Trans. Nonferrous Met. Soc. China 29(2019) 634-640
Leaching kinetics of scheelite concentrate with sodium hydroxide in the presence of phosphate
Ting-ting LI, Yan-bai SHEN, Si-kai ZHAO, Yao-yu YIN, Rui LU, Shu-ling GAO, Cong HAN, De-zhou WEI
School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China
Received 1 March 2018; accepted 9 July 2018
Abstract:
The reaction kinetics for the leaching of low-grade scheelite concentrate was investigated in an autoclave with sodium hydroxide in the presence of phosphate. The effects of stirring speed (300-600 r/min), reaction temperature (353-383 K), sodium hydroxide concentration (1.69-6.76 mol/L) and phosphate concentration (0.68-1.69 mol/L) on the WO3 dissolution ratio were studied. The results showed that the WO3 dissolution ratio was practically independent of stirring speed, while it increased with increasing the reaction temperature, and the concentrations of sodium hydroxide and phosphate. The experimental data were consistent with the shrinking core model, with a surface chemical reaction as the leaching rate-determining step. The apparent activation energy was calculated as 49.56 kJ/mol, and the reaction orders with respect to the concentrations of sodium hydroxide and phosphate were determined as 0.27 and 0.67, respectively. The kinetics equation of the leaching process was established.
Key words:
kinetics; leaching; sodium hydroxide; phosphate; scheelite;
1 Introduction
The main economic minerals of tungsten in nature are known as scheelite (CaWO4) and wolframite ((Fe,Mn)WO4) [1-4]. Among them, scheelite deposits account for approximately two-thirds of the world’s tungsten reserves, and scheelite has become a predominant raw material due to progressive exhaustion of wolframite [5,6]. Therefore, it is imperative to study and utilize scheelite efficiently and rationally [7,8].
The main methods for the extraction of tungsten from scheelite concentrate have been widely reported, including hydrochloric acid and alkali leaching methods [9-13]. As an industrial classical process, scheelite is usually decomposed to produce tungstic acid by using hydrochloric acid in the last century. However, the acid-leaching process has a fatal flaw that the dense solid layer of tungstic acid formed in acidic solution could cover the unreacted particles, and then block the contact between acidic ions and particle surface [14,15]. As a result, the reaction cannot continue, therefore results in a notably slow reaction rate and low leaching ratio [16,17].
In present commercial applications, sodium hydroxide leaching is widely used to process the wolframite and scheelite concentrates as well as the soft and hard tungsten scraps [18]. This method possesses a strong adaptability to various raw materials. It can treat not only low-grade scheelite concentrate but also wolframite-scheelite blend concentrate, thus has been successfully applied into industrial practice in China [19]. As is well known, when sodium hydroxide (NaOH) is used to decompose scheelite concentrate, the resulting sodium tungstate (Na2WO4) in the solution will react with calcium hydroxide (Ca(OH)2) in a series of subsequent operations such as unloading, dilution and filtration. Then, the calcium tungsten (CaWO4) will be formed, leading to an increase of WO3 content in the leaching residue. In order to solve the problem, some effective measures for preventing the formation of CaWO4 should be adopted. When NaOH is used as the dissolving medium in the leaching process, Ca2+ can form insoluble precipitates with some additives containing anions like 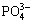 or F-. Among them, a phosphate compound, containing
or F-. Among them, a phosphate compound, containing 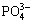 anion and an alkaline cation, is often used as the additive to inhibit the reaction between
anion and an alkaline cation, is often used as the additive to inhibit the reaction between  and Ca(OH)2 in solution. It can react with Ca2+ and OH- in alkali solution to form more stable hydroxyapatite (Ca5(PO4)3OH), thereby, reducing the loss of tungsten in the leaching residue, and improving the final dissolution ratio of scheelite. The chemical reactions of scheelite in sodium hydroxide solution without or with phosphate could be expressed as follows:
and Ca(OH)2 in solution. It can react with Ca2+ and OH- in alkali solution to form more stable hydroxyapatite (Ca5(PO4)3OH), thereby, reducing the loss of tungsten in the leaching residue, and improving the final dissolution ratio of scheelite. The chemical reactions of scheelite in sodium hydroxide solution without or with phosphate could be expressed as follows:
