DOI: 10.11817/j.issn.1672-7207.2018.08.006
喹啉-2-甲醛类希夫碱铜配合物的合成及其生物活性
胡茜,颜军,方紫薇,张寿春
(中南大学 化学与化工学院,湖南 长沙,410083)
摘 要:
啉-2-甲醛缩组胺希夫碱铜(II)配合物[Cu(Quin-Hist)Cl2],利用元素分析和红外光谱对其进行表征,通过X线单晶衍射确定其晶体结构。研究结果表明:该晶体属于单斜晶体,P21/n空间群,晶胞参数为a= 0.858 90(7) nm,b=1.282 38(10) nm,c=1.459 76(12) nm,β=99.346 0(10)°;配合物为畸变的三角双锥空间构型;该铜(II)配合物能与小牛胸腺DNA(CT-DNA)之间通过沟槽结合的方式相互作用;此外,铜(II)配合物对MCF-7人乳腺癌细胞系、A-549人非小细胞肺癌细胞系和SKOV-3人卵巢癌细胞系均有较强的体外细胞毒活性,且与顺铂相比,铜(II)配合物对MCF-7细胞系具有更强的体外细胞毒活性。
关键词:
中图分类号:O614.121 文献标志码:A 文章编号:1672-7207(2018)08-1878-06
Synthesis and bio-activity of copper(ii) complex of schiff-base derived from quinoline-2-carboxaldehyde
HU Xi, YAN Jun, FANG Ziwei, ZHANG Shouchun
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: A novel copper(II) complex, i.e. [Cu(Quin-Hist)Cl2] (Quin-Hist=2-(4-imidazolyl) ethylimino-quinoline), was prepared and structurally characterized by elemental analysis and IR spectra. The results show that the complex crystallized belongs to monoclinic space group P21/n with a=0. 858 90(7) nm, b=1.282 38(10) nm, c=1.459 76(12) nm, β=99.346 00(10)°. The copper(II) ion is situated in a trigonal bipyramid geometry. There is apparent interaction between the copper(II) complex and calf thymus DNA(CT-DNA) via a groove binding mode. Moreover, the copper(II) complex exhibits in vitro cytotoxicity against MCF-7,A549 and SKOV-3 cell lines. Especially, the copper(II) complex shows higher growth inhibitory rates than cisplatin against MCF-7 cell line.
Key words: copper complex; schiff-base; crystal structure; DNA binding; cytotoxicity
