titanium slag. The effects of particle size of high titanium slag and the mixtures of high titanium slag with different mass fractions of V2O5 on microwave absorbing properties were investigated. The optimum conditions of producing synthetic rutile from high titanium slag by microwave irradiation were obtained.
2 Experimental
2.1 Materials
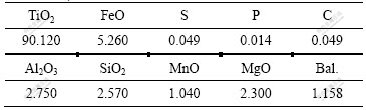
High titanium slag was obtained from Kunming City, Yunnan Province, China. High titanium slag sample was prepared from ilmenite by carbothermal reduction in an electric arc furnace. The chemical composition of high titanium slag is shown in Table 1.
Table 1 Chemical composition of high titanium slag (mass fraction, %)
2.2 Measuring principles and apparatus
The microwave sensor system was based on microwave cavity perturbation technique and digital signal processing technique. Derived from the theory of electric-magnetic field, the frequency shift and the output voltage of the microwave cavity are given by [16-17]
![]() (1)
(1)
![]() (2)
(2)
![]() (3)
(3)
where ?ω=ω-ω0, ![]() , D0,
, D0, ![]() and B0 are the fields in the interior of the sample; V and Ve are the volumes of the cavity and the sample, respectively; dV is the elemental volume; Q0 and ω0 are the quality factor and resonance frequency of cavity in the unperturbed condition, respectively; Q and ω are the corresponding parameters of the cavity loaded with the sample;
and B0 are the fields in the interior of the sample; V and Ve are the volumes of the cavity and the sample, respectively; dV is the elemental volume; Q0 and ω0 are the quality factor and resonance frequency of cavity in the unperturbed condition, respectively; Q and ω are the corresponding parameters of the cavity loaded with the sample; ![]() and
and ![]() are the real and the imaginary part of the complex permittivity of the sample, respectively; and W is the storage energy.
are the real and the imaginary part of the complex permittivity of the sample, respectively; and W is the storage energy.
According to Eqns.(1)-(3), the data of microwave absorbing properties can be acquired by measuring the output voltage and the frequency shift of the microwave sensor. A structure diagram of the microwave sensor system is illustrated in Fig.1. The computer controls the fast scanning microwave generator through multipurpose card. The microwave signals are transmitted into the microwave sensor. The output signals of the microwave sensor are picked up by the linear detector and digital signal processor (DSP). Then they are fed into the low pass filter. After that, the output signals of the low pass filter are amplified and converted by the A/D converter. The data processing of the microwave sensor system is finished on the computer [18].
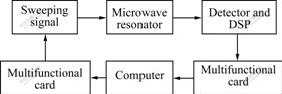
Fig.1 Sketch of microwave sensor system for measuring microwave absorbing properties
Temperature rise curve of high titanium slag was obtained with microwave reactor. The schematic diagram of the microwave reactor is shown in Fig.2. The microwave heating apparatus consists of a magnetron, a power controller, a matched load, a wave guide, and a cavity. A self-made microwave reactor has a multi-mode cavity and continuous controllable power capacity. The microwave power supply for the microwave reactor consists of two magnetrons that are cooled by water circulation, at a frequency of 2.45 GHz and a power of 1.5 kW. A ceramic tube, 50 mm (outer diameter)× 80 mm (inner diameter)×600 mm (length), is positioned at the center of the microwave oven, by drilling holes on the side faces, with ends projecting on both sides. The temperature of the sample is monitored using an infrared pyrometer (Raytek, Marathon Series, USA) with the circular crosswire focusing on the sample cross-section. The temperature is also measured using a thermocouple (Shengyun Company, China) as a reference. In case of any temperature discrepancy, the latter is used as the correct temperature. XRD patterns were obtained using a Rigaku diffractometer (D/Max 2200, Japan) with Cu Kαradiation and a Ni filter operated at 35 kV, 20 mA and a scanning rate of 0.25 (?)/min.

Fig.2 Schematic diagram of microwave reactor
2.3 Methods
The sample (100 g) was placed in the self-made microwave heating equipment and heated to 1 050 ℃ for 20 min at 3 kW, and then it was naturally cooled in the furnace to room temperature.
The high titanium slag with different mean particle sizes and the mixtures of different mass fractions of V2O5 were prepared. Each sample (2 g) was dried at 105 ℃ for 2 h. Finally, the microwave absorbing properties of the samples were obtained by putting the samples into the microwave resonant sensor in turn.
3 Results and discussion
3.1 Temperature rise curve of high titanium slag
The relationship between temperature and time of the high titanium slag under microwave irradiation is shown in Fig.3. It can be seen that the temperatures of the samples increase with increasing microwave irradiation time. The temperature reaches 1 050 ℃ after 5 min, and then the microwave power is adjusted to keep this temperature for 5 min. Fig.3 shows the temperature can in fact be kept at 1 200 ℃, which indicates that the self-made microwave heating equipment can be well controlled, thus making it suitable for other studies. Furthermore, the results indicate the high titanium slag is a good microwave absorbent.