CaWO4+2NaOH=Ca(OH)2+Na2WO4 (1)
5CaWO4+3Na3PO4+NaOH=Ca5(PO4)3OH+5Na2WO4 (2)
The above reactions demonstrate that NaOH and Na3PO4 can convert scheelite mineral into water-soluble Na2WO4 compound and simultaneously generate insoluble calcium salt. At 25 °C, the equilibrium constants, K1=[Na2WO4]/[NaOH]2 and K2=[Na2WO4]5/ ([Na3PO4]3[NaOH]), have the values of 2.59×10-4 and 1.38×1014, respectively. Therefore, it is possible to operate at a relatively low reaction temperature and obtain a higher decomposition ratio in the presence of phosphate compared to that in the absence of phosphate [20-22]. Meanwhile, this method can adopt the scheelite concentrate with high phosphorus content, and therefore can reduce tungsten loss in the physical beneficiation process. On the other hand, except for the alkali metals, almost all of metal ions exist as hydroxides in alkali solution, thereby reducing the impurities in leaching solution [23]. The leaching of scheelite with sodium hydroxide in the presence of phosphate has been successfully implemented in the industry production. Nevertheless, it is not entirely clear whether the comprehensive influence of sodium hydroxide solution containing phosphate in cooperation with various process parameters could be brought for further improvement on the leaching process and the dissolution ratio of scheelite. Therefore, it is meaningful for tungsten extraction from scheelite concentrate with sodium hydroxide in the presence of phosphate.
In this work, the leaching of scheelite with sodium hydroxide in the presence of phosphate was studied to investigate the effects of stirring speed, reaction temperature, and concentrations of sodium hydroxide and phosphate on the WO3 dissolution ratio.
2 Experimental
2.1 Raw material
Experiments were carried out with low grade scheelite concentrate, supplied by Gansu Xinzhou Mining Co. Ltd., China. Before the leaching experiments, the scheelite concentrate was dried at 90 °C for 24 h to remove free moisture water, and the average particle size of the scheelite concentrate was relatively fine with <74 μm accounting for 92%. Therefore, the fine particle scheelite concentrate was used directly without grinding and classification in all subsequent experiments. The chemical compositions of the scheelite concentrate were analyzed with an X-ray fluorescence spectrometer (XRF, ZSX Primus II, RIGAKU Corp) and listed in Table 1. It shows that the valuable composition in the sample is WO3, and the main impurities are CaO, SiO2, P2O5, MgO, SO3 and Fe2O3. An X-ray diffractometer (XRD, PANalytical X’Pert Pro, Cu Kα, λ=1.54056 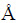 ) was utilized for the characterization of the scheelite concentrate. As shown in Fig. 1, it can be clearly seen that the sample is mainly composed of CaWO4.
) was utilized for the characterization of the scheelite concentrate. As shown in Fig. 1, it can be clearly seen that the sample is mainly composed of CaWO4.
Table 1 Chemical compositions of scheelite concentrate (wt.%)
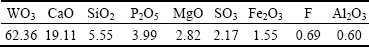
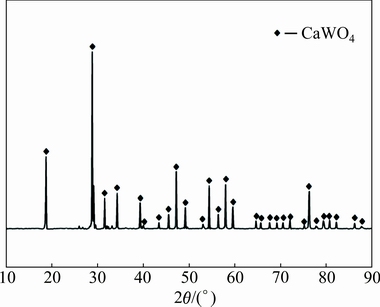
Fig. 1 XRD pattern of scheelite concentrate
2.2 Leaching experiment
The chemical reagents (NaOH and Na3PO4·12H2O) in the experiments were of analytical grade. All the leaching experiments were carried out in a 250 mL autoclave (YZPR-250 style produced by Shanghai Yanzhen Co. Ltd) equipped with a temperature and stirring speed controller. In a typical experimental procedure, 5 g of scheelite concentrate was leached with a liquid-to-solid ratio of 12:1. Scheelite concentrate, sodium hydroxide, sodium phosphate and deionized water were orderly added into the autoclave in a specific concentration. Subsequently, the experiments were run for a preset time at the required reaction temperature. After the leaching process, the obtained products were cooled down to 50 °C naturally, and the digested slurry was subsequently removed from the autoclave. After washing and filtering, the content of tungsten in the filter cake was analyzed by thiocyanate spectrometric method using a spectrophotometer (721N style produced by Shanghai Yidian Instrument Co. Ltd.) at 420 nm. The WO3 dissolution ratio was then determined based on:
 (3)
(3)
Experiments were conducted in particle size smaller than -74 μm accounting for 92%. Stirring speeds of 300, 400, 500 and 600 r/min were investigated as well as reaction temperatures from 353 to 383 K. Meanwhile, the concentrations of NaOH and Na3PO4 were also studied ranging from 1.69 to 6.76 mol/L and from 0.68 to 1.69 mol/L, respectively.
