网络首发时间: 2019-08-27 09:34
铬污染的微生物吸附技术研究进展
闫潇 刘兴宇 张明江 崔兴兰 钟娟 胡学武
摘 要:
近年来,铬在工业生产中得到了广泛的应用,随之而来的含铬污染物对周围环境造成严重的污染和破坏,铬污染的修复已成为亟待解决的环境问题。微生物在铬污染的生物修复中发挥着重要的作用。它因铬污染修复过程中环保有效,安全可靠且无二次污染等优点,引起了相关学者们的广泛关注。首先简述了铬污染危害及传统处理技术,重点综述了微生物作为生物吸附剂的吸附机理及影响因素,并分别详述了典型细菌,真菌,酵母和藻类吸附Cr(VI)污染物的机理机制及相关研究进展,然后总结了微生物吸附Cr(VI)过程中铬浓度,赋存状态,微生物营养类型,培养条件及代谢产物等的主要影响因素,最后,分析了以微生物作为生物吸附剂所存在的问题并展望了未来的研究方向,结合基因工程和酶工程选育高效工程菌、开展多种技术联合应用等方法提高微生物对铬污染的去除能力。
关键词:
铬污染 ;微生物 ;生物吸附剂 ;吸附机制 ;
中图分类号: X172;X505
作者简介: 闫潇(1991-),女,河南周口人,博士研究生,研究方向:环境污染微生物修复,E-mail:yx1443349211@163.com;; *刘兴宇,教授,电话:010-82241312,E-mail:wellwoodliu@163.com;
收稿日期: 2019-06-21
基金: 国家自然科学基金项目(U1402234,41573074); 国家重点研发计划项目(2018YFC1802702,2018YFC1801803); 广西科学研究与技术开发计划项目(桂科AB16380287,桂科AB17129025); 中组部万人计划青年拔尖人才计划项目资助;
Research Progress in Biosorption Technology for Chromium Contamination
Yan Xiao Liu Xingyu Zhang Mingjiang Cui Xinglan Zhong Juan Hu Xuewu
National Engineering Laboratory of Biohydrometallury,GRIMAT Engineering Institute Co.,Ltd.
Abstract:
The population growth and the intensification of anthropogenic activities have contributed to a worsening of environmental pollution.Such as,industrial waste was stored in an area for a long time without proper management and organization,which was including large amount of the organic and inorganic contaminants,as well as heavy metals(chromium,lead,and crop).More specifically,significant concentration of chromium from leather tanning along with other applications such as metallurgy, alloying,electroplating could be released into the environment by human activities.At present,the chromium contamination had gained substantial consideration worldwide due to its high toxicity,easy solution,strong bioavailability in the water and soil originating from numerous natural and anthropogenic activities.In addition,chromium with high migration and transformation ability could be extracted and accumulated in plant from contaminated soils,and imparted severe health risks in humans via food chain contamination,which with time reached detrimental levels in living systems,resulting in several diseases such as irritation and/or cancer in lungs and digestive tract,low growth rates in plants and death of animals,and other health alterations.For that reason,now the remediation of chromium pollution had become an urgent environmental problem to be solved.Currently,many remediation technologies were used widely,which pide into the physic and chemical methods such as tillage method,reverse osmosis,electrochemical process,ion exchange,adsorption on activated carbon,excavation and solidification/stabilization.Some of the physical and chemical treatment procedures were simple,fast and possibly help metal recovery,but,these methods had significant drawbacks including still had to meet the demand of high operational cost due to its periodical agents addition,high energy consumption,and toxic secondary pollutants generation.Therefore,it was urgently needed to consider for developing a sustainable and economical process for Cr(Ⅵ) removal,and the search for cheaper and more effective technologies had become necessary to develop of more economical,safe,and environmentally friendly methods to remove Cr(Ⅵ) from industrial wastewaters.Microbial remediation methods by biosorption were eco-friendly,efficiency,safety and had no secondary pollution,which was considered as a potential technique in remediation of Cr(Ⅵ) contaminated site.Based on the above background,in this study,the source,toxicity and the remediation technologies of chromium pollution were briefly overviewed firstly.Of these,the application limitations of traditional technologies were analyzed.Moreover,adsorbents and biosorption mechanism were reviewed and explored in detail.Knowing that biosorption was a passive,rapid,and reversible physic-chemical-biological process between heavy metal types and biological material(biosorbent).Several sensitive factors having great influences on biosorption rate,including environmental parameters and microbial culture conditions,were detailed elaborated and carefully analyzed in this study.In addition,the detailed information about research progress of biosorption by different microorganisms(e.g.bacteria,fungi,yeast and algae) were provided in this study.Different microorganisms with the differences of the capacity to remove Cr(Ⅵ) contamination were discussed,and then as well as various binding sites from the functional groups in the process of metabolism were analyzed.Furthermore,the greatest limitations factors by biosorption processes were related to the metabolic parameters and responses,which could be influenced by the Cr(Ⅵ) and by the operational conditions applied.In the process of biosorption,it payed more attention to the effects of some environmental factors of biosorption like initial concentration of chromium,nutrition types(carbon source and nitrogen source),environmental conditions(experimental pH,temperature,inoculation size) and the types,activity,population and metabolites of microorganisms(biomass,extracellular polymeric substance),as well as in processes involving heavy metals(Fe,Cu,Mn).The bio re mediation mechanism of different microorganisms suggested in this review provided an insight into a better understanding of Cr(Ⅵ) removal.This could help in developing the existing technologies of chromium remediation to be more efficient.Finally,the problem of biosorption chromium contamination was analyzed and some future research suggestions were pointed out.Still,a great gap existed between laboratory studies and successful application of biosorption technology for the treatment of chromium contaminated sites.The majority of the laboratory experiments were carried out by using synthetic chromium solutions to analyze the efficiency of the developed system for chromium remediation.Therefore,the potential of biological systems for removal Cr(Ⅵ) in the contaminated soil or industrial effluents needed to be further explored.Currently,it was difficult to carry out the biosorption and make sure the effect of bio re mediation in an open environment of the chromium contaminated sites.In addition,in the process of heavy metal biosorption by microorganisms,the growth conditions,cell activity,functional enzyme synthesis and the competition of indigenous microbes had great impacts on the biosorption rate.In order to solve the above problems,it was very necessary to further study as follows:(1) Based on the combination of genetic engineering and proteomics,the functional microbes with high biosorption capacity of Cr(Ⅵ) are isolated and identified from the chromium contaminated sites,optimized the removal conditions of Cr(Ⅵ),and revealed the biosorption mechanism of Cr(Ⅵ).(2) Through the embedding technology of microorganism,the continuous running bioreactor systems were carried out for removal Cr(Ⅵ) in the industrial effluents.(3) Selecting the combined application of different methods for removal Cr(Ⅵ),for example,the combination of plant-microbial technology.To summary,emerging techniques such as cell immobilization,the application of bioreactors operating in continuous systems,the discovery of new microorganisms and genetic alterations had been potentially indicated for biosorption processes so as to circumvent the restrictions frequently found.
