
J. Cent. South Univ. (2020) 27: 2382-2393
DOI: https://doi.org/10.1007/s11771-020-4456-1

Experimental and numerical evaluations on characteristics of vented methane explosion
SU Bin(苏彬)1, LUO Zhen-min(罗振敏)1, WANG Tao(王涛)1, LIU Lang(刘浪)2
1. School of Safety Science & Engineering, Xi’an University of Science and Technology,Xi’an 710054, China;
2. School of Energy, Xi’an University of Science and Technology, Xi’an 710054, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
To research the characteristics of vented explosion of methane-air mixture in the pipeline, coal mine tunnel or other closed space, the experiments and numerical simulations were carried out. In this work, explosion characteristics and flame propagation characteristics of methane in pipeline and coal mine tunnel are studied by using an explosion test system, combined with FLACS software, under different vented conditions. The numerical simulation results of methane explosion are basically consistent with the physical experiment results, which indicates that the numerical simulation for methane explosion is reliable to be applied to the practice. The results show that explosion parameters (pressure, temperature and product concentration) of methane at five volume fractions have the same change trend. Nevertheless, the explosion intension of 10.0% methane is the largest and that of 9.5% methane is relatively weak, followed by 11.0% methane, 8.0% methane and 7.0% methane respectively. Under different vented conditions, the pressure and temperature of methane explosion are the highest in the pipeline without a vent, followed by the pipeline where ignition or vent position is in each end, and those are the lowest in the pipeline with ignition and vent at the same end. There is no significant effect on final product concentration of methane explosion under three vented conditions. For coal mine tunnel, it is indicated that the maximum explosion pressure at the airproof wall in return airway with the branch roadway at 50 m from goaf is significantly decreased while that in intake airway does not change overwhelmingly. In addition, when the branch roadway is longer or its section is larger, the peak pressure of airproof wall reduces slightly.
Key words:
methane-air; flame propagation; CFD simulation; vented explosion; ignition position; peak pressure;
Cite this article as:
SU Bin, LUO Zhen-min, WANG Tao, LIU Lang. Experimental and numerical evaluations on characteristics of vented methane explosion [J]. Journal of Central South University, 2020, 27(8): 2382-2393.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4456-11 Introduction
Methane explosion accidents are increasingly common to happen in the natural gas and mine process industries [1-6]. It is an important way to relieve the pressure generated in restricted space through installation of a vent for preventing or minimizing damage from an explosion. In addition, the relative position of the vent and ignition has great influence on the characteristics of methane explosion. Therefore, studies on the characteristics of vented explosion of methane-air mixture under different conditions will provide the references for the prevention of methane explosion accidents.
At present, a large number of experiments and simulations were conducted to extensively investigate the characteristics of vented gas explosion [7-13]. Besides, all kinds of factors affecting vented gas explosion were investigated by experiments and numerical simulations. It is found that the quasi-static pressure in the chamber during vented explosion can be divided into three stages: pressure release, Helmholtz oscillation, and acoustic oscillation [14]. MOKHTAR et al [15] found that there is greater discrepancy in reduced explosion pressure values in the presence of vent area, ignition position and obstacles. The experimental and numerical studies on the closed and vented explosion behaviors of premixed methane-hydrogen/air mixtures were carried out by ZHANG et al [16]. WAN et al [17] investigated the effect of side vent size on explosion characteristics in a 1000 mm×100 mm×100 mm end-vented duct containing an obstacle. PHYLAKTOU et al [18] presented experimental data about the effect of the spark position on overpressure of methane-air explosion in long closed vessels. KINDRACKI et al [19] discussed the influence of ignition position on explosion development in methane–air mixture in closed vessels. They found that flame propagation and pressure variation in long tubes with or without obstacles are both extremely sensitive to the location of the ignition point. WILLACY et al [20] demonstrated that end ignition appears to be more severe than central ignition, which is contrary to the related data reported in literature. The investigation of GUO et al [21] indicated that front ignition always leads to the minimum internal and external overpressure. Additionally, the effect of vent size on vented gas explosion was examined by TOMLIN et al [22] and QI et al [23]. It is reported that reducing the vent size always increased the overpressure regardless of the degree of congestion. The experiments of BATRAEV et al [24] were performed in a tube with an open end and they measured the detonation cell size and detonation velocity. Meanwhile, the maximum flame spreading distance was mainly dominated by the vent size. An explosion venting system was established by ZHANG et al [25] to examine the influencing factors of the vented explosion position for a single vessel. The results show that the maximum overpressure for an explosion venting on the top and side does not vary for either a large or small vessel connected by pipes. A simple model was built to calculate peak pressure in vented explosions of hydrogen and hydrocarbons by SINHA et al [26]. NIE et al [27] proposed the method which can be used to calculate not only the velocity of flame front but also the velocity of the flame wherever it is in the pipe. Furthermore, researchers also studied the influence of initial pressure [28, 29], initial pressure [30, 31], obstacle configuration [32, 33], and gas concentration [34, 35] on gas explosion.