癌症是导致当今社会死亡率最高的疾病之一,它严重威胁了人们的身体健康。顺铂是目前临床上常用的一种抗癌药物,但其严重的毒副作用、耐药性及难代谢的缺陷限制了它的临床使用,因此,研发出高效、低毒、水溶性好、抗癌范围广的新型小分子金属配合物受到了人们越来越多的关注[1-4]。铜是人体必需的微量元素之一,是人体内蛋白质和酶的重要组成部分,在生物体系中具有重要作用。与铂相比,铜具有更高的生物兼容性和更低的毒性,因此,铜配合物被认为是继铂类配合物后最有应用前景的抗癌药物之一[5]。近年来,人们合成了大量铜配合物并发现这些配合物具有较强的抗癌活性[6]。在这些配合物中,希夫碱类铜配合物的研究尤为引人注目。许多希夫碱化合物本身具有一定的生物活性,希夫碱配合物也被证明具有较强的抗菌、抗炎与抗癌活性[7-8]。ZHANG等[9-10]已报道多个喹啉-2-甲醛缩氨基硫脲希夫碱配合物,这些配合物可与DNA结合,能氧化切割DNA,且对肿瘤细胞有较强的生长抑制作用。为了进一步研究喹啉-2-甲醛类希夫碱金属配合物的生物活性,本文作者选择以具有生物活性的组胺与喹啉-2-甲醛作为原料合成希夫碱配体,进而合成铜(II)配合物,以期增强铜配合物的生物兼容性,从而加强配合物的生物活性。
1 实验
1.1 实验试剂与仪器
主要试剂为:喹啉-2-甲醛,二盐酸组,三羟甲基氨基甲烷(Tris),溴化乙锭(EB),均购自Sigma公司;小牛胸腺DNA(CT-DNA),购自MBI Fermentas公司;水,为二次蒸馏水;其他试剂,均为分析纯。
主要仪器:Bruker VEC-TOR22 红外光谱仪(KBr压片);Perkin-Elmer元素分析仪;Shimadzu UV-2450 紫外-可见分光光度计;Hitachi F4600荧光光谱仪;Jasco J-815圆二色谱光谱仪;Bruker CCD X线衍射仪。
1.2 配合物[Cu(Quin-Hist)Cl2]的合成
将0.157 g(1.0 mmol)喹啉-2-甲醛和0.184 g(1.0 mmol)二盐酸组胺溶于15 mL乙醇中,滴加几滴NaOH溶液,搅拌回流2 h。然后滴加含0.085 g(0.5 mmol) CuCl2·2H2O的乙醇溶液5 mL,回流搅拌4 h。冷却至室温后过滤,将绿色沉淀用乙醇和乙醚洗涤数次。置于空气中干燥,然后将少量绿色沉淀溶于无水乙醇中,静置挥发后有亮绿色块状晶体析出,质量为0.137 g(产率为71.4%)。对C15H14N4CuCl2元素质量分数进行分析,计算值如下:C质量分数为46.91%,H为3.73%;N为14.45%。实际值(质量分数)如下:C为46.84%,H为3.70%,N为14.51%。红外图谱分析(KBr压片,cm-1)结果如下:3 111 cm-1处的峰为咪唑环上的N—H伸缩振动吸收峰;1 654 cm-1处的峰为喹啉环上的C=N伸缩振动吸收峰;1 592 cm-1处的峰为C=N伸缩振动吸收峰。
1.3 配合物[Cu(Quin-Hist)Cl2]晶体结构测定
采用Bruker CCD衍射仪收集铜配合物晶体的X线衍射数据。在温度293 K下,衍射光源为石墨单色器单色化的Mo Kα 射线,以5.0~50.0 min-1 的速度运用ω-2θ 扫描方式收集衍射结果,衍射结果均经过洛伦兹-极化因子和半经验吸收校正。所有氢原子坐标由理论计算确定,对全部非氢原子坐标及其各向异性热参数进行全矩阵最小二乘法修正。所有计算用SHELXTL程序完成。配合物的晶体学参数见表1。
1.4 配合物[Cu(Quin-Hist)Cl2]与DNA的相互作用
用Tris-HCl缓冲溶液(5 mmol/L Tris,5 mmol/L HCl,50 mmol/L NaCl,pH=7.4)溶解一定量的CT-DNA,测定该DNA溶液在260 nm和280 nm处的吸光度,这2处吸光度比值约为1.9,表明该DNA溶液中不含有蛋白质[11]。DNA溶液的浓度可通过260 nm处吸光度ε260确定(ε260=6 600 L·mol-1·cm-1)[12]。
取一定量[Cu(Quin-Hist)Cl2]溶于少量DMSO中,然后加入适量Tris-HCl缓冲溶液稀释至浓度为5.0×10-5 mol/L。取配合物溶液与DNA溶液按不同物质的量之比(r[n(Cu)/n(DNA)] 为 0,0.2,0.4和 0.6)混合,利用紫外-可见分光光度计分别测定上述各混合溶液的紫外-可见光谱。铜配合物与DNA的结合常数 kb 可由下式计算得出[13]:
