Article ID: 1003-6326(2005)05-1003-06
Wear resistance and corrosion resistance of VCp particle reinforced stainless steel composites
YAO Xiu-rong(姚秀荣)1, 2, HAN Jie-cai(韩杰才)1,
ZUO Hong-bo(左洪波)1, LIU Zhao-jing(刘兆晶)2,
LI Feng-zhen(李凤珍)2, REN Shan-zhi(任善之)2
(1. Institute of Composite Materials, Harbin Institute of Technology, Harbin 150001, China;
2. College of Materials Science and Engineering, Harbin University of Science and Technology, Harbin 150080, China)
Abstract:
The VCp reinforced stainless steel composite was produced by in-situ reaction casting. The composite was tested for its wear resistance under the wet abrasive condition and corrosion resistance, compared with the wear-resistant white iron and stainless steel. The results show that the wear resistance of the composite is slightly inferior to that of the white iron, but much better than that of the stainless steel under the wet grinding abrasive condition. The corrosion resistance of the composite is much better than that of the white iron in the acid medium, and a little worse than that of the stainless steel. Thus the composite exhibits superior properties of wear resistance and corrosion resistance.
Key words:
VCp; composite; white iron; stainles steel; wear resistance; corrosion resistance CLC number: TB331;
Document code: A
1 INTRODUCTION
Recently, the Fe-base composites prepared by in-situ reaction casting have attracted great interest[1, 2]. This technique is characterized to use the high temperature of the ferroalloy melt and promote the chemical reaction between the carbon and alloying elements in the melt, then one or several ceramic particles with high strength and elastic modulus will be generated[3, 4]. The amount and distribution of the ceramic particles, such as TiC, WC, Cr7C3 and VC, can be controlled by adjusting the technological parameters to obtain the desired particle reinforced Fe-base composites. Compared with the traditional method of addictive particle reinforcement, such simple technique with low cost will lead to the purified interface between the reinforced phase and the matrix, without adsorbed gas and oxide film. Also the reinforced phase and the matrix are well compatible and firmly combined, and these small reinforced particles (〈5μm in general) is homogenously distributed in the matrix[5]. Considering the high content of vanadium in our iron ores, spheric VC stainless steel composites can be produced by this technique to improve its hardness, corrosion resistance and wear resistance, which is much promising in the industrial application[6].
2 EXPERIMENTAL
2.1 Material preparation
The foundry ingot, vanadium iron, nickel, chromium iron and scrap steel were chosen as the main materials, and the VC particle reinforced stainless steel composites were prepared by in-situ reaction casting. Its hardness is tested to be HRC 42 and ak 9.5J/cm2, and its chemical composition is listed in Table 1.
Table 1 Chemical compositions of composite (mass fraction, %)

It can be seen from Table 1 that the composite matrix is mainly Fe together with high content of Cr, V, Ni and so on. As determined by the XRD pattern in Fig.1, the composite has an austenite base with VC as the secondary phase.
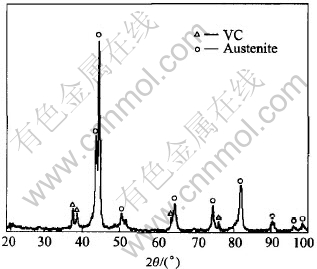
Fig.1 XRD pattern of composite
2.2 Wear test
Three VC particle reinforced stainless steel composites (VC/Fe), wear-resistant white iron(15Cr3Mo)and stainless steel(Cr18Ni9) were chosen for wear tests. They were machined into 57mm×25.5mm×6mm wear samples by linear cutting, and the wear test was performed on the MSL-225 greensand rubber wheel type wear tester.
2.3 Corrosion test
The above three materials were machined into corrosion samples of 15mm×10mm×5mm by linear cutting, and tested under electrochemical corrosion[8] and concentrated sulfuric acid corrosion, respectively.
The electrochemical corrosion was carried out in the electrochemical corrosion workstation. After the sample surface was polished by abrasive paper (Fig.2(a)), the side of 10mm×5mm was taken as corrosion electrode, and the other side of 10mm×5mm was welded by copper conductors with insulated skin. All surfaces except the corrosion electrode plane were sealed by ethoxyline resin (Fig.2(b)). The electrolytic cell with three electrodes was chosen, the testing solution was H2SO4(1mol/L, 25℃), and the scanning velocity was 5mV/s, with the platinum electrode as auxiliary electrode and the calomel electrode (Hg/Hg2SO4) as reference electrode. The anodic polarization curves were measured by the moving electrode scanning method[9].
