
Cu-based bulk metallic glasses designed by stability evaluations of liquid and amorphous phases
JI Xiu-lin(纪秀林)1, PAN Ye(潘 冶)2, ZHAO Jian-hua(赵建华)1, LIN Ping-hua(林萍华)1
1. Engineering Research Center of Dredging Technology of Ministry of Education,
Hohai University, Changzhou 213022, China;
2. School of Materials Science and Engineering, Southeast University, Nanjing 211189, China
Received 10 August 2009; accepted 15 September 2009
Abstract:
From the point of translation from liquid to amorphous phase, a new approach for composition design of bulk metallic glasses (BMGs) was suggested based on the stability estimations of liquid and amorphous phases. Gibbs free energy (ΔG) of liquid phase, which can be calculated approximately using mixing enthalpy (ΔHmix) and configuration entropy (Sconfig), was taken as a stability criterion of liquid phase. On the other hand, vacancy formation energy (ΔHh) was used to evaluate the stability of amorphous phase due to the linear relationship between ΔHh and crystallization temperature (Tx). Based on these two sides, new Cu-Zr-Nd bulk metallic glasses were designed and synthesized successfully by suction-casting into copper mold. Experimental results about glass-forming ability are consistent with the computational values, which not only proves the validity of the new approach for composition design but also shows the new BMG of Cu60.5Zr34Nd5.5 can be cast into amorphous alloys with 2 mm in diameter.
Key words:
metallic glasses; glass forming ability; vacancy formation energy; composition design;
1 Introduction
Bulk metallic glasses (BMGs) are attracting many interests for their potential engineering applications[1-7]. Though lots of BMGs of different alloy systems have been developed, how to select the elements and compositions is not very clear and their formation mechanisms still remain a puzzling mystery. Therefore, there is an urgent need to use a scientific approach to identify potential compositions of multi-component alloys for glass formation instead of empirical rules. Recently, some composition design approaches have been brought forward. By the calculation of phase diagram (CALPHD), compositions of multi-component BMGs can be identified[4-5]. The compositions of Zr-Cu-Ni-Al[6] and Cu-Ti-Zr-Ni[7] BMGs were designed in terms of deep eutectic approach. Based on the Miedema model, the methods to identify the compositions of BMGs are proposed with the linear relationship between amorphous formation energy (ΔHamor) and super-cooled liquid region (ΔTx)[8]. The calculated free energy change (ΔG) between amorphous and solid solution shows strong correlation with reduced glass transition temperature (Tg/Tl, Tg is the glass transition temperature and Tl is the liquidus temperature) in Zr-based metallic glasses[9]. Such works are all in reasonable agreement with experiment data, but the calculated results could not always present very specifical composition with good GFA due to the complexity of multi-component alloys.
From the point of translation from liquid to amorphous phase, the possibility of amorphous formation is determined in thermodynamics by the stabilities of liquid, solid and amorphous phases. Since the stability of liquid and amorphous phases can be denoted by Tl and the crystallization temperature (Tx) respectively, Tx/Tl could be regarded as a good GFA parameter[10]. However, Tx and Tl of BMGs can be detected only after sample preparation. Therefore, a new approach for composition design of BMGs was suggested recently based on the stability estimations of liquid and amorphous phases[11]. Cu-Zr-Nd ternary system was taken as an example for composition design in this work because 1) Cu-rich alloys have potential applications owing to their high strength and low cost; 2) there is more than 12% atomic radius difference between Nd and Cu, which would be in favor to glass forming
ability; 3) neodymium is an important magnetic element and Cu-Zr-Nd alloys may confer the BMGs an unique magnetic characteristic; and 4) Cu-Zr-Nd ternary system has not been reported as BMGs up to now. In this work, novel Cu-Zr-Nd bulk metallic glasses were designed and prepared.
2 Calculation method for composition design
The liquid stability can be estimated by Gibbs free energy (ΔG) of the liquid alloy system. The approximate ΔG expression can be written as
![]() (1)
(1)
![]() (2)
(2)
![]() (3)
(3)
where ΔHmix is the mixing enthalpy; Sconfig is the configuration entropy; R is the gas constant; Ωij (=4![]() ) is the regular melt interaction parameter between the ith and jth elements; ci is the molar percentage of the ith component; and
) is the regular melt interaction parameter between the ith and jth elements; ci is the molar percentage of the ith component; and ![]() is the mixing enthalpy of binary liquid alloys.
is the mixing enthalpy of binary liquid alloys.