Keyword:
chromium contamination; microorganism; biosorbent; biosorption mechanism;
Received: 2019-06-21
近几十年来世界人口剧增和工业活动加剧导致自然环境日益恶化,其中铸造厂,制革厂,纺织厂,微电子,化工厂及采矿冶炼等工业兴起过程中无机重金属污染物渗入到周边环境尤为严重,包括铬,铅,汞,镉和砷等多种重金属物质
[1 ]
。研究表明,工业活动所产生的重金属除了污染周边的水源和土壤外,还会转移到动植物中如水稻、大豆
[2 ]
,鸟类、家畜,两栖动物
[3 ]
和野生动物
[4 ]
等,甚至会通过食物链富集到人体。重金属的毒性作用机制分为4种:(1)发挥免疫抑制活性,降低免疫系统的活性或效率;(2)在酶的固定位点竞争活动因子;(3)抑制重要的酶,例如氧化磷酸化和(4)改变细胞结构,主要是在膜的脂蛋白区
[5 ]
。
环境保护局(美国)规定排入到地表的水中的总Cr不得超过0.5 mg·L-1
[6 ]
。近年来,因铬金属在合金、油漆、电镀、制革、肥料和杀菌剂等领域内广泛应用,产生大量的含铬废水、废渣,这些污染废物在不达标排放和无组织堆存的情况下释放到周边环境中,导致人为污染
[7 ]
。铬总共有Cr(Ⅱ)到Cr(VI)共九种化学形态,其中自然环境中以Cr(Ⅲ)和Cr(VI)较为稳定,但其化学性质迥然不同。Cr(Ⅲ)对人体的新陈代谢活动非常重要,可以调节葡萄糖,胆固醇甘油三酯的平衡,为生物体的营养物质代谢发挥重要作用
[8 ]
。Cr(VI)易溶于水主要以铬酸盐(Cr O4 )2- 、重铬酸盐(Cr2 O7 )2- 或氧化铬的形式存在于水及周边土壤中。Cr(VI)显示出较高的毒性,短时间接触可能会引起皮肤溃疡,过敏性反应和接触性皮炎等病症,长期暴露该环境中会造成慢性支气管炎,肺炎,颅内出血,甚至癌症,Cr(VI)还能够通过与DNA酶聚合酶的错配导致遗传性基因缺陷
[9 ]
。误食或摄取少剂量Cr(VI)化合物会引起肺癌和胃癌。通过口服Cr(VI)对健康成年大鼠的致死剂量(DL50)在50至100 mg·kg-1 之间,但Cr(Ⅲ)的DL50在1900~3300 mg·kg-1 之间,与Cr(Ⅵ)相比毒性较低。据估计,Cr(VI)的毒性比Cr(Ⅲ)高约一百倍
[10 ]
。
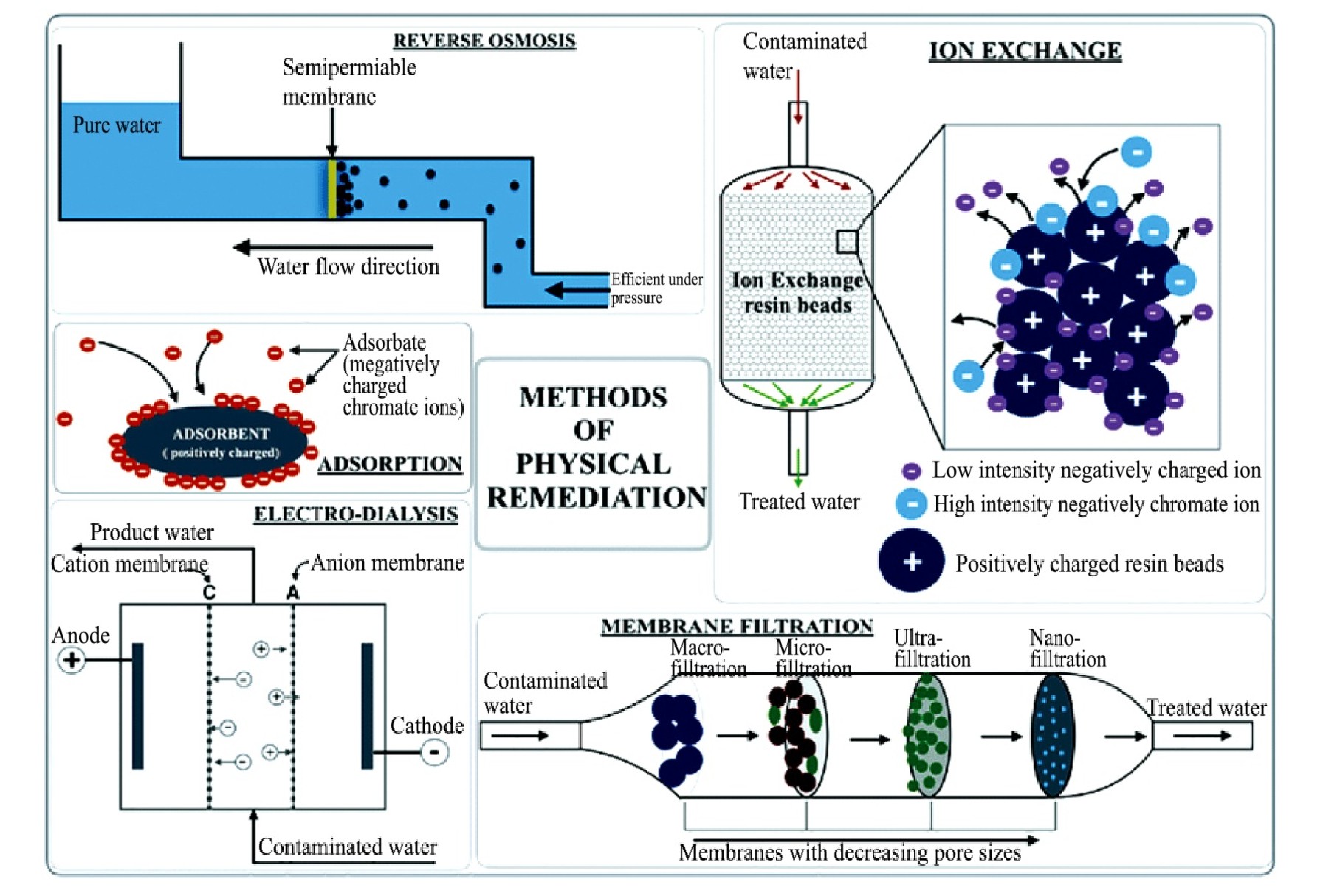
现有的技术去除重金属如Cr(VI)已在工程项目中得到广泛的应用,例如客土换土,反渗透,溶剂还原,石灰凝固,离子交换和化学沉淀等技术,其作用机制如图1所示,这些技术存在着一些不足之处,例如能源的非可再生性,高能量的需求,重金属去除的不彻底和产生二次污染的风险等
[11 ]
。因此,寻找替代解决方案和创新的技术策略已变得非常重要,例如,单独使用生物吸附剂或与已用于去除Cr(VI)的传统技术结合使用。考虑到Cr(VI)在工业效应中的存在对社会环境影响的重要性以及对人类和动物健康的潜在威胁,本综述的目的是提出并讨论实验室研究阶段利用微生物去除Cr(VI)的潜力。通过应用微生物吸附剂,讨论生物吸附过程中涉及的主要机制和影响因素。
1 生物吸附剂
用于重金属去除的吸附剂有很多种,其中包括活性炭
[12 ]
,金属氧化物,固定化纳米材料及生物吸附剂如微生物
[13 ]
和生物活性污泥等
[14 ]
。所谓的生物吸附剂是由活性或非活性微生物进行的被动,快速,可逆和独立的代谢过程聚集污染物。该过程表现为固液相接触引起表面力不平衡,在生物吸附剂上形成溶质的表面层,并通过与各种微生物细胞组分发生物化作用进行吸附重金属物质
[10 ]
。从这个意义上讲,Wang和Chen
[15 ]
认为,由于微生物细胞壁中存在官能团(羟基,羧基,氨基,苯酚),而Cr(VI)具有对生物吸附剂(如细菌,真菌和微藻)的亲和作用。所以在寻找替代方法以去除Cr(VI)的过程中,使具有吸附作用的微藻,真菌,细菌和酵母作为生物吸附剂成为可能。该生物吸附剂因环保有效和安全可靠的优点已显示出在工业应用中去除重金属方面具有巨大潜力
[16 ]
。
与传统方法相比,生物吸收剂具有低成本,高容量和高效率的特点,同时还可以减少化学药剂的残留降低二次污染的风险。生物吸附过程的机制取决于生物吸附剂的内在和外在因素。内在包括微生物自身活性,生长速度,代谢产物类型等;外在因素包括微生的营养需求和培养条件,如碳源,氮源,温度,p H和溶解氧等影响。除了以上因素作为生物吸附剂选择的重要特征外,还应考虑金属物质及其各自的浓度以及赋存状态。大多数微生物表现出双相反应,表现为当受到低浓度重金属的刺激时,微生物经过缓慢的适应期后迅速生长产生代谢产物保护自身不受重金属的侵害,代谢产物中的官能团(羟基,羧基,氨基,苯酚)有助于对重金属的吸附;当重金属浓度超过微生物最大耐受值时,细胞生长受到抑制,该过程可以直接影响种群大小,生物活性和微生物生物多样性
[17 ]
。各种类型的生物吸附剂已被用于去除重金属和其他污染物,其中包括藻类,微藻
[18 ]
,真菌
[19 ]
,酵母
[20 ]
,细菌
[21 ]
,污泥
[22 ]
和水生大型植物等。微生物作为生物吸附剂用于处理被Cr(VI)污染的有效物可以通过以下现象发生:(i)阳离子与细胞壁的结合,这被称为生物吸附,随后是阳离子在细胞内的转移,(ii)通过酶促或由于微生物与含有金属的介质的相互作用将Cr(VI)还原为Cr(III)