However, it is extremely rare to study the effect of the relative position of vent and ignition on characteristics of vented methane explosion, particularly in the pipeline and coal mine tunnel. Methane, a clean gas fuel, is widely used in various places. It is hard to avoid explosion accidents in the process of utilization and transportation. The investigation to the characteristics like explosion pressure and flame propagation velocity of vented methane explosion can help to evaluate explosion hazard of methane in restricted space. Moreover, we can obtain the relative parameters and take effective measures to prevent and mitigate the disaster.
The objective of the present work is to examine explosion characteristics of methane under vented conditions. The flame propagation of methane of three concentrations (7.0%, 9.5% and 11.0% by volume) was captured by high speed video camera in the process of explosion.Based on that, we acquired the velocity of the flame front and contrasted experiments and simulations. Additionally, the influence of ignition position on vented methane explosion was investigated by CFD simulation. Furthermore, the tunnel was modeled in FLACS software according to actual coal mine. And the effect of the branch roadway on methane explosion characteristics in coal mine tunnel was also examined. The results obtained were reliable and valuable for the prevention of vented methane explosion in the related industries.
2 Experimental
2.1 Experimental system
The experiment system consists of a flammable gas explosion limits test device and a high speed video camera, including a reaction tube, an ignition system, a circulating pump, a vacuum pump, a pressure gauge, solenoid valve and a high speed-camera, as shown in Figures 1 and 2. The experimental device diagram is displayed in Figure 3.
1) Explosion reaction tube
The explosion reaction tube was a quartz glass tube with a length of (1400±50) mm and a diameter of (60±5) mm. The thickness of the tube wall was not less than 2 mm, and the tube was equipped with a 25 mm pressure relief valve.

Figure 1 Explosion reaction tube

Figure 2 High speed viedo camera:
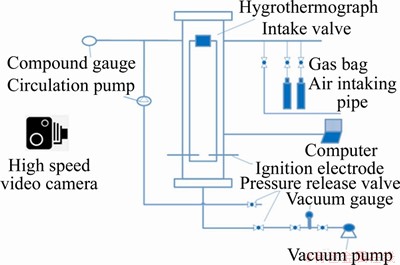
Figure 3 Experimental device diagram
2) High speed video camera
The high speed video camera with NAC Memrecam HX-6 was adopted in the experiment, and the shooting speed is as high as 4600 frame/s, as shown in Figure 3. In this work, the shooting speed is 1000 frame/s, and the shooting pixels can be up to 5×106. The image of the flame propagation process of methane explosion shot by high speed video camera can be clearly captured.
2.2 Experimental condition
In this work, methane-air mixtures with compositions of 7.0%, 9.5% and 11.0% vol were examined. The initial temperature was 20-25 °C, the initial pressure was atmospheric pressure, and the relative humidity was 58%-66%. The ignition discharging time was sustained for 500 ms. The experimental gases were prepared according to the partial-pressure method in a gas distribution system with an accuracy of 0.1%. After the gas was prepared, the circulation pump was turned on for 300 s to ensure the uniformity of the methane-air mixture. In addition, a pressure relief valve was installed at the bottom of the pipe, and it would open at the time of the explosion. In tests, ignition and vent were at the same end. The relative position map is shown in Figure 4.
3 Computational method
3.1 Mathematical model
The process of methane explosion was simulated by numerical simulation with the following reasonable assumptions considered.
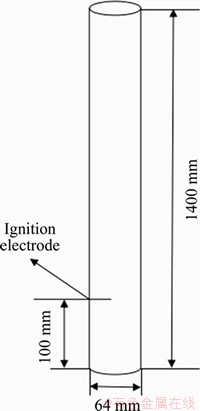
Figure 4 Relative position of ignition and vent in test
1) The explosive gas in pipeline is the ideal gas and adapts to the ideal gas equation of state;
2) The specific heat capacity of combustible gas and air mixture in pipeline varies with temperature, which adapts to the rule of mixture;
3) Methane explosion process is a single step reaction, without considering the specific primitive reaction process;
4) Explosion process is an adiabatic process, ignoring heat exchange between the restricted container device and the external environment.
Gas explosion in mathematical is approximated as heating expansion of ideal gas. Gas dynamics can be expressed by a series of basic equations such as continuity equation, momentum equation and energy equation. The basic equations in this mathematical model include continuum equation, momentum equation, energy equation, turbulent kinetic energy equation, turbulent energy dissipation rate equation, fuel component equation and mixture component equation, which can be expressed in uniform form as follows.