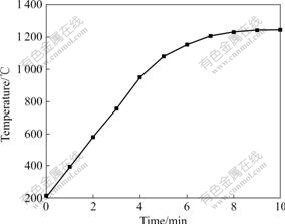
Fig.3 Temperature rise curve of high titanium slag by microwave irradiation
3.2 Characterization of high titanium slag
The high titanium slag before and after microwave irradiation is characterized by XRD, and the results are shown in Fig.4. It can be seen from Fig.4(a) that anatase and iron titanium oxide are mainly crystalline compounds in the high titanium slag. In addition, a minor amount of rutile is present. The iron titanium oxide has the strongest diffraction peak at 2θ=25.22?, which is close to the strongest diffraction peak of anatase at 2θ=25.28?, so the two peaks are overlapped. High titanium slag after microwave irradiation at 1 050 ℃ for 20 min is characterized by XRD, which is shown in Fig.4(b). It can be found from Fig.4(b) that the diffraction peaks of rutile gradually are broadened and their intensities are increased under microwave irradiation. Rutile has the strongest diffraction peak at 2θ=25.44?. All the X-ray diffraction peaks of samples well match with those of the standard XRD pattern of rutile phase. It can be inferred from Fig.4(b) that anatase is completely converted to synthetic rutile.
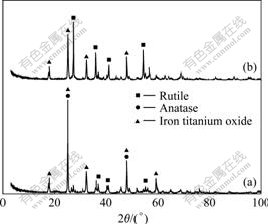
Fig.4 XRD patterns of samples: (a) Untreated by microwave irradiation; (b) Treated by microwave irradiation at 1 050 ℃ for 20 min
3.3 Absorbing properties
Eqn.(1) indicates that the real part of the permittivity is directly proportional to frequency shift. Eqn.(2) indicates that the imaginary part of the permittivity is inversely proportional to amplitude of voltage. By analyzing the changing behavior of these microwave spectra and calculating the amplitude of voltage and the frequency of the microwave spectrum’s first wave crest with computer program, the effects particle size of high titanium slag and mass fraction of V2O5 on microwave absorbing properties are obtained [19-20].
3.3.1 Effect of particle size of high titanium slag on microwave absorbing properties
The microwave spectra of particle size of high titanium slag are illustrated in Fig.5. The microwave sensor with empty chamber gives rise to the resonant curve with the highest resonant amplitude and the largest resonant frequency. The other resonant curves indicate lower resonant amplitude and smaller resonant frequency, resulted from the microwave sensor filled with different sizes of high titanium slag, respectively.
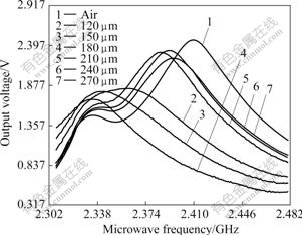
Fig.5 Microwave spectra of different particle sizes of high titanium slag
The effect of particle size of high titanium slag on the frequency shift is illustrated in Fig.6. It can be seen that the frequency shift increases gradually from about 47 to 84 kHz with increasing the particle size of high titanium slag from 120 to 180 ?m, and then it decreases from 84 to 14 kHz with increasing the particle size of high titanium slag from 180 to 270 ?m. Fig.7 shows the relationship between the amplitude of voltage of microwave sensor and particle size of high titanium slag. Similarly, the amplitude of voltage also decreases from about 1.868 to 1.701 V with increasing the particle size of high titanium slag from 120 to 180 ?m; it increases from 1.701 to 2.317 V with increasing the particle size of high titanium slag from 180 to 240 ?m; and then it decreases from 2.317 to 2.217 V with further increasing the particle size of high titanium slag from 240 to 270 ?m. Therefore, 180 ?m of particle size is chosen as the optimum size of high titanium slag in microwave field.
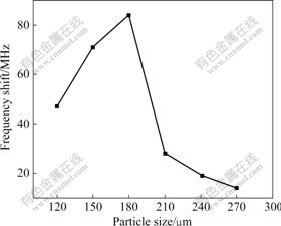
Fig.6 Relationship between frequency shift and particle size of high titanium slag
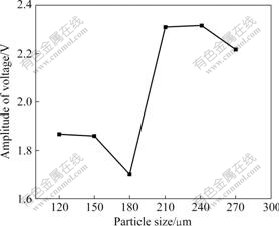
Fig.7 Relationship between amplitude of voltage of microwave sensor and particle size of high titanium slag
The result of perturbation technique is that the presence of high titanium slag in the resonant cavity causes a shift of resonant frequency and changes in the amplitude of voltage of the resonant cavity. The changes in the amplitude of voltage of the cavity exist because of dielectric loss of the sample. The dielectric constant and loss tangent of high titanium slag can be calculated from the shift of frequency and amplitude of voltage.