3 Results and discussion
3.1 Effect of stirring speed
The effect of stirring speed on the dissolution ratio of scheelite was investigated with the speed range from 300 to 600 r/min using 6.76 mol/L NaOH, 1.35 mol/L Na3PO4 and a reaction temperature of 373 K at a fixed reaction time of 120 min. The results are shown in Table 2.
Table 2 Effect of stirring speed on WO3 dissolution ratio

It can be clearly observed that the dissolution ratio of scheelite increases obviously from 44.74% to 52.50% with the increase of stirring speed from 300 to 400 r/min, and then the dissolution ratio of scheelite is not obvious with increasing stirring speed further. This demonstrates that a relatively low stirring speed is adverse to the mass transfer and dissolution of reactants during the leaching process. Thus, it is reasonable to deduce that the leaching process is controlled by chemical reaction rate in the adherent fluid film. However, the stirring speed is needed to maintain the ore particles in suspended status. Therefore, the stirring speed is fixed at 400 r/min for all subsequent experiments, which is enough to eliminate the influence of external diffusion.
3.2 Effect of reaction temperature
The effect of reaction temperature on the WO3 dissolution ratio was examined with the temperature ranging from 353 to 383 K using 6.76 mol/L NaOH and 1.35 mol/L Na3PO4 at a fixed stirring speed of 400 r/min. The results are shown in Fig. 2.
It can be clearly observed that the WO3 dissolution ratio increases as the reaction temperature increases at a fixed reaction time, indicating that the reaction temperature has a significant effect on the WO3 dissolution ratio. For example, more than 92.58% of WO3 is extracted at 383 K compared with 39.39% WO3 extracted at 353 K with the same reaction time of 360 min. Therefore, raising the reaction temperature can significantly increase the WO3 dissolution ratio.
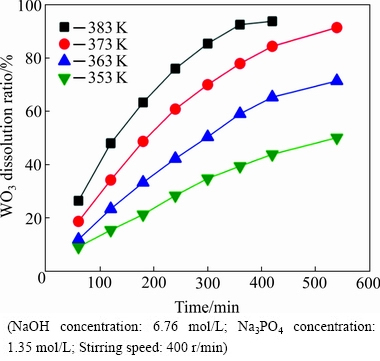
Fig. 2 Effect of reaction temperature on WO3 dissolution ratio
In order to obtain the kinetics equation and corresponding apparent activation energy, an appropriate kinetics model should be determined to describe the liquid-solid leaching process. Assuming that the scheelite particles had a spherical geometry and gradually shrunk during the leaching process, the shrinking core model with surface chemical reaction control can be utilized to describe the dissolution kinetics of the leaching reaction in this study. Results given in Table 2 demonstrate that the resistance of the external diffusion can be completely eliminated as the stirring speed is above 400 r/min, namely that the diffusion through the boundary layer does not act as a rate-determining step. Therefore, the leaching process can be controlled by the surface chemical reaction. The kinetics equation of the liquid-solid reaction at any time can be described as
1-(1-x)1/3=Kt (4)
The data of Fig. 2, plotted according to Eq. (4), give the linear relationship between 1-(1-x)1/3 and reaction time, as shown in Fig. 3. The correlation coefficient values are greater than 0.99, indicating that the kinetics data agree quite well with shrinking core model with the surface chemical reaction control in all the leaching experiments.