For the liquid temperature of the alloy system is unknown, calculation on ΔG with this expression is impossible. However, it is known that lower ΔHmix and larger Sconfig are usually favor to good GFA. Therefore, the liquid stability can be evaluated by the value of ΔHmixSconfig. On the other hand, because Tx is in direct proportion to vacancy formation energy (ΔHh) in binary alloy system[12] and the expanded ΔHh in multi-component alloys calculated by Eqs.(4) and (5) is also in proportion to Tx[11], the stability of amorphous phase can be estimated with ΔHh in multi-component alloys:
![]() (4)
(4)
where ![]() is the vacancy formation energy between the ith and jth elements.
is the vacancy formation energy between the ith and jth elements.
![]() (5)
(5)
where Vi and ![]() is the molar volume and the vacancy formation energy of pure metal of the ith element, respectively;
is the molar volume and the vacancy formation energy of pure metal of the ith element, respectively; ![]() (=
(=![]() ) is the ratio of interaction of A with B in the amorphous alloy, xA and
) is the ratio of interaction of A with B in the amorphous alloy, xA and ![]() (=
(=![]() )are the surface concentration and the molar percentage of A element, respectively.
)are the surface concentration and the molar percentage of A element, respectively.
To obtain the greatest stability of liquid and amorphous phase for BMG formation, the largest ΔHh and the biggest negative ΔHmixSconfig are advantaged. After finding out the extremes of ΔHhΔHmixSconfig in Cu-Zr-Nd ternary system at every percent of Cu contents, the predicting composition line at Zr side which is composed by the extremes in ternary composition plot is illustrated in Fig.1. The compositions of Cu49Zr48Nd3 and Cu60.5Zr34Nd5.5 with relative lower and higher Cu contents on the predicting composition line in Cu-Zr-Nd system are selected as BMG candidates, and the calculated ΔHhΔHmixSconfig values of Cu60.5Zr34Nd5.5 and Cu49Zr48Nd3 are -20183.0 and -20383.1, respectively.
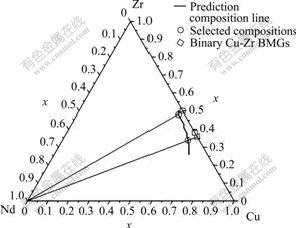
Fig.1 Predicting composition line derived by ΔHhΔHmixSconfig and selected metallic glass forming compositions in Cu-Zr-Nd alloying system
3 Experimental procedure
Two alloys of Cu49Zr48Nd3 and Cu60.5Zr34Nd5.5 (molar fraction,%) were synthesized by arc-melting a mixture of pure elements with the purity ranging from 99.5% to 99.9% in an argon atmosphere. Each of the ingots was melted four times to ensure uniformity in composition. Sample rods with diameter of 2 mm and 3 mm were synthesized, respectively, by suction casting into a copper mold under a purified Ar atmosphere. The as-cast rods were sectioned transversely and then observed by transmission electron microscope (TEM, JEM2000EX, USA). The glassy nature of the specimen was verified by analyzing the central part of their cross-sections using X-ray diffractometer (XRD, Rigaku D/max 2500, Japan) and differential scanning calorimeter (DSC, Perkin-Elmer DSC7, USA). The corresponding glass transition temperature (Tg) and crystallization temperature (Tx) were measured by DSC at a heating rate of 0.33 K/s. Vickers hardness measurements were performed on a standard micro-hardness tester at 0.98 N load and 30 s loading duration.