3.3.2 Effect of mass fraction of V2O5 on microwave absorbing properties
Fig.8 shows the microwave spectra of mixtures of high titanium slag with different mass fractions of V2O5. It is found that the microwave sensor with empty chamber gives rise to the resonant curve with the highest resonant amplitude and the biggest resonant frequency. The other curves show different results for mixtures of high titanium slag with different mass fractions of V2O5.
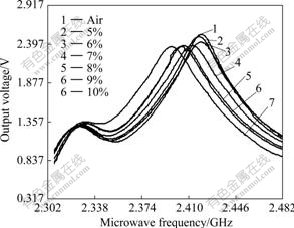
Fig.8 Microwave spectra of mixtures of high titanium slag with different mass fractions of V2O5
Fig.9 shows the relationship between the mass fraction of V2O5 and the frequency shift. It can be found
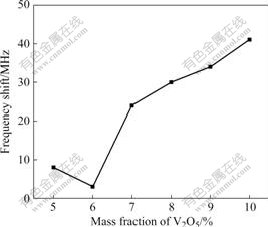
Fig.9 Relationship between frequency shift and mass fraction of V2O5
that frequency shift decreases gradually from about 8 to 3 MHz with increasing the mass fraction of V2O5 form 5% to 6%, and then it increases from 3 to 41 MHz when the mass fraction of V2O5 increases from 6% to 10%. Fig.10 shows the relationship between the amplitude of voltage and the mass fraction of V2O5. It is clear that the amplitude of voltage decreases with increasing the mass fraction of V2O5 (it decreases abruptly after 5% and then decreases smoothly after 6%). The above discussion indicates that the mass fraction of V2O5 has a significant effect on the microwave absorbing properties of high titanium slag. YU et al [21] investigated the optimum microwave absorbing of mixtures with mass fraction of V2O5 less than 10%. The optimum content, 10% of the mass fraction of V2O5, is obtained. The phase transformation from anatase to rutile in high titanium slag is irreversible. V2O5 can enhance the phase transformation of TiO2. It can also lower the phase transformation temperature of TiO2 and increase the transformation ratio.
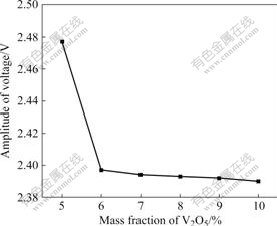
Fig.10 Relationship between amplitude of voltage and mass fraction of V2O5
4 Conclusions
(1) The temperature of high titanium slag is raised up to 1 050 ℃ after 5 min by microwave heating at a microwave frequency of 2.45 GHz and output microwave power of 3 kW. When the temperature is kept at 1 050 ℃ for 20 min, the phase transformation, from anatase to rutile of high titanium slag in microwave field, is observed. High titanium slag has good microwave absorbing properties in the microwave field.
(2) The microwave absorbing properties of high titanium slag, which are measured by microwave cavity perturbation technique, are investigated in the frequency range of 2.302-2.482 GHz. 180 ?m of particle size and 10% of the mass fraction of V2O5 are the optimum conditions for producing synthetic rutile from high titanium slag in microwave field.
(3) The microwave cavity perturbation technique is used to optimize the experimental parameters and will provide the guidance for the study of microwave heating of high titanium slag in the future.
References
[1] WANG Jun, MA Teng, ZHANG Zhao-hong, ZHANG Xiang-dong, JIANG Yue-feng, SUN Wei, LI Rong-he, ZHANG Peng. Investigation on the transition crystal of ordinary rutile TiO2 powder by microwave irradiation in hydrogen peroxide solution and its sonocatalytic activity [J]. Ultrasonics Sonochemistry, 2007, 14(5): 575-582.
[2] DONDI M, CRUCIANI G, BALBONI E, GUARINI G, ZANELLI C. Titania slag as a ceramic pigment [J]. Dyes and Pigments, 2008, 77(3): 608-613.
[3] VILLIERS J, VERRYN S, FERNANDES M. Disintegration in high-grade titania slags: Low temperature oxidation reactions of ferro-pseudobrookite [J]. Mineral Processing and Extractive Metallurgy, 2004, 113(8): 66-74.
[4] WANG Ming-yu, LI Liao-sha, ZHANG Li, ZHANG Lin-nan, TU Gan-feng, SUI Zhi-tong. Effect of oxidization on enrichment behavior of TiO2 in titanium-bearing slag [J]. Rare Metals, 2006, 25(2): 106-110.