[23 ]
。已用于Cr(VI)吸附的微生物如表1所述。
图1 物化方法修复铬污染场地示意图
Fig 1 Diagrammatic representation of various methods of physical remediation of chromium from contaminated sites
[11]
2 Cr(VI)的生物吸附
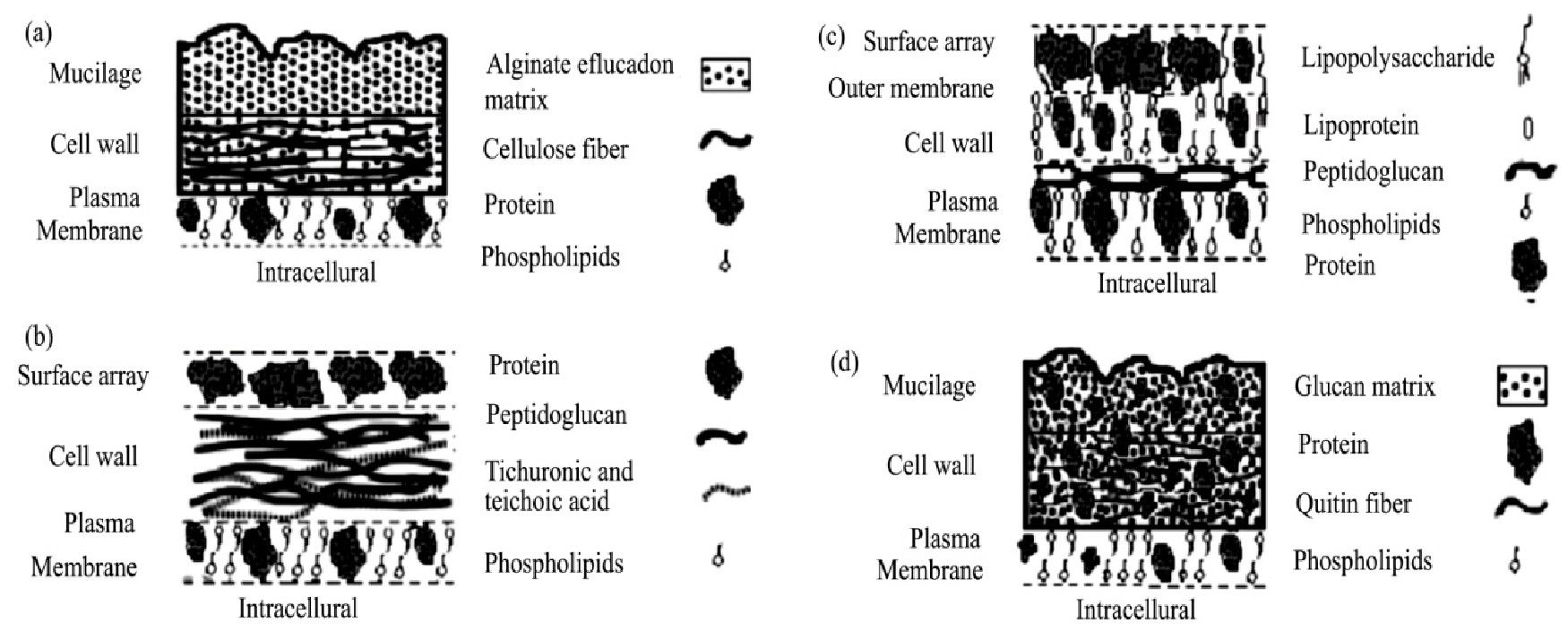
微生物作为吸附剂去除Cr(VI)的新技术已经在成本效益关系方面显示出优势,这引起相关学者们对微生物吸附技术产生极大的兴趣
[42 ]
,该方法具有较高的科学研究价值和广阔的工业应用前景。其中微生物生物质由于其细胞组成而具有吸附重金属污染物的能力,其结构如图2所示。阴离子配体磷酰基,羰基,巯基和羟基基团对生物吸附过程有很大贡献。根据物种的不同,微藻呈现出碳水化合物,蛋白质,脂类和脂肪酸的多样化成分。相比之下,真菌的细胞壁,特别是纤维细胞壁,由多糖组成,如β-葡聚糖,几丁质,壳聚糖,糖蛋白,脂质,黑色素和D-半乳糖胺聚合物,被认为是金属最为聚集的地方,存在很多的结合位点,如化学基团乙酰氨基,酰胺,磷酸,氨基,胺,巯基,羧基和羟基
[43 ]
。而革兰氏阳性细菌的细胞壁主要是由肽聚糖形成的网状结构组成,约占细胞干重的50%~80%,具有较好的柔韧性。另一方面,革兰氏阴性细菌的细胞壁具有薄层肽聚糖,约占细胞干重的5%~20%,此外,在革兰氏阴性菌细胞壁外侧聚有脂多糖、脂质双层、脂蛋白等成分,汇聚更多基团。
2.1 微藻
小球藻,栅藻,蓝绿藻和螺旋藻等物种的活体或死亡物种已被用于Cr(VI)的生物吸附。它们可以单独使用或与其他生物吸附剂结合使用,并且尽管与其他微生物相比显示出较慢的生长速度,但其生物质残余物(例如在生物柴油生产中获得脂质后剩余的生物质残余物)可以重新用作生物吸附剂。Chen等
[44 ]
确定了微藻的Cr(VI)生物吸附能力,研究了微藻对Cr(VI)时的最大耐受值,其中Scenedesmus quadricauda,Selenastrumcapriornutum和Spirulina platensis,吸附Cr(VI)浓度分别为20.9,12.4和11.7 mg·L-1 。Kwak等
[18 ]
通过在p H 2.0下加入氯化锂和二甲基亚砜以及41.1 mg·L-1 的Cr(VI)来研究螺旋藻生物吸附效果,在0.1 mol·L-1 Na OH溶液中进行5次解吸循环,Cr(VI)去除效率为70%。Chrorellaminiataals在Harnetal进行的研究中显示了生物吸附和生物还原Cr(VI)至Cr(Ⅲ)的能力。傅里叶红外光谱的结果表明,微生物中的氨基是引起Cr(VI)生物吸附的主要基团,而羧基主要吸附Cr(Ⅲ)
[24 ]
。
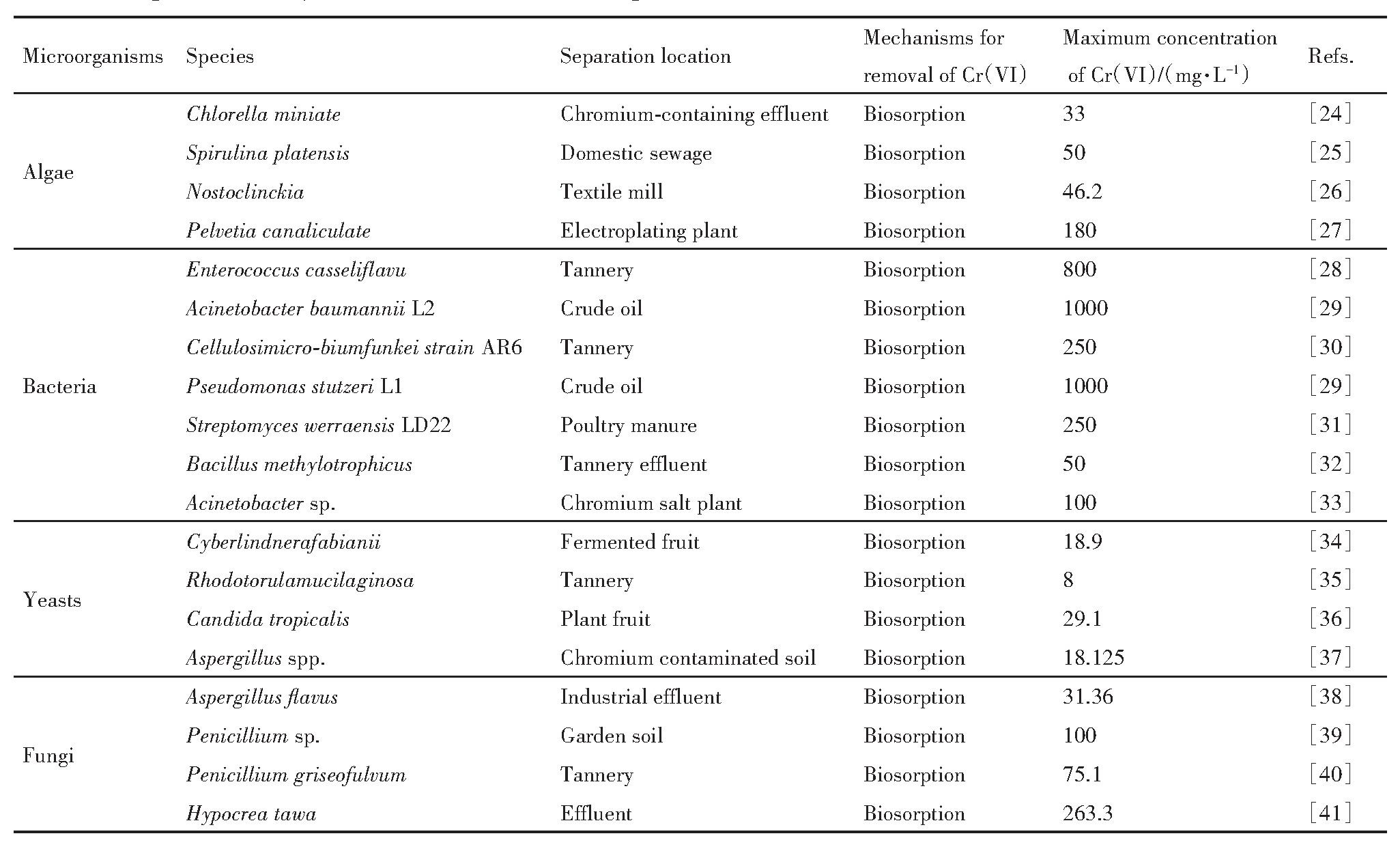
表1 用于吸附Cr(VI)的典型微生物 下载原图
Table 1 Typical microorganism used on Cr(VI)biosorption
图2 重金属生物吸附中主要生物吸附剂的结构 [10]
Fig 2 Structures of principal biosorbents used in biosorption of heavy metals
[10]
2.2 细菌
细菌在适宜的营养物质和培养条件下具有更高的生长速度。快速的繁殖会引起生物量的增加和大量的代谢产物的积累,其特异的细胞膜结构和不同的官能团类型(羟基,羧基,氨基,苯酚)是众多研究者选择细菌作为理想的生物吸附剂的最重要因素,具有非常重要的科研价值。应该指出的是,有些细菌在吸附过程中需要保持厌氧环境,然而通过严格厌氧培养活的微生物去除重金属是很困难的,这是限制部分细菌在开放环境中广泛应用的主要原因。因此,细菌作为吸附剂在工业中应用时,除了需要筛选高效功能菌株外还要保证细菌良好的生存环境。Banerjee等
[45 ]
从煤矿废水中分离得到一株Pseudomonas brenneri,通过条件优化(p H 6.0,温度为30℃),该菌可以在6天内将60mg·L-1 的Cr(VI)去除96.3%,同时,还可以耐受多种重金属离子(Fe,Cu,Mg,Mn,Zn,As和Pb)。Mathiyazhagan和Natarajan
[46 ]
使用Thiobacillusspp和Pseudomonasspp修复多种重金属离子的矿山污染物,通过比较两种菌对重金属生物吸附效果,结果表明,T.ferrooxidans能够吸附来自矿山的多种重金属(Cd,Cu,Zn,Cr,Mn和Pb),而P.Aeruginosa只能对单一重金属具有良好的吸附效果。魏长浩等
[47 ]
研究了用藻类和细菌联合吸附Cr(VI)和Cu,实验在生物反应器中进行,结果显示使用多种微生物共同作用的生物吸附剂是最有效的组合。当Cr(VI)浓度为10 mg·L-1 ,其中87%的Cr(VI)被生物吸附。Zheng等
[48 ]
还验证了枯草芽孢杆菌除了可以有效的吸附Cr(VI),在有氧条件下还具有将Cr(VI)还原为Cr(Ⅲ)的能力,并表明基因nfr A直接参与合成还原酶的过程。
