Trans. Nonferrous Met. Soc. China 27(2017) 676-685
φ-pH diagram of As-N-Na-H2O system for arsenic removal during alkaline pressure oxidation leaching of lead anode slime
Yun-long HE1, Rui-dong XU1,2, Shi-wei HE1, Han-sen CHEN1, Kuo LI1, Yun ZHU1, Qing-feng SHEN1
1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Kunming University of Science and Technology, Kunming 650093, China
Received 29 February 2016; accepted 31 August 2016
Abstract:
In order to illustrate the thermodynamic characteristics of arsenic during alkaline pressure oxidation leaching process of lead anode slime (NaNO3 as oxidant; NaOH as alkaline reagent), the φ-pH diagrams of As-Na-H2O, N-H2O, As-N-Na-H2O systems at ionic mass concentration of 0.1 mol/kg and temperatures of 298, 373, 423 and 473 K were established according to thermodynamic calculation. The results show that the existence forms of arsenic are associated with pH value, which mainly exists in the forms of H3AsO4, 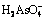 ,
, 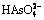 ,
, 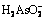 and As2O3 in lower pH region, while it mainly exists in the form of
and As2O3 in lower pH region, while it mainly exists in the form of 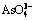 when pH>11.14. High alkali concentration and high temperature are advantageous to the arsenic leaching. The alkaline pressure oxidation leaching experiments display that the tendency of arsenic leaching rate confirms the thermodynamic analysis results obtained from the φ-pH diagrams of As-N-Na-H2O system, and the highest leaching rate of arsenic reaches 95.85% at 453 K.
when pH>11.14. High alkali concentration and high temperature are advantageous to the arsenic leaching. The alkaline pressure oxidation leaching experiments display that the tendency of arsenic leaching rate confirms the thermodynamic analysis results obtained from the φ-pH diagrams of As-N-Na-H2O system, and the highest leaching rate of arsenic reaches 95.85% at 453 K.
Key words:
φ-pH diagram; As-N-Na-H2O system; lead anode slime; leaching; arsenic removal;
1 Introduction
Lead anode slime is an important secondary product from the process of lead electrolytic refining, which is rich in valuable elements such as bismuth, antimony, arsenic, lead, gold and silver [1,2]. As we know, the environmental pollution, low quality of production and complicated process will be caused during the lead anode slime treatment because arsenic is a harmful element. Therefore, the arsenic removal is very essential in pyrometallurgical and hydrometallurgical processes in order to extract other valuable metals from lead anode slime [3]. At present, the treatment process of lead anode slime can be classified in the following two methods as pyrometallurgy and hydrometallurgy: the pyrometallurgy mainly includes reduction roasting [4], volatilization roasting or eliminating arsenic in vacuum [5], and the hydrometallurgy mainly includes oxidizing acid leaching [6], oxidation alkaline leaching [7] or chloridization leaching [8,9]. Pressure leaching is an effective method and widely used for metal extraction from different raw materials [10-13], and alkaline pressure oxidation leaching of lead anode slime is proposed in order to remove arsenic in this research.
The φ-pH diagram (potential-pH diagram) is an useful tool in thermodynamic characteristics analysis during the hydrometallurgy process [14,15]. A rapid and reliable method for intuitive assessment of thermodynamic equilibriums and reaction feasibility can be provided easily according to φ-pH diagram. In addition, it can also be widely used in many other fields such as corrosion and anticorrosion of metal, mineral geology [16-18]. The φ-pH diagram of As-H2O system at different temperatures has been investigated [19], but the φ-pH diagrams of As-N-Na-H2O system for pressure oxidation alkaline leaching of arsenic-rich lead anode slime have never been reported. This research has done some works in order to explain the thermodynamic characteristics of arsenic in the pressure oxidation leaching progress by means of the construction of the φ-pH diagrams of As-N-Na-H2O system. Based on φ-pH diagrams of As-N-Na-H2O system, an originally thermodynamic guidance can be obtained for the arsenic removal during alkaline pressure oxidation leaching of lead anode slime.
2 Thermodynamics
2.1 Thermodynamic calculation method
All the chemical reactions in the hydrometallurgical process can be presented simply as follows:
aA+nH++ze=bB+cH2O (1)
where a, n, b and c are the stoichiometric coefficients for species A, H+, B and H2O in the electrochemical reaction, respectively, and z is the electron transfer number of the electrode reaction. The Gibbs free energy of Eq. (1) can be shown explicitly as

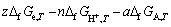 (2)
(2)
Some interrelated equations about Gibbs free energy of Eq. (1) can be shown as follows:
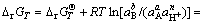
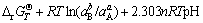 (3)
(3)
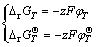 (4)
(4)
According to Eqs. (2)-(4), Nernst equation can be deduced as [20]
-zFφT =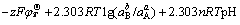 (5)
(5)
Equation (5) can be divided into the following three situations and the φ-pH equations in different situations can be shown as follows:
Only electron is involved in the reaction (z≠0, n=0):
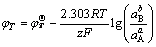 (6)
(6)
Only H+ is involved in the reaction (n≠0, z=0):
 (7)
(7)
Both electrons and H+ are involved in the reaction (n≠0, z≠0):
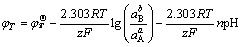 (8)
(8)
where △fGT is the formation Gibbs free-energy of a certain specie at temperature T; △rGT and 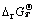 are the free energy and standard free energy of reaction at temperature T, respectively; φT and
are the free energy and standard free energy of reaction at temperature T, respectively; φT and 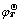 are the electrochemical potential and standard electrochemical potential for the reaction at temperature T, respectively; F is the Faraday’s constant and R is the molar gas constant; aA and aB are the overall activities of species A and B, respectively. So, the φ-pH diagram based on the Eqs. (6)-(8) can be established in different situations as long as the φr or
are the electrochemical potential and standard electrochemical potential for the reaction at temperature T, respectively; F is the Faraday’s constant and R is the molar gas constant; aA and aB are the overall activities of species A and B, respectively. So, the φ-pH diagram based on the Eqs. (6)-(8) can be established in different situations as long as the φr or 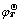 or aA and aB at the certain temperature can be known.
or aA and aB at the certain temperature can be known.