Fig.2 Samples for electrochemical corrosion
The sulfuric acid corrosion test used the 90% sulfuric acid that was prepared by pure concentrated sulfuric acid with little de-ionized water. Sample masses (m) before and after wear or corrosion tests were measured by the photoelectric analytical balance. The wear surface and corrosion surface were observed by the scanning electronic micro-scopy(SEM).
3 RESULTS AND DISCUSSION
3.1 Wet wear test
The wear capacity can be used to describe the material abradability directly in the way to denote the mass loss of wearing samples, namely, the wear capacity represents the mass difference before and after a wear test.
mloss=mbefore-mafter
where mbefore is the mass before wear and mafter is the mass after wear.
The sample masses after wear under different loads are listed in Table 2, and the wear capacity variations are shown in Fig.3. For the abraded samples, their surfaces were observed by SEM and shown in Fig.4.
Table 2 Sample masses after wear under different loads
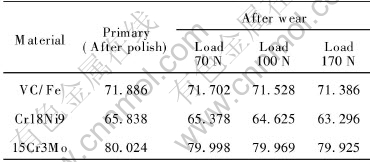

Fig.3 Wear capacity under different loads

Fig.4 SEM images of sample surfaces after wear
As seen from Fig.4, after they are worn under the wet abrasive condition, the white iron has the minimum wear capability under the same load, the VC particle reinforced stainless steel composite shows a small wear capability, and the stainless steel has the maximum wear capability. With the increasing of loads, the wear capability of the white iron has a slight increment, and that of the stainless steel increases largely, while for the VC particle reinforced stainless steel composite, the wear capability decreases gradually.
The wear under the wet abrasive condition[10, 11] can be regarded as the complex effects of wear under both the dry grinding material and the electrochemical corrosion. The wear mechanism for VC particle reinforced stainless steel composites, wear-resistant white irons and stainless steels will be discussed respectively according to their wear and corrosion behaviors.
3.1.1 Grinding abrasion
Seen from the matrix microstructure, the wear-resistant white iron contains high content of Cr, and a large amount of massive M7C3-type carbides are embedded in the matrix. In the VC particle reinforced stainless steel composite, VC particles are spheric or granular. During wear, the matrix of lower hardness is first abraded by the scouring effects of grindings. Then carbides expose gradually, and with the increasing grinding abrasion, the exposed part of carbides increases. Under a certain load, the size of exposed carbides reaches a critical value and the carbides will be stripped. Fig.4 describes the wear difference among the VC particle reinforced stainless steel composite, wear-resistant white iron and stainless steel.
For the VC particle reinforced stainless steel composite, the matrix is heavily abraded and there exist deep scratches and pits on the worn surface, which represents the severe grinding abrasion. As for the milder stripping of VC particles, it directly relates to both the firm combination between the VC particles and the matrix, and the high microhardness of particles[12] (Fig.4(a)). For the wear-resistant white iron, the high hardness of the matrix (HRC≥60) leads to the typical grinding abrasion so that the matrix has low wear capability and neither furrows nor deep nicks exist in the worn surface (Fig.4(b)). For the stainless steel, its seriously abraded that furrows and heavy plastic deformation can be observed on its wear surface (Fig.4(c)).
During wearing process, the massive M7C3 carbides are embedded in the deep matrix of the wear-resistant white iron, which are firmly combined with the matrix and can not scale off easily. While in the VC particle reinforced stainless steel composite, VC particles are spherically distributed in the matrix, and then a slight exposure of them will result in their scouring off. As seen from Fig.4(a), there exist many spherical pits on the wear surface.
Under the grinding abrasion, the matrix of wear-resistant white iron exhibits the maximum hardness, and the corresponding abrasion mechanism is synchronized grinding abrasion of both the matrix and carbides. For the VC particle reinforced stainless steel composite, the abrasion mechanism is the cyclic process that the matrix surface is first abraded, followed by VC particles exposion, and then the particles are abraded to scale off. As for the austenite stainless steel, its low hardness together with the nonexistence of hard reinforced particles leads to the poor wear resistance.
In our experiment, the major wear type is the grinding abrasion and the corrosion wear is minor, so the white iron exhibits the optimal wear resistance. When the material serves in a corrosion environment for a long time, the electrochemical corrosion will become important and then the VC particle reinforced stainless steel composite shows the best resistance to wear and corrosion.
3.1.2 Electrochemical corrosion
The experimental materials will be used in mud pumps, where the excellent wear-resistant property and good resistance to long-term seawater corrosion (12 months turnaround) are required. Accordingly, the corrosion behavior under the grinding abrasion conditions must be considered.