2.3 酵母菌
近年来,有些报道中提出用酵母作为生物吸附剂用于去除Cr(VI)。酵母菌作为多功能微生物不仅广泛应用于食品行业,而且还可以用于污染土壤的修复。它们具有宽广的培养条件,低成本的营养需求和安全可靠的应用空间等特征,被作为理想的生物吸附剂应用于各行业
[49 ]
。Bahafid等
[36 ]
发现毕赤酵母去除Cr(VI)涉及吸附在官能团(例如,酰胺I,酰胺II,酰胺III,多糖,磺酸盐和羧基)上,然后在细胞内积累的Cr(VI)生物转化为Cr(Ⅲ),从而得到吸附过程与还原相结合的结论。同一作者还报道了其他3种酵母(Cyberlindnerafabianii,Wickerhamomycesanomalus和Candida tropicalis)能够通过吸附作用从铬污染场地中去除Cr(VI)。Pas等
[50 ]
使用Candida intermedia对不同浓度Cr(VI)进行生物吸附,并通过扫描电子显微镜观察,显示低浓度的Cr(VI)吸附在细胞周围且不影响细胞特征,当Cr(VI)超过其抑制浓度时,引起细胞结构的损害。酵母可用作单一生物吸附剂应用于低浓度Cr(VI)的吸附。Sathvika等
[51 ]
使用微波和纤维素生物聚合物来固定酵母并制成生物吸附剂材料,该材料不仅可以集中地处理污染物而且填充材料还可以为微生物提供营养素物质,实现最大化吸附Cr(VI)的目的。
2.4 真菌
真菌在生物圈中起着重要的作用。它们是多功能的微生物,能够迅速适应不利的环境,快速繁殖且可以循环利用有机物,这些特性使它们成为去除Cr(VI)的主要微生物,真菌吸附Cr(VI)机制主要表现为阴离子(Cr O4 2- 或Cr2 O7 2- )与真菌生物质上的带正电荷的基团结合(葡聚糖,几丁质,功能基团,蛋白酶和纤维类等)。Padma和Bajpai
[52 ]
研究发现,Cr(VI)在Trichoderma sp.表面的结合位点是细胞的羧基和胺基团。Chakraborty等
[53 ]
从印度红树林中分离得到一株具有去除Cr(VI)能力的真菌Aspergillus sp.,通过条件优化,该菌可以对50mg·L-1 的Cr(VI)去除98%以上,另外通过傅立叶变换红外结果显示,氨基,羧基,磺酸基等官能团参与主要的生物吸附过程。Singh等
[38 ]
研究表明,曲霉(Aspergillus flavus)吸附的Cr(VI)的最大浓度为16.1 mg·g-1 ,表明该微生物可用于中试工厂,用于处理含Cr(VI)的电解质。在由Chhikara等
[54 ]
进行的一项研究中使用的真菌生物吸附剂是用酸处理并固定在藻酸钙基质中的黑曲霉(Aspergillus niger)。通过傅立叶变换红外检测光谱显示由于细胞壁中存在的羟基,羧基和胺基团是吸附Cr(VI)的主要基团。Mohite等
[55 ]
对Schwanniomyces和Trichoderma吸附Cr(VI)的研究中发现,微生物所产生的胞外聚合物质(EPS)与对金属离子的吸附耦合还原过程相关联。Carol等
[42 ]
研究生物吸附剂平菇(Pleurotusostreatus)作为一种廉价且有前景的吸附剂用于从工业废水中去除Cr(VI)。该真菌的吸附作用是由于其细胞结构中存在的壳聚糖负荷,这是一种衍生自脱乙酰几丁质的化合物。结果显示由于真菌细胞壁中存在很高浓度的壳聚糖,对Cr(VI)具有良好的吸附作用,吸附值达5.2 mg·g-1 。
3 微生物吸附铬的影响因素
微生物作为吸附剂对Cr(VI)的吸附受许多物理,化学和环境参数的影响。如污染物的浓度,赋存状态,营养物质,培养条件及代谢产物等因素
[56 ]
。
3.1 铬的赋存形态及浓度
与其他重金属修复原理相似,当微生物经过驯化后随着铬浓度增加时,其吸附效率随之增加,但当铬浓度超过微生物的最大耐受值时,铬的吸附明显受到抑制,吸附效率降低,这是因为高浓度的铬对微生物有毒害作用。铬对微生物毒害的强弱不仅取决于铬的浓度,还取决于微生物的种类以及铬的赋存状态等。研究表明不同的微生物对铬的吸附能力明显不同。例如,Bacillus methylotrophicus对低浓度的Cr(VI)具有良好的吸附作用,但当Cr(VI)的浓度超过800 mg·L-1 时能抑制其生长降低吸附效率
[56 ]
。Chug等
[57 ]
通过条件优化Bacillus subtilis产生的更多的胞外聚合物,可以在两天内将初始浓度为10 mg·L-1 的Cr(VI)去除48%。铬的不同赋存形态也影响着生物吸附的效果,这主要因为不同赋存形态对微生物的毒害作用各不相同。在自然环境中,铬虽然存在九个化合价,但只有Cr(Ⅲ)和Cr(VI)较为稳定,它们与不同物质形成多种化合物,其中以氧化铬酸盐合物、重铬酸盐、铬的氧化物和铬的氢氧化物最为常见,这些化合物因Cr(VI)毒性较强而对微生物的吸附作用影响最大。Pas等
[50 ]
进行了Candida intermedia对Cr(Ⅲ)和Cr(VI)的不同吸附研究,结果表明,Cr(Ⅲ)影响着其出芽方式,参与其代谢活动且不会抑制微生物的生长;而在吸附Cr(VI)的过程中,Cr(VI)大量聚集在细胞,抑制微生物的生长,且高浓度的Cr(VI)使细胞变性甚至破裂。Fernández等
[58 ]