c[DNA]/(εa – εf)=c[DNA]/(εb–εf)+1/[kb(εb – εf)] (1)
式中:c[DNA]为纯DNA的浓度;εa为配合物的表观摩尔吸光系数;εf为纯配合物的摩尔吸光系数;εb为配合物与DNA充分结合后的摩尔吸光系数;kb为结合常数,即以c[DNA]/(εb-εf)对c[DNA]作图得到的直线斜率与截距的比值。
表1 配合物[Cu(Quin-Hist)Cl2]的晶体学参数及其值
Table 1 Crystal and structure refinement parameters and their values for complex [Cu(Quin-Hist)Cl2]

在CT-DNA(1.0×10-4 mol/L)和EB(1.0×10-5 mol/L)混合溶液中缓慢滴加[Cu(Quin-Hist)Cl2]溶液(5.0×10-5 mol/L),在室温下测定每次加样后荧光光谱的变化(激发波长为530 nm,发射波长为600 nm)。猝灭常数k可由Stern-Volmer式计算得出[14]:
I0/I=1+k·r[n(Cu)/n(DNA)] (2)
式中:I0和I分别为有无金属配合物时荧光光谱的发射强度;r[n(Cu)/n(DNA)]为铜配合物与DNA的物质的量之比。以I0/I对r[n(Cu)/n(DNA)]作图,猝灭常数k为该直线的斜率。
在25 ℃测定[Cu(Quin-Hist)Cl2]与DNA(1.0×10-4 mol/L)以不同物质的量之比混合所得溶液的圆二色光谱。扫描波长为220~320 nm,扫描速度为10 nm/min。
1.5 体外细胞毒活性测试
配合物[Cu(Quin-Hist)Cl2]的体外细胞毒活性选用人乳腺癌细胞系(MCF-7)、人非小细胞肺癌细胞系(A-549)和人卵巢癌细胞系(SKOV-3)并用MTT(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)法进行测试,并以顺铂为阳性对照。肿瘤细胞于96孔板中在体积分数为5%的CO2和37 ℃培养20 h,细胞贴壁后,加入含有不同浓度梯度的铜配合物的培养液100 μL培养48 h。然后在每孔中加入含MTT的PBS缓冲溶液(20 μL,5 g/L),孵育4 h。最后在每孔中加入200 μL DMSO振荡摇匀10 min,采用酶标仪检测。
2 结果与讨论
2.1 晶体结构
喹啉-2-甲醛与二盐酸组胺以物质的量比为1:1的比例混合反应生成希夫碱化合物,然后加入CuCl2·2H2O,通过原位反应得到配合物产物[Cu(Quin-Hist)Cl2]。[Cu(Quin-Hist)Cl2]的分子结构如图1所示,主要键长和键角如表2所示。配合物中氢键的键长和键角如表3所示。该配合物中心金属Cu(II)离子处于变形三角双锥配位环境中,其τ为0.65(τ=(β-α)/60,其中,α为Cl(2)-Cu(1)-Cl(1)的键角,β为N(1)-Cu(1)-N(3)的键角,α和β分别为130.8°和169.8°)。当τ为0或1时,配合物分别为规则的四方锥或三角双锥构型;当τ为0~1时,配合物为变形三角双锥构型)[15]。喹啉环上N(1)原子和咪唑环上N(3)原子位于三角双锥的2个极轴顶点,亚胺基N(2)原子和2个氯原子组成三角平面。三角平面上N(2)-Cu(1)-Cl(1),N(2)-Cu(1)-Cl(2)和Cl(2)-Cu(1)-Cl(1)的键角均不相等,其值分别为106.2(6)°,122.5(6)°和130.8(3)°。Cu(1)-Cl(1) 和Cu(1)-Cl(2)的键长也不相等,其值分别为0.241 0(6) nm和0.229 4(7) nm。极轴上N(1)-Cu(1)-N(3)的键角为169.8(7)°,比规则的三角双锥中的该键角180°更小。