4 Results and discussion
Fig.2 shows the XRD patterns of Cu49Zr48Nd3 and Cu60.5Zr34Nd5.5 rod samples with diameter of 2 mm and 3 mm. The XRD patterns of 2 mm-diameter rods exhibit only a broad scattering peak characteristic of metallic glass without any detectable crystalline phases, indicating the formation of single amorphous phase. But there are several sharp Bragg characteristic peaks of crystalline phases superimposed on the XRD pattern of 3 mm-diameter rods, as shown in Fig.2, suggesting that there are crystallizations during the formation of the sample. By comparing the XRD patterns of 3 mm- diameter rods, the characteristic of crystallization of Cu60.5Zr34Nd5.5 is more evident than that of Cu49Zr48Nd3. So, lower negative value of ΔHhΔHmixSconfig means higher GFA for alloys.
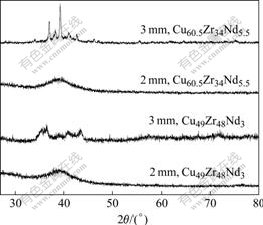
Fig.2 XRD patterns of as-cast Cu60.5Zr34Nd5.5 and Cu49Zr48Nd3 rods with diameter of 2 mm and 3 mm
More works have been put on Cu60.5Zr34Nd5.5 because it is more difficult to form BMG than Cu49Zr48Nd3. The TEM image shows that there is no hint of the presence of any distinguishable crystallites in the Cu60.5Zr34Nd5.5 rods with 2 mm in diameter, as shown in Fig.3. The inserted selected area electron diffraction pattern reveals a full ring, which is inherent to an amorphous phase. Therefore, the results of TEM are in agreement with the XRD patterns and indicate that Cu60.5Zr34Nd5.5 can be prepared as full amorphous rod with 2 mm in diameter. The stability of amorphous phase was analyzed by DSC experiments in Cu60.5Zr34Nd5.5. Fig.4 shows DSC trace obtained from as-cast specimen of Cu60.5Zr34Nd5.5 with 2 mm in diameter, which shows a clear glass transition temperature (Tg) at about 713 K and a crystallization temperature (Tx) at about 756 K. Using the DSC characteristic data, the supercooled liquid region ΔTx(=Tx-Tg) as a commonly used GFA indicator can be calculated to be 43 K for Cu60.5Zr34Nd5.5.
The Vickers hardness of the two representative alloys varies from HV615 to HV672. The average hardness is 643 kg/mm2, which is considerably higher than that of other Cu-based bulk glasses[13-15]. The yield strength of these amorphous alloys is estimated from the approximation scaling relationship to be 1.93 GPa.

Fig.3 Bright-field electron micrograph and selected-area diffraction pattern of Cu60.5Zr34Nd5.5 rod with diameter of 2 mm
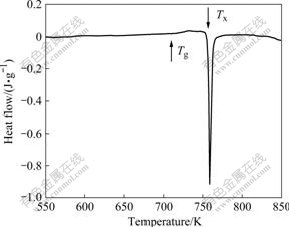
Fig.4 DSC trace of as-cast Cu60.5Zr34Nd5.5 rod with diameter of 2 mm
Besides Cu49Zr48Nd3 and Cu60.5Zr34Nd5.5, other alloys on the present predicting composition line need to have more experiments. Even now, using the composition design method based on the stability estimations of liquid and amorphous phase, the new BMGs of Cu60.5Zr34Nd5.5 and Cu49Zr48Nd3 in Cu-Zr-Nd ternary system are derived. Actually, the composition design method with the calculation of ΔHhΔHmixSconfig is consistent with Inoue’s empirical rules, because 1) increasing the amount of constituent elements will increase Sconfig in an alloy system; 2) enlarging atomic radius difference among constituent elements will increase ΔHh; and 3) they both consider that larger negative heat of mixing (ΔHmix) among main constituent elements is more favorable to glass formation. The distinct difference between them is that a predicting composition line can be obtained using the method with the calculation of ΔHhΔHmixSconfig, but Inoue’s empirical rules could not provide any specific alloy composition. Therefore, ΔHhΔHmixSconfig is more practicable for composition design of BMGs.
5 Conclusions
1) Based on the stability of liquid and amorphous phases estimated by Gibbs free energy (ΔG) and vacancy formation energy (ΔHh), the method of composition design for bulk metallic glasses is suggested.
2) For the amount of ΔG can be evaluated approximately by ΔHmixSconfig and the calculation of ΔHh can be expended to multi-component alloys, the predicting composition line in ternary system can be derived by the extremes of ΔHhΔHmixSconfig. Using this method, the composition line which is favorable to BMG’s formation is obtained in Cu-Zr-Nd ternary system.