2.2 Species and thermodynamic equilibrium
The leaching process of arsenic from lead anode slime is to make insoluble arsenic turn into dissolvable forms. NaOH and NaNO3 were employed as leaching agents during the alkaline oxidation leaching, leading to the fact that nitrogen and sodium were involved in the system. Therefore, many elements and species must be involved [21]. The data of the thermodynamic calculation in this research are obtained from Ref. [22] except as otherwise indicated, and the thermodynamic data of the main substances in As-N-Na-H2O system are listed in Table 1.
Table 1 Thermodynamic data of main substances in As-N-Na-H2O system for lead anode slime leaching process
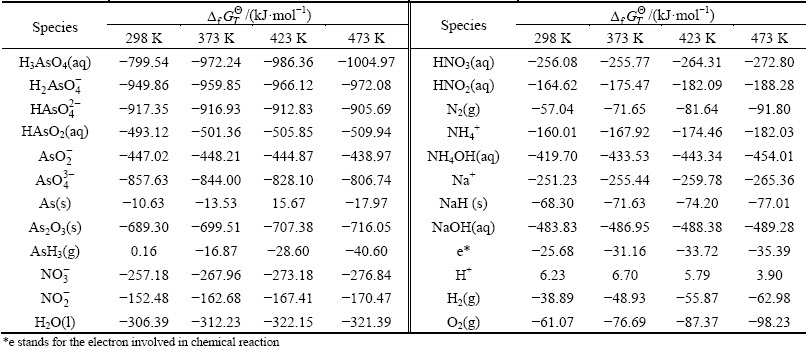
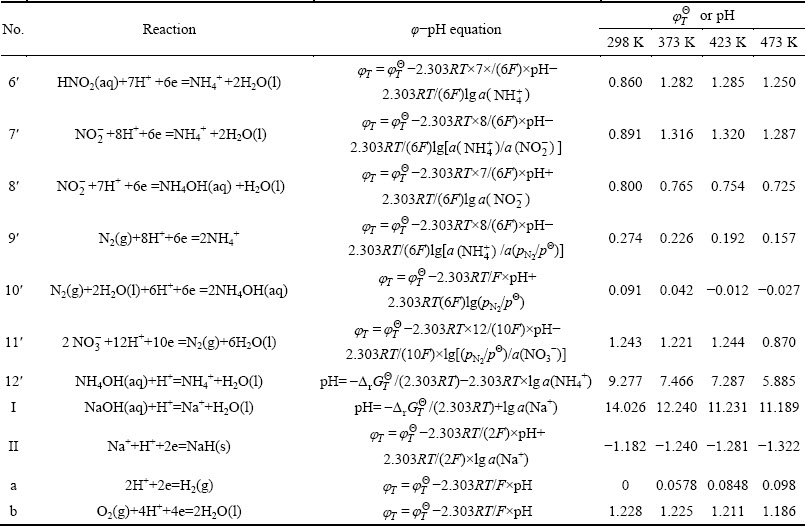
The arsenic leaching is available based on the chemical reactions among the species. Nernst equation can be used to illustrate the thermodynamic equilibrium. The φ-pH diagrams for the leaching progress can be established according to the Nernst equation. The relationship expressions between φ and pH value were calculated according to Eqs. (2)-(8). The considered chemical reactions and their φ-pH formulas are listed in Table 2.
Table 2 Electrode reaction and standard potential value in As-N-Na-H2O system for lead anode slime leaching process
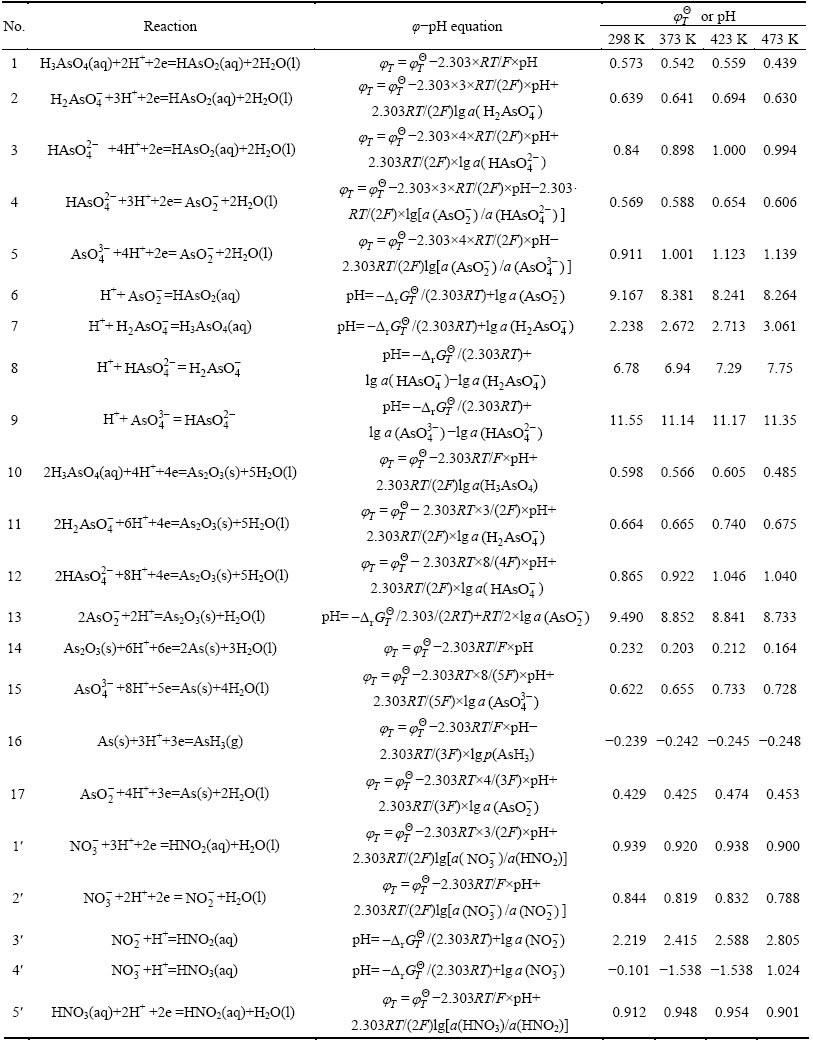
to be continued
continued
2.3 φ-pH diagram of As-Na-H2O system
The φ-pH diagrams of As-Na-H2O system at different temperatures are constructed in Fig. 1 according to equilibrium equations listed in Table 2. The condition parameters are established as the molality of all the species is 0.1 mol/kg and temperatures range from 298 to 473 K.