键轴上Cu(1)-N(1)和Cu(1)-N(3)的键长分别为0.203 9(17) nm和0.196 5(17) nm,比三角平面上Cu(1)-N(2)键长(0.207 4(18) nm)更短。该配合物与一些含咪唑环Cu(II)配合物中的Cu-N咪唑环键长基本相等[16],但比另一些希夫碱铜(II)配合物中Cu-N亚胺基键长略长[17-18]。[Cu(Quin-Hist)Cl2]的晶体结构堆积图如图2所示,分子间存在氢键,N(4)···Cl(1)的D···A距离为0.315 0 nm。除此之外,配合物中的喹啉环与相邻的配体之间存在π-π 堆积作用,相邻平面的间距为0.331 5(3) nm。配合物通过氢键和π-π堆积作用形成具有链状结构的超分子体系。
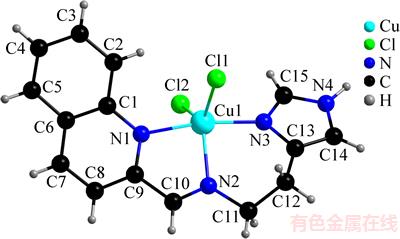
图1 配合物[Cu(Quin-Hist)Cl2]的分子结构
Fig. 1 Molecular structure of [Cu(Quin-Hist)Cl2]
表2 配合物的主要键长和主要键角
Table 2 Selected bond distances (nm) and angles (°) of [Cu(Quin-Hist)Cl2]

表3 配合物中的氢键的键长和键角
Table 3 Hydrogen bond lengths (nm) and bond angles (°) of [Cu(Quin-Hist)Cl2]


图2 配合物分子通过氢键和π-π堆积形成的一维超分子链状结构
Fig. 2 [Cu(Quin-Hist)Cl2] crystal structure with relevant one-dimensional supramolecular structure formed by hydrogen-bonding and π-π stacking interactions
2.2 配合物与DNA相互作用方式的研究
2.2.1 紫外吸收光谱
DNA被普遍认为是许多抗癌药物的主要作用靶点,研究金属配合物与DNA之间的相互作用对于开发新型高效的抗癌试剂具有重要意义。本文利用紫外-可见吸收光谱、荧光光谱与圆二色光谱等方法对配合物[Cu(Quin-Hist)Cl2]与DNA之间相互作用进行研究。当小分子金属配合物以嵌入方式与DNA碱基对结合时,其紫外吸收光谱会出现峰位红移和减色效应。紫外吸收光谱变化程度与其结合能力有关。减色效应越大,说明金属配合物与DNA作用越强,以嵌入方式结合的可能性越高。吸收峰红移越大,则说明配合物与DNA嵌入程度越大[19]。配合物与DNA作用的紫外吸收光谱如图3所示。从图3可见:随着DNA浓度增加,配合物的紫外吸收光谱在251 nm处发生了减色效应,减色率约为8.42%,但没有出现红移现象。为了定量比较铜配合物与DNA结合能力,利用式(1)求得配合物与DNA的结合常数kb为6.70×104 mol/L。该常数远小于经典的嵌入试剂EB的结合常数(EB-DNA结合常数为1.00×106 mol/L)[20],说明该铜配合物与DNA的结合能力比EB与DNA的结合能力低,且该配合物可能是通过非经典的作用方式如沟槽方式与DNA结合[21]。

图3 不同浓度配合物与DNA相互作用的紫外吸收光谱
Fig. 3 Electronic absorption spectra of complex (5.0×10-5 mol/L) absence (a) and presence (b-d) of increasing amounts of CT-DNA at the ratio r=0, 0.2, 0.4, 0.6
2.2.2 荧光光谱
DNA和EB本身的荧光性都很弱,但当EB插入到DNA小沟处的碱基对后,其荧光强度就会大大增强。当EB从DNA碱基对中游离出来时,荧光强度又会大大减弱。因此,可以根据配合物对EB-DNA复合物荧光强度的影响来研究该配合物与DNA间的相互作用。配合物[Cu(Quin-Hist)Cl2]与DNA相互作用的荧光光谱如图4所示(每次向EB-DNA体系中增加20 μL配合物溶液)。从图4可见:随着配合物浓度增加,EB-DNA复合物在600 nm处的荧光强度逐渐减弱,减弱率为49%。这表明铜配合物可以取代EB-DNA复合物中的EB,与DNA进行有效结合。铜配合物可能是以沟槽方式与DNA结合[22],这与紫外吸收光谱测定结果一致。