3) Experimental results not only prove the validity of the new approach for composition design but also show that the new BMGs of Cu60.5Zr34Nd5.5 and Cu49Zr48Nd3 can be cast into 2 mm in diameter by suction casting method. The Vickers hardness of the two kinds of alloys varies from HV615 to HV 672.
Acknowledgments
The authors would like to thank Dr. H. CAO at Department of Materials Science and Engineering of University of Wisconsin-Madison, USA, for DSC analysis and Prof. FU Ming-xi & Dr. LI Dong-sheng at School of Materials Science and Engineering of Jiangsu University, China, for preparation of samples.
References
[1] LIU Y H, WANG G, WANG R J, ZHAO D Q, PAN M X, WANG W H. Super plastic bulk metallic glasses at room temperature [J]. Science, 2007, 315: 1385-1388.
[2] WANG Wei-hua. Roles of minor additions in formation and properties of bulk metallic glasses [J]. Progress in Materials Science, 2007, 52(4): 540-596.
[3] LU Z P, MA D, LIU CT, CHANG Y A. Competitive formation of glasses and glass-matrix composites [J]. Intermetallics, 2007, 15(3): 253-259.
[4] MA D, CAO H, CHANG Y A. Identifying bulk metallic glass-formers from multi-component eutectics [J]. Intermetallics, 2007, 15: 1122-1126.
[5] GORSSE S, ORVEILLON G, SENKOV O N, MIRACLE D B. Thermodynamic analysis of glass-forming ability in a Ca-Mg-Zn ternary alloy system [J]. Phys Rev B, 2006, 73: 224202.
[6] LU Z P, SHEN J, XING D W, SUN J F, LIU C T. Binary eutectic clusters and glass formation in ideal glass-forming liquids [J]. Appl Phys Lett, 2006, 89: 071910.
[7] YANG Y J, XING D W, LI C P, WEI S D, SUN J K, SHEN Q K. A new way of designing bulk metallic glasses in Cu-Ti-Zr-Ni system [J]. Mater Sci Eng A, 2007, 448: 15-19.
[8] XIA M X, ZHANG S G, LI J G, MA C L. Thermal stability and its prediction of bulk metallic glass systems [J]. Appl Phys Lett, 2006, 88: 261913.
[9] RAO B S, BHATT J, MURTY B S. Identification of compositions with highest glass forming ability in multi-component systems by thermodynamic and topological approaches [J]. Mater Sci Eng A, 2007, 449/451: 211-214.
[10] MONDAL K, MURTY B S. On the parameters to assess the glass forming ability of liquids [J]. J Noncryst Solids, 2005, 351: 1366-1371.
[11] JI X L, PAN Y. Predicting alloy compositions of bulk metallic glasses with high glass-forming ability [J]. Mater Sci Eng A, 2008, 485(1/2): 154-159.
[12] BUSCHOW K H J, BEEKMANS N M. Thermal stability of amorphous alloys [J]. Solid State Commun, 1980, 35: 233-236.
[13] CAO H B, PAN Y, DING L, ZHANG C, ZHU J, HSIEH K C, CHANG Y A. Discovery of copper-rich amorphous alloys by computational thermodynamics [J]. Acta Mater, 2008, 56: 2032-2039.
[14] Ji X L, Pan Y, Cai A H. The effect of Al on glass forming ability of Cu-based bulk metallic glasses [J]. Journal of Central South University of Technology, 2007, 14(S2): 20-23.
[15] PARK E S, CHANG H J, KIM D H, OHKUBO T, HONO K. Effect of the substitution of Ag and Ni for Cu on the glass forming ability and plasticity of Cu60Zr30Ti10 alloy [J]. Scripta Materialia, 2006, 54(9): 1569-1573.
(Edited by YANG Hua)
Foundation item: Project (XZY/08B007-02) supported by Scientific Research Starting Foundation for Doctors in Hohai University, China
Corresponding author: JI Xiu-lin; Tel: +86-519-85191969; E-mail: xiulinji@gmail.com