Arsenic exists as acid radical in the system when φ is positive. H3AsO4(aq) and 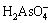 exist in the acid region. They exist in the forms of
exist in the acid region. They exist in the forms of 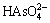 and HAsO2 when pH values are between 7 and 11.
and HAsO2 when pH values are between 7 and 11. 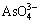 is stable when pH value is higher than 11. The stable region of H3AsO4(aq), HAsO2(aq),
is stable when pH value is higher than 11. The stable region of H3AsO4(aq), HAsO2(aq), 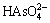 , As2O3(s),
, As2O3(s), 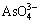 and As(s) superimposes largely in the stable region of water, which approves the feasibility of acid or alkali leaching of arsenic in the arsenic-rich lead anode slime in water solution [23].
and As(s) superimposes largely in the stable region of water, which approves the feasibility of acid or alkali leaching of arsenic in the arsenic-rich lead anode slime in water solution [23].
Based on the φ-pH diagrams of As-Na-H2O system in Fig. 1, thermodynamics behaviors of arsenic in alkaline pressure oxidation leaching process were discussed. The soluble arsenic ions should be the main research object. The main features of the φ-pH diagrams of As-Na-H2O system are summarized as follows.
1) The stability region of NaOH(aq) increases with increasing leaching temperature.
2) The stability region of 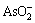 increases with the rise of temperature. More
increases with the rise of temperature. More 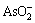 will change into
will change into 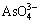 in the high pH region. Therefore, high leaching temperature and high pH value or high alkali concentration will be beneficial to the arsenic leaching. Consequently, high temperature and high alkali concentration are very essential at the beginning of the leaching.
in the high pH region. Therefore, high leaching temperature and high pH value or high alkali concentration will be beneficial to the arsenic leaching. Consequently, high temperature and high alkali concentration are very essential at the beginning of the leaching.
3) There is a large area of As2O3(s) containing in the stability region of water. In these superimposed areas, there is a thermodynamic equilibrium region between water and As2O3(s). The chemical reaction can be written as As2O3(s)+H2O(aq)=2HAsO2(aq), so, As2O3(s) and HAsO2(aq) will coexist in these regions [21].
2.4 φ-pH diagrams of N-H2O system
Dissolved nitrate with several different valences can be involved in many reactions in the solution, and it can be transformed among various forms according to the situation. During the leaching process of arsenic from lead anode slime, NaNO3 is employed as oxidant, nitrogen atom in 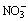 will get electron in the reaction and its valence will decrease. Most insoluble arsenic in the lead anode slime mainly exists as the lower valence state, while
will get electron in the reaction and its valence will decrease. Most insoluble arsenic in the lead anode slime mainly exists as the lower valence state, while 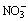 plays an electron acceptor role in the electrochemical reaction, which promotes the dissolution of arsenic during the alkaline oxidation leaching, and solvable arsenic is usually at higher valence state in the solution.
plays an electron acceptor role in the electrochemical reaction, which promotes the dissolution of arsenic during the alkaline oxidation leaching, and solvable arsenic is usually at higher valence state in the solution.
In order to analyze the thermodynamic equilibriums of nitrogenous compounds in the solution, the φ-pH diagrams of N-H2O system are established and shown in Fig. 2. The condition parameters are established when the molality of all the species is 0.1 mol/kg and temperatures range from 298 to 473 K.