研究表明,微生物对Cr(VI)的吸附作用不仅与铬的赋存形态有关,还受到Cr(VI)的浓度的直接影响,Cr(VI)去除速率随Cr(VI)初始浓度的增大而逐渐降低。
3.2 营养类型(碳源,氮源)及培养条件(温度,p H)
合适的碳源氮源不仅可以为微生物提供必要的营养物质,增加生物量,还能够为微生物吸附还原Cr(VI)提供电子供体,促进微生物对Cr(VI)的吸附或还原。微生物在吸附Cr(VI)的过程中能利用不同类型的碳源氮源,如葡萄糖、丙三醇、酵母膏等有机物和乳酸盐、草酸和铵盐等无机物。当提供给微生物不同类型的营养物质时,微生物对Cr(VI)的吸附效果也是不同的。研究表明,相比无机物,以有机物为营养物质时,微生物生长更迅速,更有利于对Cr(VI)的吸附。Enterbacter cloacae HOI去除Cr(VI)的研究发现,添加蔗糖作为其碳源时,去除Cr(VI)的效果比以其他类型的碳源较为显著,且该碳源最终可分解为葡萄糖和果糖。其中Enterococcus casseliflavu以酵母膏和蛋白胨为营养物质时吸附Cr(VI)的速率就明显高于以丙三醇和氯化铵为营养物质时的速率
[28 ]
。
p H和温度也对微生物吸附Cr(VI)存在重要的影响。p H和温度可以改变微生物的存活状况和生理代谢活动,又因微生物对Cr(VI)的吸附作用主要表现在细胞膜和代谢产物中,所以保证微生物生活在一个适宜的条件下产生足够的生物量和代谢产物显得十分重要。Yue等
[59 ]
研究了Desulfoviobiodesulfuricans对Cr(VI)的吸附时,发现该菌株在p H为8.9-9.8,35℃时产生较多的胞外聚合物,24 h内达到最大的吸附效率为85.1%。Kumar等
[60 ]
比较了Aspergillus niger,Aspergillus sydoni和Penicillium janthinellum对Cr(VI)的吸附能力,结果表明Aspergillus niger在p H 2.0时,对Cr(VI)的吸附率高达91.3%。Poopal和Laxman
[61 ]
研究Scenedesmus sp.对Cr(VI)的吸附能力时发现,在p H 2.7条件下最大吸附效率达90%以上,且随温度升高吸附率短暂增加。一些文献表明,Penicilliumsp.BS-1在30?C时对Cr(VI)的去除率最高达86.3%,而Streptomyces griseus还原Cr(VI)的最适温度为28℃。以上的研究均表明在适宜的p H和温度范围内,微生物对Cr(VI)的吸附均有理想的效果
[39 ]
。
3.3 接种量和胞外聚合物
微生物对Cr(VI)的吸附要取得良好的效果,就需要接种适量微生物为前提,在最优条件下培养产生足够的微生物数量共同作用。在一定浓度的微生物接种量范围内,微生物吸附Cr(VI)的速率会随着接种量的增长而升高,但当接种的微生物数量超过某一数值时,其吸附速率出现短时间内迅速升高而后缓慢下降的趋势。研究表明,Bacillus methylotrophicus吸附Cr(VI)时,在微生物接种量2%-8%(v/v)范围内,吸附速率随微生物接种量的增加而提高,但是吸附速率达到最高值后随时间逐渐降低,这是因为Cr(VI)不仅毒害微生物本身,还会因接种的微生物数量过多使相互竞争营养物质加剧,不利于微生物的健康成长和代谢产物的产生
[56 ]
。
胞外聚合物质(EPS)是由微生物自身在生长代谢活动中分泌的生物聚合物或大分子物质,其附着在微生物表面且化学成分复杂。EPS的主要成分是蛋白质和碳水化合物占据80%以上。另外,它还含有核酸,脂质,多糖,有机官能团和腐殖质等物质
[62 ]
。根据研究发现,因EPS中有多种功能官能团的存在,其具有吸附、螯合和络合Cr(VI)的潜力。例如羧基,巯基,酚基和磷酰基等
[63 ,64 ,65 ]
。Azotobacterbeijreinckii是一种好氧革兰氏阴菌,该菌株产生的EPS参与Cd和Cr的吸附。如EPS的傅立叶变换红外光谱所揭示的,羧基(αCOOH)和羟基(αOH)官能团主要参与金属结合
[57 ]
。Rasulov等
[66 ]
从Azotobacterchroococcum XU1中提取的EPS用于去除Pb和Hg。该研究通过设计不同的因子实验发现微生物的营养类型和培养条件等因素对EPS生产的质量和总量具有深远的影响。Chug等
[57 ]
研究了不同p H,温度和生长期对Bacillus subtilis产生EPS产生的影响。结果表明在p H 7.0 37?C条件下,该菌株在96 h内可产生35.33 mg的EPS用于吸附Cr(VI)。该实验印证了可以通过优化微生物产EPS的条件提高吸附Cr(VI)的潜力。
3.4 其他影响因素
除以上因素以外,还存在很多影响对Cr(VI)吸附效果的因素。例如,微生物的需氧量对吸附作用也存在着很大的影响。研究表明,许多微生物都可在厌氧条件下进行重金属的去除
[67 ]
。如sulfate reducing bacteria可在厌氧条件下去除Cr(VI),且显著高于好氧条件下的去除率。因此控制好溶解氧的浓度也十分重要。在铬污染区域中还有其他共存离子,这些离子也会在某种条件下影响微生物对Cr(VI)的吸附
[59 ]
。Mathiyazhagan和Natarajan
[46 ]