[5] LI Chun, LIANG Bin, GUO Ling-hong. Dissolution of mechanically activated Panzhihua ilmenites in dilute solutions of sulphuric acid [J]. Hydrometallurgy, 2007, 89(1/2): 1-10.
[6] SAMAL S, RAO K K, MUKHERJEE P S, MUKHERJEE T K, Statistical modelling studies on leachability of titania-rich slag obtained from plasma melt separation of metallized ilmenite [J]. Chemical Engineering Research and Design, 2008, 86(2): 187-191.
[7] BESSINGER D, GELDENHUIS J M A, PISTORIUS P C, MULABA A, HERANE G. The decrepitation of solidified high titania slags [J]. Journal of Non-Crystalline Solids, 2001, 282(1): 132-142.
[8] PISTORIUS P C, MOTLHAMME T. Oxidation of high-titanium slags in the presence of water vapour [J]. Minerals Engineering, 2006, 19(3): 232-236.
[9] LI Wei, PENG Jin-hui, ZHANG Li-bo, ZHANG Ze-biao, LI Lei, ZHANG Shi-min, GUO Sheng-hui. Pilot-scale extraction of zinc from the spent catalyst of vinyl acetate synthesis by microwave irradiation [J]. Hydrometallurgy, 2008, 92(1/2): 79-85.
[10] LI Wei, ZHANG Li-bo, PENG Jin-hui, LI Ning, ZHANG Shi-min, GUO Sheng-hui. [J]. Effects of microwave irradiation on the basic properties of wood-ceramics made from carbonized tobacco stems impregnated with phenolic resin [J]. Industrial Crops and Products, 2008, 28(2): 143-154.
[11] VERMA A, SAXENA A K, DUBE D C. Microwave permittivity and permeability of ferrite–polymer thick films [J]. Journal of Magnetism and Magnetic Materials, 2003, 263(1/2): 228-234.
[12] KRASOVITSKY V, TERASAWA D, NAKADA K, KOZUMI S, SAWADA A, SATO N. Microwave cavity perturbation technique for measurements of the quantum hall effect [J]. Cryogenics, 2004, 44(3): 183-186.
[13] SHEEN J. Measurements of microwave dielectric properties by an amended cavity perturbation technique [J]. Measurement, 2009, 42(1): 57-61.
[14] HUANG Ming, YANG Jing-jing, WANG Jia-qiang, PENG Jin-hui. Microwave sensor for measuring the properties of a liquid drop [J]. Measurement Science and Technology, 2007, 18(7): 1934-1938.
[15] HUANG Meng-yang, PENG Jin-hui, HUANG Ming, ZHANG Shi-min, LI Yu, LEI Ying. Microwave-absorbing characteristics of mixtures about different proportions of carbonaceous reducer and ilmenite in microwave field [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(3): 476-480. (in Chinese)
[16] HUANG Ming, PENG Jin-hui, YANG Jing-jing, WANG Jia-qiang. Microwave cavity perturbation technique for measuring the moisture content of sulphide minerals concentrates [J]. Minerals Engineering, 2007, 20(1): 92-94.
[17] CARTER R G. Accuracy of microwave cavity perturbation measurements [J]. IEEE Transactions on Microwave Theory and Techniques, 2001, 49(5): 918-923.
[18] HUANG Ming, PENG Jin-hui, YANG Jing-jing, WANG Jia-qiang. A new equation for the description of dielectric losses under microwave irradiation [J]. Journal of Physics D: Applied Physics, 2006, 39(10): 2255-2258.
[19] CRISTALLOG, RONCARIE, RINALDOA, TRIFIROF. Study of anatase-rutile transition phase in monolithic catalyst V2O5/TiO2 and V2O5-WO3/TiO2 [J]. Applied Catalysis A: General, 2001, 209(1/2): 249-256.
[20] HABERJ, NOWAKP. A catalysis related electrochemical study of the V2O5/TiO2 (rutile) system [J]. Langmuir, 1995, 11(3): 1024-1032.
[21] YU Xiao-feng, WU Nian-zu, XIE You-chang, TANG You-qi. The monolayer dispersion of V2O5 and its influence on the anatase-rutile transformation [J]. Journal of Materials Science Letters, 2001, 20(4): 319-321.
(Edited by CHEN Wei-ping)
Foundation item: Project(2007CB613606) supported by the Major State Basic Research and Development Program of China; Project(50734007) supported by the National Natural Science Foundation of China
Received date: 2008-11-20; Accepted date: 2009-02-27
Corresponding author: PENG Jin-hui, Professor, PhD; Tel: +86-871-5191046; E-mail: jhpeng@kmust.edu.cn
- Microwave absorbing properties of high titanium slag