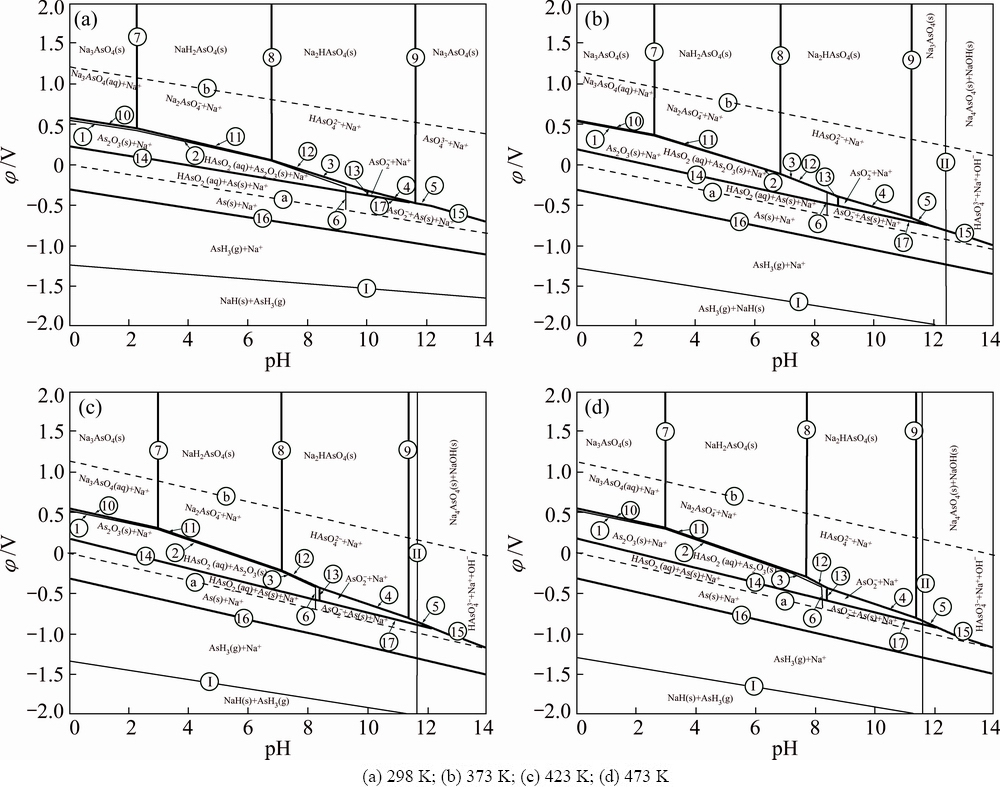
Fig. 1 φ-pH diagrams of As-Na-H2O system at different temperatures
The thermodynamic characteristics of N-H2O at different temperatures are summarized as follows.
1) The stable regions of NH4OH(aq) and 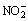 increase apparently with increasing temperature.
increase apparently with increasing temperature.
2) The stable regions of 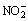 ,
, 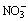 and N2 superimpose with the stable region of water, which indicates the thermodynamic possibility of the redox reactions among nitrogenous compounds in the solution. So, it is reasonable and feasible to use NaNO3 as the oxidant during the alkaline oxidation leaching of lead anode slime.
and N2 superimpose with the stable region of water, which indicates the thermodynamic possibility of the redox reactions among nitrogenous compounds in the solution. So, it is reasonable and feasible to use NaNO3 as the oxidant during the alkaline oxidation leaching of lead anode slime.
3) Large proportion of N2 stable region superimposes with the stable region of water, which indicates the possibility of N2 formation during the leaching process.
2.5 φ-pH diagram of As-N-Na-H2O system
The φ-pH diagrams of As-N-Na-H2O system are constructed at different temperatures for the leaching of arsenic from lead anode slime, and shown in Fig. 3. It can be seen from Fig. 3 that many stable regions of arsenic compounds and nitrate superimpose with the water stable region, therefore, the occurrence of various reactions among the considered species is possible in the solution.
The main thermodynamic features of As-N-Na- H2O system can be summarized as follows.
1) Various forms of arsenic are associated with pH value. It mainly exists as H3AsO4(aq), 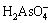 ,
, 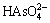 , H2AsO2(aq) and As2O3(s) in lower pH value regions, and it mainly exists as
, H2AsO2(aq) and As2O3(s) in lower pH value regions, and it mainly exists as 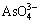 when pH value is higher than 11.14.
when pH value is higher than 11.14.
2) In lower pH region, when φ is less than -0.137 V, As2O3 will transform into AsH3 according to electrode reactions (Eqs. (14) and (16) listed in Table 2).
3) The stable areas of 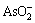 and NaOH(aq) increase with the rise of temperature and pH value, which indicates that more
and NaOH(aq) increase with the rise of temperature and pH value, which indicates that more 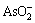 will generate when temperature and alkali concentration are high, meaning that more soluble trivalent arsenic will be oxidized into high valence states such as H3AsO4(aq),