The apparent activation energy can be determined by Eq. (5):
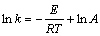 (5)
(5)
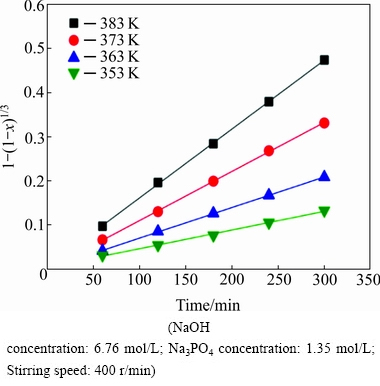
Fig. 3 Relationship between 1-(1-x)1/3 and reaction time for tungsten leaching at various reaction temperatures
The overall reaction rate constants obtained from the slopes in Fig. 3, are used to draw Arrhenius plot. According to Eq. (5), the Arrhenius plot of ln k vs T -1 at four reaction temperatures is shown in Fig. 4. The Arrhenius plot shows a relatively good linear tendency with a correlation coefficient of 0.9956. Based on the slope of the straight line, the apparent activation energy for the leaching reaction is calculated to be 49.56 kJ/mol. According to the previous report, the apparent activation energy for a diffusion-controlled process in the leaching reaction is usually between 4 and 12 kJ/mol, while for a surface chemical reaction controlled process, the value is typically greater than 40 kJ/mol [24]. Therefore, the result clearly confirms that the leaching process is controlled by the surface chemical reaction and the leaching is strongly influenced by the reaction temperature.
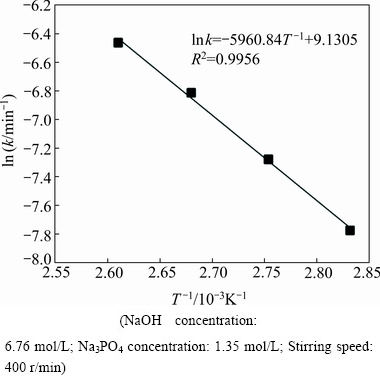
Fig. 4 Arrhenius plot for WO3 dissolution in reaction temperature range of 353-383 K
3.3 Effect of NaOH concentration
A series of experiments were performed with different NaOH concentrations under the conditions of Na3PO4 concentration 1.35 mol/L, reaction temperature 373 K and stirring speed 400 r/min. The NaOH concentrations ranged from 1.69 to 6.76 mol/L. The experimental data are given in Fig. 5.
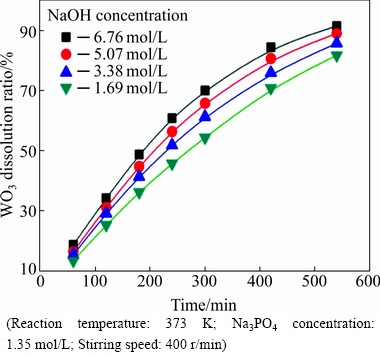
Fig. 5 Effect of NaOH concentration on WO3 dissolution ratio
It can be seen from Fig. 5 that the WO3 dissolution ratio increases significantly with the increase of the NaOH concentration. After 540 min, the WO3 dissolution ratio reaches 91.47% at 6.76 mol/L NaOH, whereas it is only 81.74% at 1.69 mol/L NaOH. Hence, increasing the NaOH concentration is an effective method to facilitate the leaching process. The data of Fig. 5, plotted according to Eq. (4), give the linear relationship between 1-(1-x)1/3 and reaction time, as shown in Fig. 6. The results further confirm the kinetics assumption of the shrinking core model with a surface chemical-controlled step.
The reaction order can be obtained via the logarithm relationship between the overall rate constant and NaOH concentration. As summarized in Fig. 7, the reaction order with respect to NaOH concentration is determined as 0.27, indicating a weak dependence of reaction on the NaOH concentration.