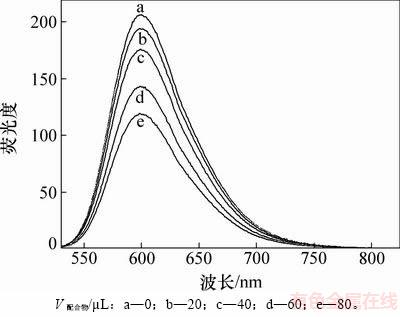
图4 不同体积配合物与EB-DNA体系相互作用的荧光光谱图
Fig. 4 Fluorescence emission spectra of ethidium bromide (EB)-CT-DNA system in absence and presence of complex
2.2.3 圆二色谱
DNA是具有双螺旋结构的手性分子,在圆二色谱上会出现2个峰:275 nm处的正峰和245 nm处的负峰。正峰是由DNA碱基对π-π堆积形成的,正峰上升意味着碱基堆积紧实;负峰是由DNA的右旋性产生的,负峰上升意味着配合物对DNA有解旋能力[23]。配合物[Cu(Quin-Hist)Cl2]与DNA相互作用的圆二色谱如图5所示。从图5可见:随着r[n(Cu)/n(DNA)]增大,圆二色谱中275 nm处的正峰强度稍增大,而245 nm处的负峰强度显著减小,但并没有发生谱带红移或蓝移。这表明铜配合物与DNA发生相互作用,导致DNA构象发生了明显变化。在247 nm处负峰下降,可能是由于配合物对DNA产生解旋作用,使DNA转化为A型构型[24]。

图5 不同浓度配合物与DNA相互作用的圆二色谱图
Fig. 5 CD spectra of CT-DNA in absence and presence of increasing amounts of complex at the ratio r
2.3 体外细胞毒性研究
IC50为杀死半数肿瘤细胞所需的药物浓度,其值越低,则药物的细胞毒性越高。本文以顺铂作为阳性对照,通过MTT法测试配合物[Cu(Quin-Hist)Cl2]对3种不同肿瘤细胞系(MCF-7,A-549和SKOV-3)的细胞毒活性,结果如图6所示。从图6可见:该铜配合物对MCF-7,A-549和SKOV-3这3种细胞系的IC50分别为15.2,27.2和25.8 μmol/L,由此说明该配合物对MCF-7细胞系具有最强的体外细胞毒活性,对其他2种细胞系也有不同程度的生长抑制作用。此外,该铜配合物对MCF-7细胞系的IC50比顺铂的低(IC50=20.4 μmol/L),即表现出强于顺铂的体外细胞毒活性,这表明配合物[Cu(Quin-Hist)Cl2]有望发展成为一种潜在的抗癌试剂。

图6 配合物对3种癌细胞株的细胞毒活性图 (以顺铂为对照)
Fig. 6 Cytotoxic activity of [Cu(Quin-Hist)Cl2] against three human tumor cell lines (cisplatin is used as positive control)
3 结论
1) 以喹啉-2-甲醛、二盐酸组胺、CuCl2·2H2O为原料,通过原位反应合成了一个新的希夫碱类铜配合物[Cu(Quin-Hist)Cl2]。
2) 该希夫碱类铜配合物结构为畸变的三角双锥构型,且配合物分子通过氢键和π-π堆积作用形成具有链状结构的超分子体系。
3) 配合物[Cu(Quin-Hist)Cl2]以沟槽结合的方式和CT-DNA结合,并且对MCF-7细胞系具有比顺铂更高体外细胞毒活性,表明该配合物可作为一种潜在的抗癌试剂。
参考文献:
[1] GARBUTCHEONSINGH K B, GRANT M P, HARPER B W, et al. Transition metal based anticancer drugs[J]. Current Topics in Medicinal Chemistry, 2011, 11(5): 521-542.