将分离出的Thiobacillus spp.和Pseudomonas spp.用于Cr(VI)的去除时,发现Pseudomonas spp.除了具有去除Cr(VI)的能力外,还可以同时作用于Cd,Ca,Zn,Cr,Mn和Pb等重金属离子,而Thiobacillus spp.却不具有此项能力。Kabir等
[68 ]
研究了从铬污染土壤和废水中分离的Kosakoniacow-anii MKPF2,Klebsiella pneumonia MKPF5,Acineto-bacter gerneri MKPF7,Klebsiella variicola MKPF8和Serratia marcescens MKPF12能够耐受Cr(VI)达到2000 mg·L-1 ,同时耐受Ni2+ 和Zn2+ ,但有Cd2+ 和Hg2+ 存在时Cr(VI)去除效率显著降低。该结果表明微生物对其他重金属离子的耐受能力不同,同时影响着Cr(VI)的去除。
4 生物吸附的局限性和潜力
综上所述,以微生物作为吸附剂修复铬污染,因其经济环保,安全高效且无二次污染等优点引起了业内学者的关注,并取得了理想的吸附效果。但生物吸附过程也存在着一定的局限性。例如微生物吸附Cr(VI)的过程中,因修复区域处于一个开放环境中,不易于各影响因素的协调,从而无法保证理想的修复效果,更难保证长期稳定的吸附作用。再者在重金属吸附的过程中,涉及微生物生长速度,细胞活性,酶合成和土著菌的竞争等因素,微生物对Cr(VI)的吸附也会受到不同程度的影响。
现阶段以微生物为生物吸附剂处理Cr(VI)的技术还处于实验室的研究阶段,想要利用生物吸附剂在工业应用中发挥重要的作用仍需我们进一步的研究和探索。其中未来值得我们进一步探索的方向包括以下几个方面:
1.结合基因工程和蛋白组学,分离和选育出对Cr(VI)具有高效吸附能力的菌株,并研究其吸附Cr(VI)的机制。通过探索该菌株的营养类型,培养条件和吸附机制,进一步提高微生物活性,增加代谢产物质量,增强吸附稳定性,从而提高对Cr(VI)的吸附能力。
2.通过微生物的包埋技术,以填料的方式将其应用于连续运行的生物反应器系统中,一方面微生物可以利用废水中的能源物质提供营养,另一方面通过充分接触废水中的Cr(VI)进行吸附。
3.探索多种Cr(VI)去除方法联合应用,例如植物—微生物联合吸附,微生物—化学沉淀,微生物—物理吸等技术,该方法既避开单一吸附的各种局限性又可以提高Cr(VI)的去除效率。
参考文献
[1] Wen J K,Chen B W,Shang H,Zhang G C. Research progress in biohydrometallurgy of rare metals and heavy nonferrous metals with an emphasis on China[J]. Rare Metals,2016,35(6):433.
[2] Silva B,Figueiredo H,Quintelas C. Improved biosorption for Cr(VI)reduction and removal by Arthrobacter viscosus using zeolite[J]. International Biodeterioration&Biodegradation,2012,74:116.
[3] Fernando V A K,Weerasena J,Lakraj G P,Perera I C,Dangalle C D,Handunnetti S,Premawansa,S,Wijesinghe M R. Lethal and sub-lethal effects on the Asian common toad Duttaphrynus melanostictus from exposure to hexavalent chromium[J]. Aquatic Toxicology,2016,177:98.
[4] Curi N H,Brait C H,Antoniosi Filho N R,Talamoni S A. Heavy metals in hair of wild canids from the Brazilian Cerrado[J]. Biological Trace Element Research,2012,147(3):97.
[5] Strandberg G W,Shumate S E,Parrott Junior J R. Microbial cells as biosorbents for heavy metals:accumulation of uranion by Saccharomyces cerevisiae and Pseudomonas aeruginosa[J]. Applied and Environmental Microbiology,1981,41(1):237.
[6] Baral A,Engelken R D. Chromium-based regulations and greening in metalfinishing industries in the USA[J]. Environmental Science&Policy, 2002, 5(2):121.