will generate when temperature and alkali concentration are high, meaning that more soluble trivalent arsenic will be oxidized into high valence states such as H3AsO4(aq), 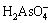 ,
, 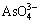 or
or 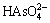 , this transversion complies with the following equations respectively.
, this transversion complies with the following equations respectively.
HAsO2(aq)+2H2O(l)-2e=H3AsO4(aq)+2H+ (9)
HAsO2(aq)+2H2O(l)-2e=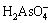 +3H+ (10)
+3H+ (10)
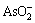 +2H2O(l)-2e=
+2H2O(l)-2e=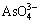 +4H+ (11)
+4H+ (11)
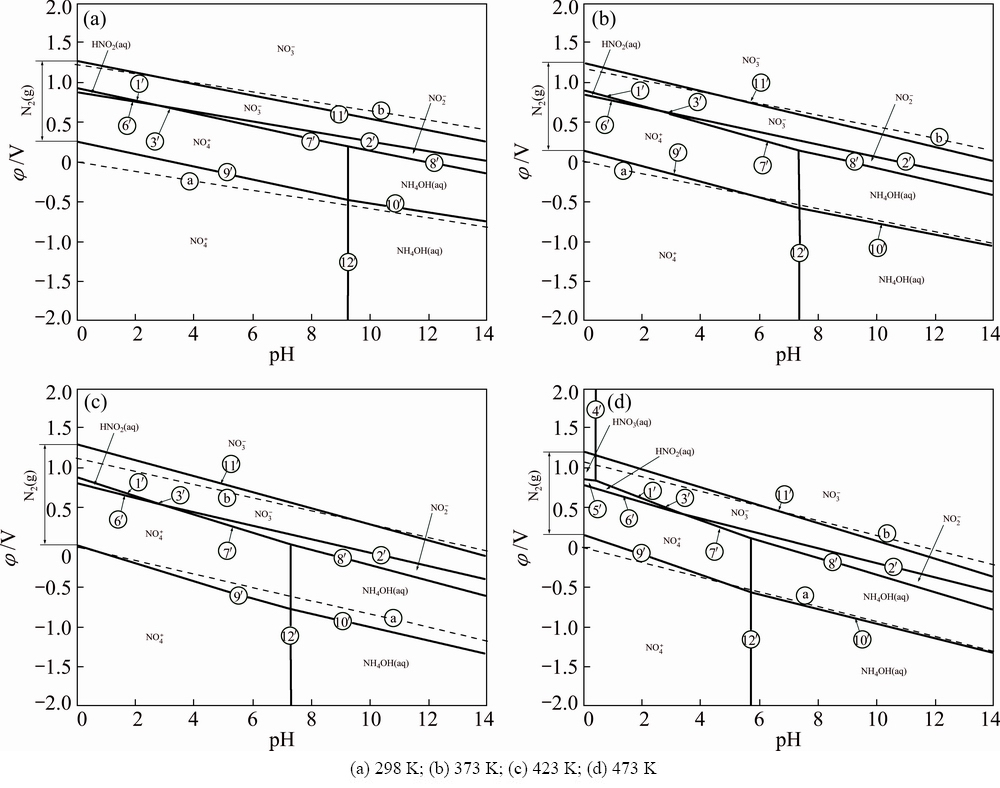
Fig. 2 φ-pH diagram of N-H2O system at different temperatures
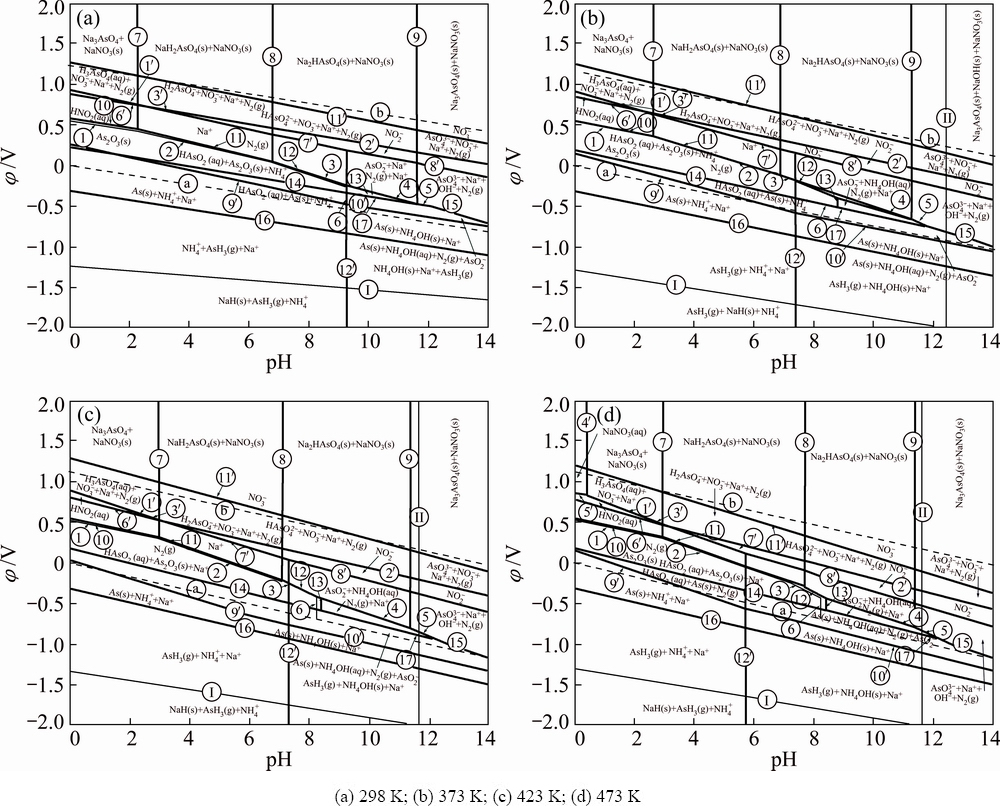
Fig. 3 φ-pH diagrams of As-N-Na-H2O system at different temperatures
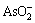 +2H2O(l)-2e=
+2H2O(l)-2e=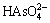 +3H+ (12)
+3H+ (12)
HAsO2(aq)+2H2O(l)-2e=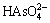 +4H+ (13)
+4H+ (13)
4) More As2O3(s) will dissolve with the rise of temperature and pH value. Main chemical reaction of trioxide arsenic dissolution in alkaline condition is shown as follows:
As2O3(s)+2OH-=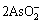 +H2O(l) (14)
+H2O(l) (14)
There is a large amount of OH- when pH value is high, and As2O3(s) can dissolve more efficiently in the alkaline condition according to the above equation.
5) During the alkaline oxidation leaching of lead anode slime, arsenic will be removed in the form of Na3AsO4. The formation reaction of 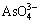 can be shown as follows:
can be shown as follows:
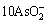 +16OH-+4
+16OH-+4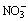 =10
=10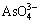 +8H2O(l)+N2(g) (15)
+8H2O(l)+N2(g) (15)
The transformation reaction between As2O3 (s) and Na3AsO4(aq) in the alkaline leaching system can be written as
5As2O3(s)+26NaOH(aq)+4NaNO3(aq)=10Na3AsO4(aq)+13H2O(l)+2N2(g) (16)
Based on the above description, more As2O3(s) will transform into 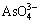 when OH- concentration is high. Consequently, alkaline pressure oxidation leaching is feasible to remove arsenic from lead anode slime because As2O3 (s) is the main phase. The usage of NaNO3 (aq) and NaOH (aq) as oxidant and alkaline reagent respectively during the leaching process is reasonable because an advantageous condition for the arsenic leaching can be provided.
when OH- concentration is high. Consequently, alkaline pressure oxidation leaching is feasible to remove arsenic from lead anode slime because As2O3 (s) is the main phase. The usage of NaNO3 (aq) and NaOH (aq) as oxidant and alkaline reagent respectively during the leaching process is reasonable because an advantageous condition for the arsenic leaching can be provided.