[2] OLIVERI V, VECCHIO G. 8-Hydroxyquinolines in medicinal chemistry: a structural perspective[J]. European Journal of Medicinal Chemistry, 2016, 120(14): 252-274.
[3] 覃姣兰, 曹倩倩, 覃其品, 等. 8-羟基喹啉及其衍生物金属配合物抗肿瘤和抗菌活性研究进展[J]. 中国科学: 化学, 2017, 47(2): 172-182.
QIN Jiaolan, CAO Qianqian, QIN Qipin, et al. Progress on antitumor and antibacterial activity of 8-hydroxyquinoline and its derivatives metal complexes[J]. Scientia Sinica Chimica, 2017, 47(2): 172-182.
[4] QIN Qipin, CHEN Zhenfeng, QIN Jiadan, et al. Studies on antitumor mechanism of two planar platinum(II) complexes with 8-hydroxyquinoline: synthesis, characterization, cytotoxicity, cell cycle and apoptosis[J]. European Journal of Medicinal Chemistry, 2015, 92(6): 302-313.
[5] MARZANO C, PELLEI M, TISATO F, et al. Copper complexes as anticancer agents[J]. Anti-Cancer Agents in Medicinal Chemistry, 2009, 9(2): 185-211.
[6] ZHANG Zhen, WANG Huiyun, YAN Maocai, et al. Novel copper complexes as potential proteasome inhibitors for cancer treatment (Review)[J]. Molecular Medicine Reports, 2017, 15(1): 3-11.
[7] LIAN Wenjing, WANG Xintian, XIE Chengzhi, et al. Mixed-ligand copper(ii) Schiff base complexes: the role of the co-ligand in DNA binding, DNA cleavage, protein binding and cytotoxicity[J]. Dalton Transactions, 2016, 45(22): 9073-9087.
[8] SHEBLL M. Synthesis,spectroscopic characterization and antimicrobial activity of binuclear metal complexes of a new asymmetrical Schiff base ligand: DNA binding affinity of copper(II) complexes[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2014, 117(1): 127-137.
[9] ZHANG Shouchun, DONG Juanjuan, FAN Xiaorui, et al. Cobalt(II) complexes with thiosemicarbazone as potential antitumor agents:synthesis,crystal structures, DNA interactions, and cytotoxicity[J]. Spectrochimica Acta: Part A (Molecular and Biomolecular Spectroscopy), 2013, 66(24): 4268-4279.
[10] ZHANG S C, DONG J J, FAN X R, et al. A new nickel(II) complex with the thiosemicarbazone of quinoline-2- carboxaldehyde: structure, DNA-binding, cleavage, and cytotoxic activities[J]. Journal of Coordination Chemistry, 2012, 65(17): 3098-3110.
[11] MARMUR J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms[J]. Journal of Molecular Biology, 1961, 3(1): 208-218.
[12] REICHMANN M E, RICE S A, THOMAS C A, et al. A further examination of the molecular weight and size of desoxypentose nucleic acid[J]. Journal of American Chemical Society, 1954, 76(11): 3047-3053.
[13] WOLFE A, JR SHIMER G H, MEEHAN T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA[J]. Biochemistry, 1987, 26(20): 6392-6396.
[14] EFTINK M R, GHIRON C A. Fluorescence quenching studies with proteins[J]. Anal Biochem, 1981, 114(2): 199-227.