3.4 Effect of Na3PO4 concentration
A series of experiments were carried out with different Na3PO4 concentrations under the conditions of NaOH concentration 6.76 mol/L, reaction temperature 373 K and stirring speed 400 r/min. The Na3PO4 concentrations ranged from 0.68 to 1.69 mol/L. The experimental data are given in Fig. 8.
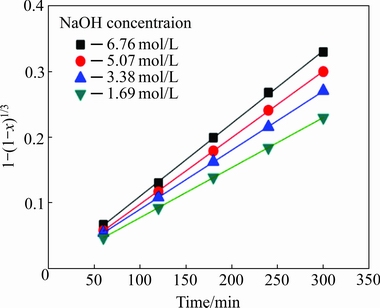
Fig. 6 Relationship between 1-(1-x)1/3 and reaction time for tungsten leaching at various NaOH concentrations
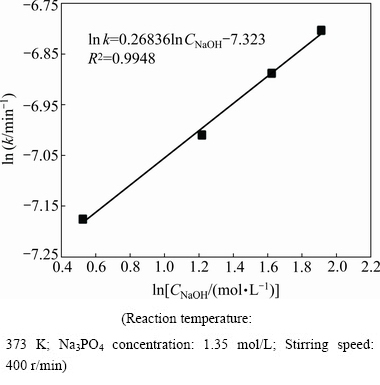
Fig. 7 Arrhenius plot for ln k vs ln CNaOH
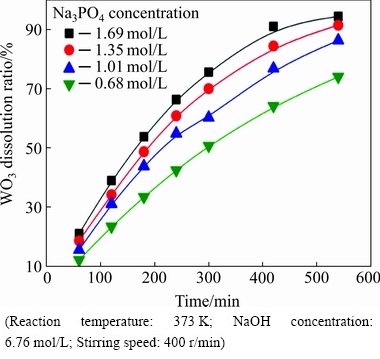
Fig. 8 Effect of Na3PO4 concentration on WO3 dissolution ration
It can be seen from Fig. 8 that the WO3 dissolution ratio increases significantly with increasing Na3PO4 concentration. After 540 min, the WO3 dissolution ratio reaches 94.45% at 1.69 mol/L Na3PO4, whereas it is only 74.04% at 0.68 mol/L Na3PO4. The results indicate that the Na3PO4 concentration has a positive effect on the decomposition of the scheelite. The data of Fig. 8, plotted according to Eq. (4), give the linear relationships, as shown in Fig. 9. The results further indicate that the leaching process is controlled by the surface chemical reaction.
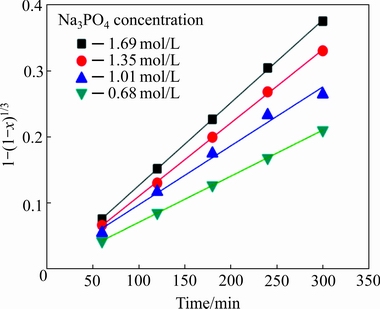
Fig. 9 Relationship between 1-(1-x)1/3 and reaction time for tungsten leaching at various Na3PO4 concentrations
The reaction order can be determined via the logarithm relationship between the overall rate constant and Na3PO4 concentration. The results are summarized in Fig. 10, from which the reaction order of Na3PO4 concentration in the leaching process is determined as 0.67. The empirical reaction order for Na3PO4 concentration is 0.67, implying a strong dependence of the leaching process on the Na3PO4 concentration.