AsH3 is a poisonous substance which is generated easily in the acid environment. It can be seen from Fig. 3 that there are large stable areas of AsH3(g) in the low pH region, which demonstrates a high thermodynamic possibility of AsH3 formation. Therefore, it is not commonly recommendable to remove arsenic by strong acid leaching process in order to avoid the formation of AsH3(g).
3 Arsenic removal experiment during alkaline pressure oxidation leaching of lead anode slime
Lead anode slime containing arsenic was employed as the raw materials, and its chemical compositions are shown in Table 3. It can be seen that bismuth (Bi) is the highest content metal and its content is 45.25%, the contents of arsenic (As), antimony (Sb) and lead (Pb) are 10.68%, 11.8% and 11.43%, respectively, the contents of gold (Au) and silver (Ag) are 24.3 and 3408 g/t respectively. In addition, there are slight tin (Sn) and copper (Cu). Arsenic must be removed before extracting bismuth and enriching gold and silver from lead anode slime.
Table 3 Chemical composition of metallic element in lead anode slime (mass fraction, %)

The anode slime was ground to less than 250 μm and then put into the autoclave, the alkaline pressure oxidation leaching process of arsenic was carried out under the following conditions of pressure of 0.8 MPa, liquid to solid ratio of 7:1, leaching time of 180 min, stirring speed of 300 r/min, NaOH concentration of 200 g/L and NaNO3 concentration of 15% (the mass ratio of NaNO3 to lead anode slime is 15%). In addition, the temperatures of the alkaline pressure oxidation leaching were controlled from 313 to 493 K. The schematic diagram of the experimental apparatus is shown in Fig. 4.
The effect of temperature on leaching rate of arsenic from lead anode slime is shown in Fig. 5. The result indicates that the arsenic leaching rate increases from 13.86% to 95.55% when the temperature increases from 313 to 433 K, and it reaches the highest value of 95.85% at 453 K. Thereafter, the leaching rate begins to decrease.
The above leaching rate of arsenic confirms the thermodynamic analysis based on the φ-pH diagrams of As-N-Na-H2O system at different temperatures. As shown in φ-pH diagrams of As-N-Na-H2O from 298 to 473 K, the stabile region of 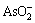 gradually increases with the increase of temperature, displaying that more As2O3(s) will transform into the soluble
gradually increases with the increase of temperature, displaying that more As2O3(s) will transform into the soluble 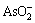 in the high pH region, complying with Eqs. (14) and (17).
in the high pH region, complying with Eqs. (14) and (17).
As2O3(s)+H2O=2HAsO2(aq) (17)
Therefore, high temperature and high pH value will be advantageous to the arsenic leaching, and higher leaching rate of arsenic can be obtained during the alkaline pressure oxidation leaching of lead anode slime.
Lixivium was cooled down and crystallized after finishing alkaline pressure oxidation leaching of lead anode slime, and the phase structures of crystals were measured by XRD and the result is shown in Fig. 6. It can be concluded from Fig. 6 that the phase compositions of the crystals obtained from lixivium are Na3AsO4.10H2O. During the course of alkaline pressure oxidation leaching, As2O3 in lead anode slime is dissolved in the form of 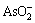 according to Eq. (14). Thereafter,
according to Eq. (14). Thereafter, 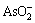 will be oxidized to
will be oxidized to 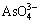 according to Eq. (15) when NaNO3 is used as oxidant in the alkaline conditions, leading to the fact that large amounts of
according to Eq. (15) when NaNO3 is used as oxidant in the alkaline conditions, leading to the fact that large amounts of 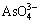 and Na+ exist in the system.
and Na+ exist in the system.
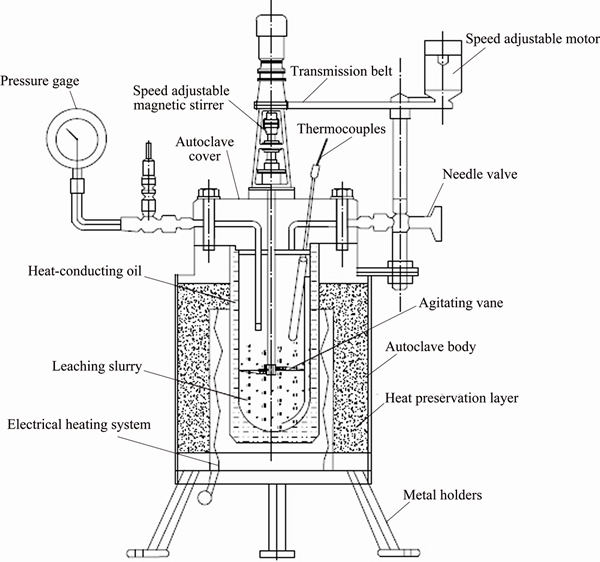
Fig. 4 Schematic diagram of experimental apparatus
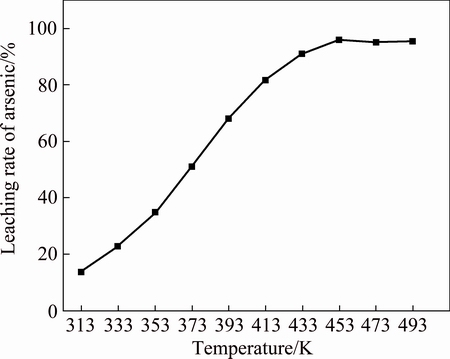
Fig. 5 Effect of temperature on leaching rate of arsenic from lead anode slime
Therefore, the main phase composition of arsenic is Na3AsO4(aq) in the lixivium. The transformation reaction between As2O3(s) and Na3AsO4 (aq) can be written as Eq. (16). The above research results agree well with the thermodynamic prediction according to φ-pH diagrams of As-N-Na-H2O system.