When the polyphase wear-resistant materials, especially that with carbides, serve under the wet abrasive condition, large electric potential differences will occur between the carbide and the matrix due to the existence of water and other mixed liquid. Then corrosion batteries will form and interphase corrosion will happen, which extremely weakens the binding force between the carbide and matrix. So the carbides will easily part or scale off from the matrix under the effect of grinding materials or hard particles.
For metal materials, the electrochemical corrosion in solution is the process that metals release electrons and transform into ions entering the solution. There only exist the ion or compound of the oxidized metal in the solution, so during the electrochemical corrosion, the metal is placed in the water solution of its own ions. However, reversible reaction alone between the metal and its ions seldom happen; in fact, metals in the solution will act in two or more processes between cathodes and anodes.
The corrosion parameters of the three materials in 1mol/L H2SO4 (25℃) are listed in Table 3, and their anodic polarization curves are shown in Fig.5.
It can be seen that the passivation potential Ecr of the composite approximates that of the stainless steel but it is largely lower than that of the white iron, which implies that the composite tends towards passivation. Such phenomenon is caused by the high content of Cr (above 16%, mass fraction) in the composite and the stronger passivation of Cr than Fe[12]. The passivation current density icr of the VC particle reinforced stainless steel composite is between that of the white iron and stainless steel, but closer to that of the stainless steel. The wide passivation region means that the VC particle reinforced stainless steel composite has good corrosion resistance, and with the increasing wear time and load, its resistant properties to both wear and corrosion will be more remarkable under the wet abrasive condition.
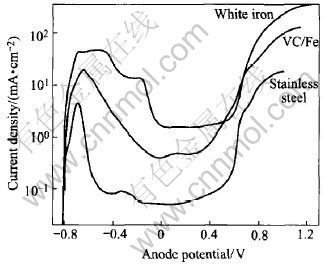
Fig.5 Anodic polarzation curve of three materials in 1mol/L H2SO4
During the wet grinding abrasion, the corrosion wear is directly proportional to the electric potential value, namely, the higher the potential difference, the heavier the corrosion wear. Then, its deduced that under the long-term wet grinding abrasion, the composite has both the grinding abrasion and the electrochemical corrosion with medium water. The wear phenomenon is the complex results from the wear and corrosion.
3.2 Evaluation of corrosion rate
To well investigate the corrosion character of the composite[13, 14], the corrosion experiments immersion for 168h were performed in 90% concentrated sulfuric acids. The mass loss method is taken to evaluate the corrosion rate using corrosion depth B. The calculation is as below:
v=(m0-m1)/(At)
B=8.76v/ρ
where m0 is the primary mass of samples(g), m1 is sample mass after corrosion (g), A denotes the sample area (m2), t is the corrosion time (h), v is the mean corrosion rate (g/(m2·h)), ρ represents the materials density (g/cm3) and B is the corro sion depth described by depth (mm/a).
Table 3 Corrosion parameters of three materials in 1mol/L H2SO4

After immersed in the above acid for 168h, the materials approach to the calculated corrosion rates shown in Fig.6. It can be seen that the three materials are all eroded and lose masses, among which the stainless steel has the minimum loss, the VC particle reinforced stainless steel loses less (approximating the stainless steel), while the white iron has the most serious corrosion.
Fig.7 describes the SEM images of the three materials after wear. Seen from Fig.7(a), the VC particle reinforced stainless steel composite exhibits slight corrosion on the surface, while the white iron has heavy pitting corrosion on the surface shown in Fig.7(b). There also exist some corrosion traces on the stainless steel surface, but the appearance of reticular protective film leads to good corrosion resistance.
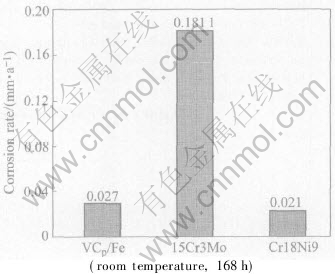
Fig.6 Corrosive rate in 90%H2SO4
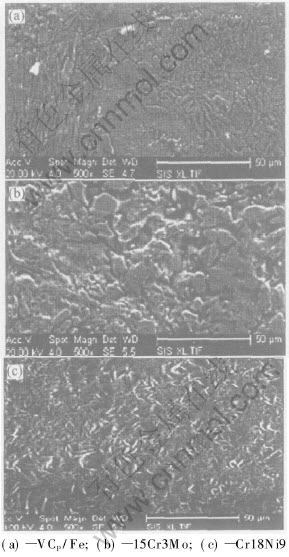
Fig.7 SEM images of sample surfaces
As seen from the corrosion rate and surface morphology, the VC particle reinforced stainless steel composite exhibits the corrosion resistance in concentrated sulfuric acid much better than that of the wear-resistant white iron, while approximating that of the stainless steel. Thus it can be used as wear-resistant materials with good corrosion resistance.