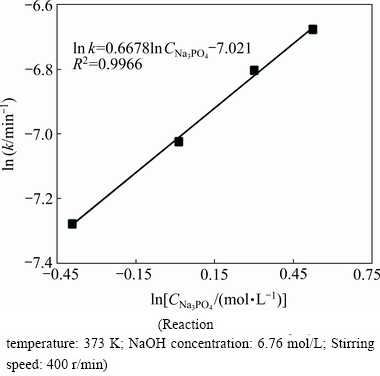
Fig. 10 Arrhenius plot for 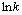 versus
versus 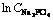
3.5 Establishment of kinetics equation
The results of stirring speed, apparent activation energy and reagent concentrations of this research demonstrate that the interface reaction between phosphate and scheelite in the alkaline solution is the rate-determining step. Therefore, according to Eqs. (4) and (5), an empirical kinetics equation can be expressed as
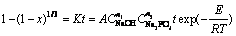 (6)
(6)
The relationship between 1-(1-x)1/3 and 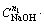
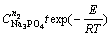 is shown in Fig. 11. Although the data points are somewhat scatter in the plot, they surround a straight line with a correlation coefficient of over 0.97. Thus, based on Fig. 11, A value is determined from the slope of the straight line to be 6.03.
is shown in Fig. 11. Although the data points are somewhat scatter in the plot, they surround a straight line with a correlation coefficient of over 0.97. Thus, based on Fig. 11, A value is determined from the slope of the straight line to be 6.03.
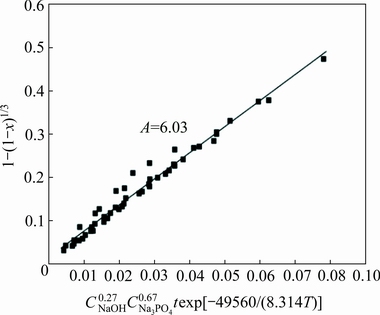
Fig. 11 Relationship between 1-(1-x)1/3 and 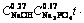
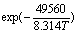
According to the obtained apparent activation energy, reaction orders and A value, the kinetics equation of sodium hydroxide leaching of scheelite in the presence of phosphate can be expressed concretely as
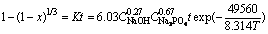 (7)
(7)
The establishment of the scheelite leaching kinetics will be helpful to identify the rate-determining factors in the alkaline leaching in the presence of phosphate, optimize the process parameters, and consequently accelerate leaching reaction and obtain a high yield of tungsten. Therefore, the leaching kinetics will be promising to guide the practical operation and industrial production of the alkaline leaching of low-grade scheelite concentrate in the presence of phosphate.
4 Conclusions
(1) Reaction temperature, NaOH concentration and Na3PO4 concentration have significant effects on the WO3 dissolution ratio. The WO3 dissolution ratio increases with the increase of reaction temperature, NaOH concentration and Na3PO4 concentration, while it is independent of stirring speed at an elevated range.
(2) The experimental data agree quite well with the shrinking core model, with the chemical reaction control as the rate-determining step. The apparent activation energy is determined as 49.56 kJ/mol. Furthermore, the reaction orders with respect to sodium hydroxide and phosphate are determined to be 0.27 and 0.67, respectively. The established kinetics equation could well describe the leaching process.
(3) This leaching method can greatly improve the dissolution ratio of the scheelite as well as effectively reduce tungsten loss. The leaching kinetics will be promising to guide the practical operation and industrial production of the alkaline leaching of low-grade scheelite concentrate in the presence of phosphate.
Nomenclatures
ε
WO3 dissolution ratio, %;
m1
Mass of filter cake, g;
β
WO3 content in filter cake, %;
m
Mass of sample, g;
α
WO3 content in solid sample, %;
x
Fraction of dissolution, %;
K
Overall reaction rate constant, min-1;
t
Reaction time, min;
k
Rate constant for surface reaction, min-1;
E
Apparent activation energy, J/mol;
R
Mole gas constant, 8.314 J/(K·mol);
T
Reaction temperature, K;
A
Frequency factor;
n1
NaOH reaction order;
n2
Na3PO4 reaction order;
C
Concentration of leaching reagent, mol/kg.
References
[1] BOHLOULI A, AFSHAR M R, ABOUTALEBI M R, SEYEDEIN S H. Optimization of tungsten leaching from low manganese wolframite concentrate using response surface methodology (RSM) [J]. International Journal of Refractory Metals & Hard Materials, 2016, 61: 107-114.
[2] MARTINS J I. Leaching systems of wolframite and scheelite: A thermodynamic approach [J]. Mineral Processing & Extractive Metallurgy Review, 2014, 35: 23-43.