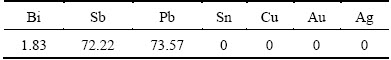
In addition, under the following alkaline pressure oxidation leaching from lead anode slime as NaOH concentration of 200 g/L, NaNO3 concentration of 15%, pressure of 0.8 MPa, liquid to solid ratio of 7:1, leaching time of 180 min, leaching temperature of 453 K and stirring speed of 300 r/min, the highest leaching rate of arsenic is obtained and it reaches 95.85%. In addition, the leaching rates of other elements in the lead anode slime are shown in Table 4. As shown in Table 4, the leaching rates of antimony and lead are 72.22% and 73.57%, respectively, and the leaching rate of bismuth is only 1.83%. The following metals as tin, copper, gold and silver all enter the leaching residue.
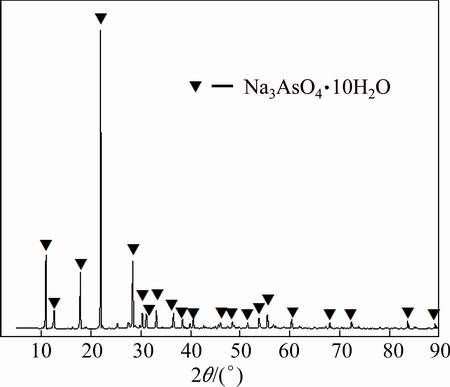
Fig. 6 XRD pattern of crystals from arsenic lixivium
Table 4 Leaching rates of other elements in lead anode slime (%)
Therefore, the removal of arsenic, antimony and lead by alkaline pressure oxidation leaching method provides the condition for the extracting bismuth and enriching gold and silver from lead anode slime.
4 Conclusions
1) The φ-pH diagrams of As-N-Na-H2O systems at different temperatures were established to illustrate the thermodynamic characteristics of arsenic during alkaline oxidation leaching process of lead anode slime.
2) The existing forms of arsenic are associated with pH value. It mainly exists as H3AsO4(aq), 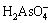 ,
, 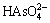 , H2AsO2(aq) and As2O3(s) in lower pH value region; whereas it mainly exists as
, H2AsO2(aq) and As2O3(s) in lower pH value region; whereas it mainly exists as 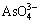 when pH value is higher than 11.14. High alkali concentration and high thermodynamic temperature are advantageous to the arsenic leaching.
when pH value is higher than 11.14. High alkali concentration and high thermodynamic temperature are advantageous to the arsenic leaching.
3) The tendency of arsenic leaching rate confirms the thermodynamic analysis results obtained from the φ-pH diagrams of As-N-Na-H2O system, and the highest leaching rate of arsenic reaches 95.85% at thermodynamic temperature of 453 K during the course of alkaline pressure oxidation leaching.
References
[1] LIN De-qiang, QIU Ke-qiang. Removing arsenic from anode slime by vacuum dynamic evaporation and vacuum dynamic flash reduction [J]. Vacuum, 2012, 86: 1155-1160.
[2] LIU Chao. Process study on enrichment and recovery of tellurium from lead anode slime and its production practice [J]. China Nonferrous Metallurgy, 2012, 41(2): 25-29. (in Chinese)
[3] LIU Wei-feng, YANG Tian-zu, LIU You-nian, CHEN Lin, ZHANG Du-chao, TANG Mo-tang. Selection of pretreatment process for removing base metals from lead anode slime [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(2): 549-558. (in Chinese)
[4] WANG Chun-guang, HU Liang, CHEN Jia-xi. Comprehensive recovery technique for lead anode slime [J]. Yunnan Metallurgy, 2008, 37(6): 78-80. (in Chinese)
[5] LI Liang, TIAN Yang, LIU Da-chun, ZHOU Hou-jun, DAI Yong-nian, YANG Bin. Pretreatment of lead anode slime with low silver by vacuum distillation for concentrating silver [J]. Journal of Central South University, 2013, 20(3): 615-621.
[6] YANG Xi-yun, GONG Zhu-qing, LI Yi-bing. Review on the hydrometallurgical process of recovery lead from lead anode slime [J]. Mining and Metallurgical Engineering, 2002, 22(4): 73-75. (in Chinese)
[7] CAI Lian-bin,LIU Wei,CHAI Li-yuan. Study on Pre-treatment process of arsenic removal for arsenic-rich lead anode slime [J]. Mining and Metallurgical Engineering, 2007, 27(6): 44-47. (in Chinese)
[8] CHEN Jin-zhong, YANG Tian-zu. Chlorination-leaching of lead anode slime with high antimony and low silver contents at controlled potential [J]. Journal of Central South University (Science and Technology), 2010, 41(1): 44-49. (in Chinese)
[9] RUAN Shu-feng, YIN Fei, WANG Cheng-yan, WANG Jun, WANG Zhen-wen. Study on selective leaching from lead anode slime by H2SO4+NaCl [J]. Mining and Metallurgy, 2012, 21(3): 30-32. (in Chinese)
[10] WANG Si-fu, WEI Chang, DENG Zhi-gan, LI Cun-xiong, LI Xin-bing, WU Jun, WANG Ming-shuang, ZHANG Fan. Extraction of molybdenum and nickel from Ni-Mo ore by pressure acid leaching [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3083-3088.
[11] WU Cheng-you, YU Hong-fa, ZHANG Hui-fang. Extraction of aluminum by pressure acid-leaching method from coal fly ash [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2282-2288.
[12] PADILLA R, VEGA D, RUIZ M.C. Pressure leaching of sulfidized chalcopyrite in sulfuric acid-oxygen media [J]. Hydrometallurgy, 2007, 86: 80-88.
[13] XU Zhi-feng, NIE Hua-ping, LI Qiang, LU Qiu-hu, WANG Wei, YUE Ri-hui. Pressure leaching technique of smelter dust with high-copper and high-arsenic [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(S1): s59-s63.