[15] ADDISON A W, RAO T N, DEEDIJK J, et al. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol- 2'-yl)-2,6-dithiaheptane]copper(II) perchlorate[J]. J Chem Soc Dalton Trans, 1984(7): 1349-1356.
[16] SADHUKHAN D, RAY A, DAS S, et al. Effect of ligand substitution on DNA binding ability of two new square planar copper(II)-Schiff base complexes[J]. J Mol Struc, 2010, 975(1): 265-273.
[17] DONG Jianfang, LI Lianzhi, LIU Guihua, et al. Synthesis, crystal structure and DNA-binding properties of a new copper(II) complex with l-valine Schiff base and 1,10-phenanthroline[J]. J Mol Struc, 2011, 986(1/2/3): 57-63.
[18] QIAO Xin, MA Zhongying, XIE Chengzhi, et al. Study on potential antitumor mechanism of a novel Schiff Base copper(II) complex: synthesis, crystal structure, DNA binding, cytotoxicity and apoptosis induction activity[J]. J Inorg Biochem, 2011, 105(5): 728-737.
[19] NAIR R B, TENG E S, KIRKLAND S L, et al. Synthesis and DNA-binding properties of [Ru(NH3)4dppz]2+[J]. Inorganic Chemistry, 1998, 37(1): 139-141.
[20] WARING M J. Complex formation between ethidium bromide and nucleic acids[J]. Journal of Molecular Biology, 1965, 13(1): 269-282.
[21] XU Zhihong, CHEN Fengjuan, XI Pinxian, et al. Synthesis, characterization, and DNA-binding properties of the cobalt(II) and nickel(II) complexes with salicylaldehyde 2-phenylquinoline-4-carboylhydrazone[J]. J Photochem Photobiol A, 2008, 196(2): 77-83.
[22] KRISHNA A G, KUMAR D V, KHAN B, et al. Taxol-DNA interactions:fluorescence and CD studies of DNA groove binding properties of taxol[J]. Biochimica et Biophysica Acta (BBA)-General Subjects, 1998, 1381(1): 104-112.
[23] COLLINS J G, SHIELDS T P, BARTON J K. 1H-NMR of Rh(NH3)4phi3+ Bound to d(TGGCCA)2:classical intercalation by a nonclassical octahedral metallointercalator[J]. Journal of the American Chemical Society, 1994, 116(22): 9840-9846.
[24] CHAUHAN M, BANERJEE K, ARJMAND F. DNA binding studies of novel copper(ii) complexes containing L-tryptophan as chiral auxiliary: in vitro antitumor activity of Cu-Sn2 complex in Human neuroblastoma cells[J]. Inorg Chem, 2007, 46(8): 3072-3082.
(编辑 陈灿华)
收稿日期:2017-10-23;修回日期:2017-12-20
基金项目(Foundation item):国家自然科学基金资助项目(21301194)(Project(21301194) supported by the National Natural Science Foundation of China)
通信作者:张寿春,博士,副教授,从事金属配合物的生物活性研究;E-mail:zhang_shch@sina.cn
摘要:合成一种新的喹啉-2-甲醛缩组胺希夫碱铜(II)配合物[Cu(Quin-Hist)Cl2],利用元素分析和红外光谱对其进行表征,通过X线单晶衍射确定其晶体结构。研究结果表明:该晶体属于单斜晶体,P21/n空间群,晶胞参数为a= 0.858 90(7) nm,b=1.282 38(10) nm,c=1.459 76(12) nm,β=99.346 0(10)°;配合物为畸变的三角双锥空间构型;该铜(II)配合物能与小牛胸腺DNA(CT-DNA)之间通过沟槽结合的方式相互作用;此外,铜(II)配合物对MCF-7人乳腺癌细胞系、A-549人非小细胞肺癌细胞系和SKOV-3人卵巢癌细胞系均有较强的体外细胞毒活性,且与顺铂相比,铜(II)配合物对MCF-7细胞系具有更强的体外细胞毒活性。