[3] SRINIVAS K, SREENIVAS T, NATARAJAN R, PADMANABHAN N P H. Studies on the recovery of tungsten from a composite wolframite-scheelite concentrate [J]. Hydrometallurgy, 2000, 58: 43-50.
[4] WANG Lin-sheng, HUANG Xiao-jing, DENG Deng-fei, LI Hong-chao, CHEN Yao-ming. Synthesis of scheelite from sodium tungstate solution by calcium hydroxide addition [J]. Hydrometallurgy, 2015, 154: 17-19.
[5] LI Jiang-tao, ZHAO Zhong-wei. Kinetics of scheelite concentrate digestion with sulfuric acid in the presence of phosphoric acid [J]. Hydrometallurgy, 2016, 163: 55-60.
[6] ZHAO Zhong-wei, LIANG Yong, LIU Xue-heng, CHEN Ai-liang, LI Hong-gui. Sodium hydroxide digestion of scheelite by reactive extrusion [J]. International Journal of Refractory Metals & Hard Materials, 2011, 29: 739-742.
[7] HE Gui-xiang, ZHAO Zhong-wei, WANG Xiao-bo, LI Jiang-tao, CHEN Xing-yu, HE Li-hua, LIU Xu-heng. Leaching kinetics of scheelite in hydrochloric acid solution containing hydrogen peroxide as complexing agent [J]. Hydrometallurgy, 2014, 144: 140-147.
[8] ZHAO Z W, DING W T, LIU X H, LIANG Y. Effect of ultrasound on kinetics of scheelite leaching in sodium hydroxide [J]. Canadian Metallurgical Quarterly, 2013, 52: 138-145.
[9] GURMEN S, TIMUR S, ARSLAN C, DUMAN I. Acidic leaching of scheelite concentrate and production of hetero-poly-tungstate salt [J]. Hydrometallurgy, 1999, 51: 227-238.
[10] LI Xiao-bin, SHEN Lei-ting, ZHOU Qiu-sheng, PENG Zhi-hong, LIU Gui-hua, QI Tian-gui. Scheelite conversion in sulfuric acid together with tungsten extraction by ammonium carbonate solution [J]. Hydrometallurgy, 2017, 171: 106-115.
[11] MARTINS J I. Leaching of synthetic scheelite by nitric acid without the formation of tungstic acid [J]. Hydrometallurgy, 2003, 70: 131-141.
[12] PAULINO J F, AFONSO J C, MANTOVANO J L, VIANNA C A. Recovery of tungsten by liquid-liquid extraction from a wolframite concentrate after fusion with sodium hydroxide [J]. Hydrometallurgy, 2012, 127-128: 121-124.
[13] LI Hong-gui. Production of high purity APT from scheelite and complex tungsten raw material with high Mo content [J]. Transactions of Nonferrous Metals Society of China, 2004, 14: 366-369.
[14] LIU Liang, XUE Ji-lai, LIU Kang, ZHU Jun, WANG Zeng-jie. Complex leaching process of scheelite in hydrochloric and phosphoric solutions [J]. JOM, 2016, 68: 1-8.
[15] ZHANG Wen-juan, LI Jiang-tao, ZHAO Zhong-wei, HUANG Shao-bo, CHEN Ying-xue, HU Kai-long. Recovery and separation of W and Mo from high-molybdenum synthetic scheelite in HCl solutions containing H2O2 [J]. Hydrometallurgy, 2015, 155: 1-5.
[16] KAHRUMAN C, YUSUFOGLU I. Leaching kinetics of synthetic CaWO4 in HCl solutions containing H3PO4 as chelating agent [J]. Hydrometallurgy, 2006, 81: 182-189.
[17] ZHAO Zhong-wei, LI Jiang-tao, WANG Shi-bo, LI Hong-gui, LIU Mao-sheng, SUN Pei-mei, LI Yun-jiao. Extracting tungsten from scheelite concentrate with caustic soda by autoclaving process [J]. Hydrometallurgy, 2011, 108: 152-156.