[14] YOU Hai-xia, XU Hong-bin, ZHANG Yi, ZHENG Shi-li, GAO Yi-ying. Potential-pH diagrams of Cr-H2O system at elevated temperatures [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): s26-s31.
[15] MU Wang-zhong, ZHANG Ting-an, LIU Yan, GU Yan, DOU Zhi-he,  Guo-zhi, BAO Li, ZHANG Wei-guang. E-pH diagram of ZnS-H2O system during high pressure leaching of zinc sulfide [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 2012-2019.
Guo-zhi, BAO Li, ZHANG Wei-guang. E-pH diagram of ZnS-H2O system during high pressure leaching of zinc sulfide [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(10): 2012-2019.
[16] ANDERKO A, SANDERS S J, YOUNG R D. Real-solution stability diagrams: A thermodynamic tool for modeling corrosion in wide temperature and concentration ranges [J]. Corrosion Science, 1997, 53(1): 43-53.
[17] KINNIBURGH D G, COOPER D M. Predominance and mineral stability diagrams revisited [J]. Environmental Science & Technology, 2004, 38(13): 3641-3648.
[18] FISHTIK I. Thermodynamic stability relations in redox systems [J]. Environmental Science & Technology, 2006, 40: 1902-1910.
[19] JIN Zhe-nan, JIANG Kai-xi, WEI Xu-jun, WANG Hai-bei. Potential-pH diagrams of As-S-H2O system at high temperature [J]. Mining and Metallurgy, 1999, 4(8): 46-50. (in Chinese)
[20] FU Xian-cai. Physical chemistry [M]. Beijing: Higher Education Press, 2006: 89-96. (in Chinese)
[21] POURBAIX M. Atlas of electrochemical equilibria in aqueous solutions [M]. Houston: National Association of Corrosion Engineers, 1974.
[22] YANG Xian-wang. Handbook of thermodynamic data in aqueous solutions at high temperature [M]. Beijing: Metallurgical Industry Press, 1983. (in Chinese)
[23] MU Wang-zhong, ZHANG Ting-an, DOU Zhi-he,  Guo-zhi, LIU Yan. φ-pH diagram of V-Ti-H2O system during pressure acid leaching of converter slag containing vanadium and titanium [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(9): 2078-2086.
Guo-zhi, LIU Yan. φ-pH diagram of V-Ti-H2O system during pressure acid leaching of converter slag containing vanadium and titanium [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(9): 2078-2086.
铅阳极泥碱性加压氧化浸出脱砷过程中As-N-Na-H2O系的φ-pH图
何云龙1,徐瑞东1,2,何世伟1,陈汉森1,李 阔1,朱 云1,沈庆峰1
1. 昆明理工大学 冶金与能源工程学院,昆明 650093;
2. 昆明理工大学 省部共建复杂有色金属资源清洁利用国家重点实验室,昆明 650093
摘 要:为阐明砷在铅阳极泥碱性加压氧化浸出过程(NaNO3为氧化剂; NaOH为碱性试剂)中的热力学特性,通过热力学计算绘制体系离子质量浓度为0.1 mol/kg,温度为298、373、423和473 K条件下的As-Na-H2O、N-H2O和As-N-Na-H2O系的φ-pH图。结果表明,砷的存在形态与pH值有关。当pH值较低时,砷主要以H3AsO4、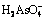 、
、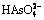 、
、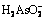 及As2O3的形式存在;当pH>11.14时,砷主要以
及As2O3的形式存在;当pH>11.14时,砷主要以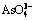 形式存在,高碱浓度及高温对砷浸出有利。碱性加压氧化浸出实验表明,砷浸出率变化趋势与As-N-Na-H2O系φ-pH图的热力学分析结果一致,在453 K时砷的最佳浸出率为95.85%。
形式存在,高碱浓度及高温对砷浸出有利。碱性加压氧化浸出实验表明,砷浸出率变化趋势与As-N-Na-H2O系φ-pH图的热力学分析结果一致,在453 K时砷的最佳浸出率为95.85%。
关键词:φ-pH 图;As-N-Na-H2O系;铅阳极泥;浸出;脱砷
(Edited by Wei-ping CHEN)
Foundation item: Project (51564031) supported by the National Natural Science Foundation of China; Project (0201352042) supported by the Cooperation between School and Enterprise of China
Corresponding author: Rui-dong XU; Tel: +86-871-65198154; E-mail: rdxupaper@aliyun.com
DOI: 10.1016/S1003-6326(17)60075-X
Abstract: In order to illustrate the thermodynamic characteristics of arsenic during alkaline pressure oxidation leaching process of lead anode slime (NaNO3 as oxidant; NaOH as alkaline reagent), the φ-pH diagrams of As-Na-H2O, N-H2O, As-N-Na-H2O systems at ionic mass concentration of 0.1 mol/kg and temperatures of 298, 373, 423 and 473 K were established according to thermodynamic calculation. The results show that the existence forms of arsenic are associated with pH value, which mainly exists in the forms of H3AsO4, 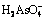 ,
, 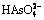 ,
, 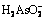 and As2O3 in lower pH region, while it mainly exists in the form of
and As2O3 in lower pH region, while it mainly exists in the form of 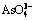 when pH>11.14. High alkali concentration and high temperature are advantageous to the arsenic leaching. The alkaline pressure oxidation leaching experiments display that the tendency of arsenic leaching rate confirms the thermodynamic analysis results obtained from the φ-pH diagrams of As-N-Na-H2O system, and the highest leaching rate of arsenic reaches 95.85% at 453 K.
when pH>11.14. High alkali concentration and high temperature are advantageous to the arsenic leaching. The alkaline pressure oxidation leaching experiments display that the tendency of arsenic leaching rate confirms the thermodynamic analysis results obtained from the φ-pH diagrams of As-N-Na-H2O system, and the highest leaching rate of arsenic reaches 95.85% at 453 K.