4 CONCLUSIONS
1) Under the same load and grinding abrasion, the wear rate of the wear-resistant white iron is the minimal, that of the VC particle reinforced stainless steel composite is higher, and that of the stainless steel is the maximum.
2) During the wet grinding abrasion, both grinding wear and electrochemical corrosion occur, wherein the VC particle reinforced stainless steel composite shows optimal resistance to wear and corrosion.
3) During the corrosion experiments, the VC particle reinforced stainless steel composite shows excellent corrosion resistance that approximates that of the stainless steel, far better than the wear-resistant white iron.
4) The obtained composite exhibits good resistance to both wear and corrosion, which has wide application potential in wear and corrosion resistant fields.
REFERENCES
[1]Terry B S, Chinyamakobvu O S. In situ production of Fe-TiC composites by reactions in liquid iron alloys [J]. Journal of Materials Science Letters, 1991, 10: 628-629.
[2]YAN You-wei, WEI Bo-kang, MEI Zhi, et al. A new preparing technique of in-situ particle intensified ferro-composite—reaction casting process [J]. Modern Cast Iron, 1998, 2: 50-54.(in Chinese)
[3]ZHANG De-ming, LI Feng-zhen, LIU Zhao-jing, et al. Optimization of preparing technology of in situ TiCp/Fe composites[J]. Journal Harbin Univ Sci & Tech, 2002, 7(4): 22-26.(in Chinese)
[4]YAO Xiu-rong, LIU Zhao-jing, LI Feng-zhen, et al. Effect of heat-treatment on microstructures and mechanical properties of in-situ TiCp/Fe composites [J]. The Chinese Journal of Nonferrous Metals, 2003,13(3): 640-644.(in Chinese)
[5]Pagounis E, Lindroos V K. Processing and properties of particulate reinforced steel matrix composites[J]. Materials Science and Engineering, 1998, A246: 221-234.
[6]XI Gan, LEI Ying, HU Ke-jun. Overseas application of vanadium [J]. World Nonferrous Metals, 2000, 2:13-21.(in Chinese)
[7]YAO Xiu-rong, HAN Jie-cai, GAO Yue-gang, et al. Optimization of preparing technology of steel-base composite [J]. Materials Engineering, 2005.(in Press)
[8]Edition Committee for Metal Corrosion Manual in Chinese Association of Corrosion and Protection, Metal Corrosion Manual [M]. Shanghai: Science and Technology Press, 1987.(in Chinese)
[9]KE Jian-hong, LIU Mao. Anodic polarization analysing alloys corrosion resistance [J]. J Wenzhou Teachers College (Nat Sci), 1998, 19(3): 36-39. (in Chinese)
[10]WANG Ke-fei, WANG Shi-jun. The abrasive wear behavior and wear resistance of white cast iron[J]. J East China Univ of Metallurgy, 1997,14(2): 110-115. (in Chinese)
[11]WANG Yi-san, ZHANG Xin-yuan, HUANG Wen, et al. Structure and wear-resistance of Fe-VC surface composite produced by liquid reactions [J]. Acta Materiae Compositae Sinica, 2000, 17(1): 71-75.(in Chinese)
[12]WU Cheng-jian, CHEN Guo-liang, QIANG Wen-jiang. Metal Material Science [M]. Beijing: Metallurgical Industry Press, 2001.(in Chinese)
[13]GAO Yi-min, ZHANG Feng-hua, XING Jian-dong, et al. Erosive wear resistance of particle reinforced stainless steel composites [J]. J Xian Jiaotong Univ, 2001, 35(7): 727-731.(in Chinese)
[14]TANG Wu, GAO Yi-min, BAO Chong-gao. Study on corrosion of aluminium oxide/ stainless steel composites [J]. J Xian Jiaotong Univ, 2000, 34(5): 106-108.(in Chinese).
Foundation item: Project(E0216) supported by the Natural Science Foundation of Heilongjiang Province; Project(GCO4A211) supported by the Key Technologies R&D Program of Heilongjiang Province
Received date: 2005-02-17; Accepted date: 2005-08-18
Correspondence: YAO Xiu-rong, Professor, PhD candidate; Tel: +86-451-86402392; E-mail: yaoxiurong1@sina.com.cn