[7] Cheung K H,Gu J D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential:a review[J]. International Biodeterioration&Biodegradation,2007,59(1):8.
[8] Frois S R,Grassi M T,Fernandes T C,Barreto R AS,Abate G. Preconcentration of Cr(III)and speciation analysis of chromium employing montmorillonite saturated with potassium ions[J]. Química Nova,2011,34(3):462.
[9] Fernandez P M,Cruz E L,Figueroa L I C. Perspective in bioremediation:enhancing the hexavalent chromium removal using native yeasts from Tucuman,Argentina[J]. Bioremediation in Latin America,2014,209.
[10] Vendruscolo F,Ferreira G L D R,Filho N R A. Biosorption of hexavalent chromium by microorganisms[J]. International Biodeterioration&Biodegradation,2016,119:87.
[11] Jobby R,Jha P,Yadav A K. Biosorption and biotransformation of hexavalent chromium[Cr(VI)]:a comprehensive review[J]. Chemosphere,2018,207:255.
[12] Jung C,Heo J,Han J,Her N,Lee S J,Oh J,Ryu J,Yoon Y. Hexavalent chromium removal by various adsorbents:powdered activated carbon,chitosan,and single/multi-walled carbon nanotubes[J]. Separation and Purification Technology,2013,106:63.
[13] Fadel M,Hassanein N M,Elshafei M M,Mostafa A H,Ahmed M A,Khater H M. Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae[J]. Housing and Building National Research Center,2017,13(1):106.
[14] Chen T,Zhou Z,Xu S,Wang H,Lu W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge[J]. Bioresource Technology,2015,190:388.
[15] Wang J,Chen C. Biosorbents for heavy metals removal and their future[J]. Biotechnology Advances,2009,27(2):195.
[16] Fu F,Wang Q. Removal of heavy metal ions from wastewaters:a review[J]. Journal of Environmental Management,2011,92(3):407.
[17] Kavamura V N,Esposito E. Biotechnological strategies applied to the decontamination of soils polluted with heavy metals[J]. Biotechnology Advances,2010,28(1):61.
[18] Kwak H W,Kim M K,Lee J Y,Yun H,Kim MH,Park Y H,Lee K H. Preparation of bead-type biosorbent from water-soluble Spirulina platensis extracts for chromium(VI)removal[J]. Algal Research,2015,7:92.
[19] Khani M H,Pahlavanzadek H,Alizadeh K. Biosorption of strontium from aqueous solution by fungus Aspergillus terreus[J]. Environmental Science and Pollution Research International,2012,19(6):2408.
[20] Mahmoud M S,Mohamed S A. Calcium alginate as an eco-friendly supporting material for Baker's Yeast strain in chromium bioremediation[J]. Housing and Building National Research Center,2017,13(3):245.
[21] Huang H,Wu K,Khan A,Jiang Y,Ling Z,Liu P,Chen Y,Tao X,Li X. A novel Pseudomonas gessardii strain LZ-E simultaneously degrades naphthalene and reduces hexavalent chromium[J]. Bioresource Technology,2016,207:370.
[22] Michailides M K,Tekerlekopoulou A G,Akratos C S,Coles S,Pavlou S,Vayenas D V. Molasses as an efficient low-cost carbon source for biological Cr(VI)removal[J]. Journal of Hazardous Materials, 2015,281:95.
[23] Gokhale S V,Jyoti K K,Lele S S. Kinetic and equilibrium modeling of chromium(VI)biosorption on fresh and spent Spirulina platensis/Chlorella vulgaris biomass[J].Bioresource Technology,2008,99(9):3600.
[24] Han X,Wong Y S,Wong M H,Tam N F Y.Biosorption and bioreduction of Cr(VI)by a microalgal isolate,Chlorella miniata[J]. Journal of Hazardous Materials,2007,146(2):65.
[25] Finocchio E,Lodi A,Solisio C. Chromium(VI)removal by methylated biomass of Spirulina platensis:the effect of methylation process[J]. Chemical Engineering Journal,2010,156(2):264.
[26] Mona S,Kaushik A,Kaushik C P. Biosorption of chromium(VI)by spent cyanobacterial biomass from a hydrogen fermentor using Box-Behnken model[J]. International Biodeterioration&Biodegradation, 2011, 65(4):656.
[27] Hackbarth F V,Maass D,Souza A A U,Vilar V J P,Souza S M A G U. Removal of hexavalent chromium from electroplating wastewaters using marine macroalga Pelvetiacanaliculata as natural electron donor[J]. Biochemical Engineering Journal,2016,290:477.
[28] Saranraj P,Stella D,Reetha D. Bioabsorption of chromium resistant Enterococcus casseliflavusisolated from tannery effluent[J]. Journal of Ecobiotechnology,2010,2:17.
[29] Sathishkumar K,Murugan K,Benelli G. Bioreduction of hexavalent chromium by Pseudomonas stutzeri L1 and Acinetobacter baumannii L2[J]. Annals of Microbiology,2016,67(1):91.
[30] Karthik C,Ramkumar VS,Pugazhendhi A,Gopalakrishnan K,Arulselvi P I. Biosorption and biotransformation of Cr(VI)by novel Cellulosimicrobiumfunkeistrain AR6[J]. Journal of the Taiwan Institute of Chemical Engineers,2017,70:282.
[31] Latha S,Vinothini G,Dhanasekaran D. Chromium[Cr(VI)]biosorption property of the newly isolated actinobacterial probiont Streptomyces werraensis LD22[J]. 3Biotech,2015,5(4):423.
[32] Srinath T,Verma T,Ramteka P W,Garg S K. Chromium(VI)biosorption and bioaccumulation by chromate resistant bacteria[J]. Chemophere,2002,48(4):427.
[33] Srivastava S,Ahmad AH,Thakur IS. Removal of chromium and pentachlorophenol from tannery effluents[J].Bioresource Technology,2007,98(5):1128.
[34] Joutey N T,Sayel H,Bahafid W E,Ghachtouli N.Mechanism of hexavalent chromium resistance and removal by microorganisms[J]. Reviews of Environmental Contamination and Toxicology,2015,233:45.
[35] Chatterjee S,Chatterjee N C,Dutta S. Bioreduction of chromium(VI)tochromium(III)by a novel yeast strain Rhodotorulamucilaginosa(MTCC 9315)[J]. African Journal of Biotechnology,2012,11:14920.
[36] Bahafid W,Joutey N T,Sayel H,Ghachtouli N E L.Mechanism of hexavalent chromium detoxification using Cyberlindnerafabianii yeast isolated from contaminated site in Fez(Morocco)[J]. Journal of Materials and Environmental Science,2013,4(6):840.
[37] Sivakumar D. Biosorption of hexavalent chromium in a tannery industry wastewater using fungi species[J].Journal of Environmental Management, 2016, 2(2):105.
[38] Singh R,Kumar M,Bishnoi N R. Development of biomaterial for chromium(VI)detoxification using Aspergillus flavus system supported with iron[J]. Ecological Chemistry and Engineering,2016,91:31.
[39] Barsainya M,Chandra P,Singh D P. Investigation of Cr(VI)uptake in saline condition using psychrophilic and mesophilic Penicilliumsp.[J]. International Journal of Current Microbiology and Applied Sciences,2016,5(1):274.