[18] ILHAN S, KALPAKLI A, KAHRUMAN C, YUSUFOGLU I. The investigation of dissolution behavior of gangue materials during the dissolution of scheelite concentrate in oxalic acid solution [J]. Hydrometallurgy, 2013, 136: 15-26.
[19] ZHANG Wen-juan, LI Jiang-tao, ZHAO Zhong-wei. Leaching kinetics of scheelite with nitric acid and phosphoric acid [J]. International Journal of Refractory Metals & Hard Materials, 2015, 52: 78-84.
[20] ZHAO Zhong-wei, LI Hong-gui. Thermodynamics for leaching of scheelite-pseudo-ternary-system phase diagram and its application [J]. Metallurgical & Materials Transactions B, 2008, 39: 519-523.
[21] ZHAO Zhong-wei, CAO Cai-fang, LI Hong-gui. Thermodynamics on soda decomposition of scheelite [J]. The Chinese Journal of Nonferrous Metals, 2008, 18: 356-360. (in Chinese)
[22] WANG Shi-bo, ZHAO Zhong-wei, LI Hong-gui. Thermodynamic analysis on phosphate decomposition of scheelite [J]. Rare Metals & Cemented Carbides, 2005, 1: 1-4 (in Chinese).
[23] MARTINS J P. Kinetics of soda ash leaching of low-grade scheelite concentrates [J]. Hydrometallurgy, 1996, 42: 221-236.
[24] ASHRAF M, ZAFAR Z I, ANSARI T M. Selective leaching kinetics and upgrading of low-grade calcareous phosphate rock in succinic acid [J]. Hydrometallurgy, 2005, 80: 286-292.
氢氧化钠-磷酸盐浸出白钨矿精矿动力学
李停停,沈岩柏,赵思凯,殷尧禹,卢 瑞,高淑玲,韩 聪,魏德洲
东北大学 资源与土木工程学院,沈阳 110819
摘 要:以低品位白钨精矿为研究对象,在高压反应釜体系中研究白钨精矿在氢氧化钠-磷酸盐溶液中的反应动力学,考察搅拌速度(300~600 r/min)、反应温度(353~383 K)、氢氧化钠浓度(1.69~6.76 mol/L)和磷酸盐浓度(0.68~1.69 mol/L)对WO3浸出率的影响。结果表明,WO3浸出率与搅拌速度无关,但随着反应温度、氢氧化钠浓度和磷酸盐浓度的增加而升高。实验结果遵循收缩核模型,即浸出速率由原料和产物的表面化学反应控制。浸出反应的表观活化能为49.56 kJ/mol,氢氧化钠浓度和磷酸盐浓度的反应级数分别为0.27和0.67。该浸出过程动力学方程可以根据相关结果和数据建立。
关键词:动力学;浸出;氢氧化钠;磷酸盐;白钨矿
(Edited by Xiang-qun LI)
Foundation item: Projects (51674067, 51422402) supported by the National Natural Science Foundation of China; Projects (N150101001, N160106004, N170106005) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Yan-bai SHEN; Tel/Fax: +86-24-83687381; E-mail: shenyanbai@mail.neu.edu.cn
DOI: 10.1016/S1003-6326(19)64973-3
Abstract: The reaction kinetics for the leaching of low-grade scheelite concentrate was investigated in an autoclave with sodium hydroxide in the presence of phosphate. The effects of stirring speed (300-600 r/min), reaction temperature (353-383 K), sodium hydroxide concentration (1.69-6.76 mol/L) and phosphate concentration (0.68-1.69 mol/L) on the WO3 dissolution ratio were studied. The results showed that the WO3 dissolution ratio was practically independent of stirring speed, while it increased with increasing the reaction temperature, and the concentrations of sodium hydroxide and phosphate. The experimental data were consistent with the shrinking core model, with a surface chemical reaction as the leaching rate-determining step. The apparent activation energy was calculated as 49.56 kJ/mol, and the reaction orders with respect to the concentrations of sodium hydroxide and phosphate were determined as 0.27 and 0.67, respectively. The kinetics equation of the leaching process was established.