[40] Abigail E A M,Samuel M S,Chidambaram R. Hexavalent chromium biosorption studies using Penicillium griseofulvum MSR1 a novel isolate from tannery effluent site:Box-Behnken optimization,equilibrium,kinetics and thermodynamic studies[J]. Journal of the Taiwan Institute of Chemical Engineers,2015,49:156.
[41] Barrera L M,Urbina E C. Bioreduction of hexavalent chromium by Hypocrea tawa in a concentric draft-tube airlift bioreactor[J]. Journal of Environment&Biotechnology Research,2015,1(1):37.
[42] Carol D,Kingsley S J,Vincent S. Hexavalent chromium removal from aqueous solutions by Pleurotusostreatus spent biomass[J]. International Journal of Engineering Sciences&Research Technology,2012,4:7.
[43] Das S,Mishra J,Das S K,Pandey S,Rao D S,Chakraborty A,Sudarshan M,Das N,Thatoi H. Investigation on mechanism of Cr(VI)reduction and removal by Bacillus amyloliquefaciens,a novel chromate tolerant bacterium isolated from chromite mine soil[J]. Chemosphere,2014,96:112.
[44] Chen H L,Pan G,Yan H,Qin Y W. Toxic effects of hexavalent chromium on the growth of blue-green microalgae[J]. Environmental Science,2003,24(2):13.(陈海柳,潘纲,闫海,秦延文.六价铬抑制淡水蓝绿藻生长的毒性效应[J].环境科学, 2003, 24(2):13.)
[45] Banerjee S,Kamila B,Barman S,Joshi S R,Mandal T,Halder G.Interlining Cr(VI)remediation mechanism by a novel bacterium Pseudomonas brenneriisolated from coalmine wastewater[J]. Journal of Environmental Management,2019,233:271.
[46] Mathiyazhagan N,Natarajan D. Bioremediation on effluents from magnesite and bauxite mines using Thiobacillus spp and Pseudomonas spp.[J]. Journal of Bioremediation&Biodegradation,2011,2(1):115.
[47] Wei C H,Bai W H,Zhen C Y,Yang L,Liu W Q. Preliminary study on adsorption of copper and chromium by bacteria and algae[J]. China Brewing, 2016, 35(12):129.(魏长浩,白汶洪,甄春燕,杨柳,刘文群.几种细菌和藻类吸附铜、铬重金属离子初步研究[J].中国酿造,2016,35(12):129.)
[48] Zheng Z,Li Y,Zhang X,Liu P,Ren J,Wu G,Zhang Y,Chen Y,Li X.A Bacillus subtilis strain can reduce hexavalent chromium to trivalent and an nfrA gene is involved[J]. International Biodeterioration&Biodegradation,2015,97:90.
[49] Wang J,Chen C. Biosorption of heavy metals by Saccharomyces cerevisiae:a review. biotechnol[J]. Biotechnology Advances,2006,24(5):427.
[50] Pas M,Milacic R,Draslar K,Pollak N,Raspor P. Uptake of chromium(III)and chromium(VI)compounds in the yeast cell structure[J]. Bio. Metals,2004,17(1):25.
[51] Sathvika T,Rajesh V,Rajesh N. Microwave assisted immobilization of yeast in cellulose biopolymer as a green adsorbent for the sequestration of chromium[J]. Biochemical Engineering Journal,2015,279:38.
[52] Padma S V,Bajpai D. Phyto-remediation of chrome-VI of tannery effluent by Trichoderma species[J]. Desalination,2008,222(3):255.
[53] Chakraborty V,Sengupta S,Chaudhuri P,Das P. Assessment on removal efficiency of chromium by the isolated manglicolous fungi from Indian Sundarban mangrove forest:removal and optimization using response surface methodology[J]. Environmental Technology&Innovation,2018,10(1):335.
[54] Chhikara S,Hooda A,Rana L,Dhankhar R. Chromium(VI)biosorption byimmobilized Aspergillus Niger in continuous flow system with special referenceto FTIR analysis[J]. Journal of environmental biology,2010,31(5):561.
[55] Mohite P T,Kumar A R,Zinjarde S S. Biotransformation of hexavalentchromium into extracellular chromium(III)oxide nanoparticles using Schwanniomycesoccidentalis[J]. Biotechnology Letters,2015,38(3):441.
[56] Boopathy R. Factors limiting bioremediation technologies[J]. Bioresource Technology. 2000,74(1):63.
[57] Chug R,Gour V S,Mathur S,Kothari S L. Optimization of extracellular polymeric substances production using Azotobacterbeijreinckii and Bacillus subtilis and its application in chromium(VI)removal[J]. Bioresource Technology,2016,214:604.
[58] Fernández P M,Vinarta S C,Bernal A R,Cruz E L,Figueroa L C. Bioremediation strategies for chromium removal:current research,scale-up approach and future perspectives[J]. Chemosphere,2018,208:139.
[59] Yue Z B,Li Q,Li C C,Chen T H,Wang J. Component analysis and heavy metal adsorption ability of extracellular polymeric substances(EPS)from sulfate reducing bacteria[J]. Bioresource Technology, 2015,194:399.
[60] Kumar R,Bishnoi N R,Garima K B. Biosorption of chromium(VI)from aqueous solution and electroplating wastewater using fungal biomass[J]. Chemical Engineering Journal,2008,135(3):202.
[61] Poopal A C,Laxman R S. Hexavalent chromate reduction by immobilized Streptomyces griseus[J]. Biotechnology Letters,2008,30(6):1005.
[62] Yuan D Q,Wang Y L. Study on the stratification components of extracellular polymeric substances(EPS)in activated sludge and their variation characteristics in physicochemical properties[J]. Environmental Science,2012,33(10):3522.(袁冬琴,王毅力.活性污泥胞外聚合物(EPS)的分层组分及其理化性质的变化特征研究[J].环境科学,2012,33(10):3522.)
[63] Chien C C,Lin B C,Wu C H. Biofilm formation and heavy metal resistanceby an environmental Pseudomonas sp.[J]. Biochemical Engineering Journal, 2013,78:132.
[64] Ha J,Gélabert A,Spormann A M,Brown G E. Role of extracellularpolymeric substances in metal ion complexation on Shewanellaoneidensis:batch uptake,thermodynamic modeling,ATR-FTIR,and EXAFS study[J].Geochimica et Cosmochimica Acta,2010,74(1):1.
[65] Li G Y,Tao L,Sun J,Wang Y D,Li Y F. Morphology properties of Aspergillus niger during bioleaching of uranium and its effect on uranium extraction[J]. Chinese Journal of Rare Metals,2019,43(10):1085.(李广悦,陶露,孙静,王永东,李艳芳.黑曲霉浸铀过程中的形态特征及其对铀浸出的影响[J].稀有金属,2019,43(10):1085.)
[66] Rasulov B A,Yili A,Aisa H A. Biosorption of metal ions by exopolysaccharide produced by Azotobacterchroococcum XU1[J]. Journal of Environmental Protection,2013,4:989.
[67] Luo Y,Wen J K,Wu B. Activity of bacteria and leaching properties by sodium carboxymethyl cellulose[J].Chinese Journal of Rare Metals,2018,42(11):1199.(罗毅,温健康,武彪.羧甲基纤维素钠对细菌活性及其浸矿性能的影响[J].稀有金属,2018,42(11):1199.)
[68] Kabir M M,Fakhruddin A N M,Chowdhury M A Z. Isolation and characterization of chromium(VI)-reducing bacteria from tannery effluents and solid wastes[J].World Journal of Microbiology&Biotechnology,2018,34(9):126.