I II III IV(1)
where ρ is density; t is time; u is the component of velocity in x axial; Γφ is the exchange coefficient of φ; Sφ is the energy source term; μeff is effective viscosity;  is Prandtl value; φ is general variable, representing the velocity component u, v, w, turbulent kinetic energy k, dissipation rate of turbulent flow energy dissipation ε, enthalpy h, the mass fraction Yfu of combustible gas; I is an unsteady term; II is the convection term; III is the diffusion term; IV is the source term. The details of the controlling equations are shown in Table 1.
is Prandtl value; φ is general variable, representing the velocity component u, v, w, turbulent kinetic energy k, dissipation rate of turbulent flow energy dissipation ε, enthalpy h, the mass fraction Yfu of combustible gas; I is an unsteady term; II is the convection term; III is the diffusion term; IV is the source term. The details of the controlling equations are shown in Table 1.
3.2 Mesh generation and parameters setup
1) Pipeline
The mesh model of pipeline is displayed in Figure 5. The initial condition is at room temperature and atmospheric pressure. The boundary conditions were set where the velocity of each direction on the wall is 0; the radial pressure and temperature are set to 0; the gas density is uniform and the default is EULER. Output parameters are consistent with the above parameters. Methane is selected as explosive gas with different concentrations (7.0%, 8.0%, 9.5%, 10.0% and 11.0%). The gas filling area is arranged as the whole pipeline. The ignition position is in the axis of circular tube from the bottom 100 mm, and the starting time of ignition is set to 0.
2) Coal mine tunnel
According to a coal mine, a U-type working face with the cross section which is 5 m in width and 4 m in height is selected. There are 8 m long airproof walls at 450 m on the intake side and return side of the roadway, and there are three rows of single props with an interval of 1.5 m and an extension of 50 m on both sides of the goaf, totaling 204, with a diameter of 15 cm. It is equipped with electromechanical equipment which is 35 m long, 1.5 m high, 1.1 m wide, and 0.5 m away from the outside of the roadway. The length of the goaf is 180 m; the distance between the hydraulic support and the back wall is 0.8 m; the diameter of the hydraulic prop is 30 cm with the spacing of 90 cm, totaling 201, as shown in Figure 6. In the course of numerical simulation, the whole tunnel is set up as the calculation area. The uniform mesh division and the dividing principle of 400×200×4 at x, y and z axis were used. In addition, two pressure transducers were installed at the airproof wall located at the ends of intake and return airways respectively. The fuel area is set to be filled with combustible gas in the goaf. The filling length of the combustible gas in the return airway is 300 m, and there is no combustible gas in the intake airway.
Table 1 Controlling equations
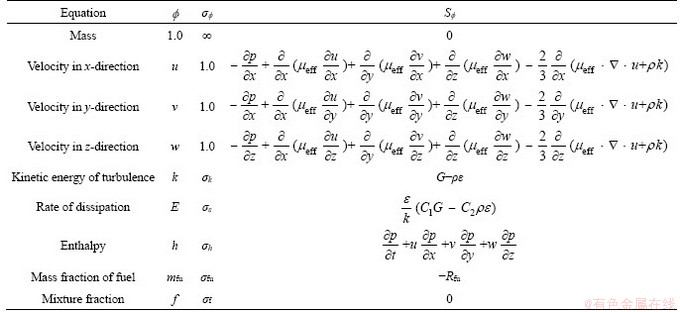
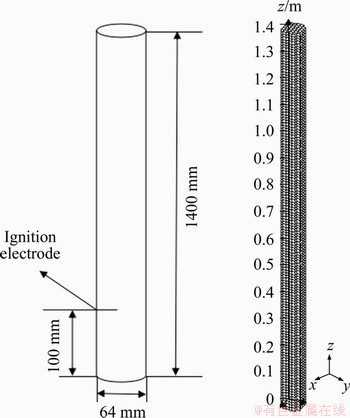
Figure 5 Geometry model and mesh model of pipeline
It is assumed that the concentration of methane was 10.0% and ignition position was set to the center of goaf. The mesh model of coal mine tunnel is displayed in Figure 7.
4 Results and analysis
4.1 Flame front velocity of methane explosion in pipeline
The flame propagation of methane explosion in pipeline was captured by the high-speed camera with a working speed 1000 frame/s. The interval of two pictures obtained was 1 ms. The primary pictures are shown in Figure 8. In order to analyze quantitatively, the data of the pictures were calculated according to the principle of similarity to obtain the propagation velocity and the displacement of the flame front. The calculation formula is shown as follows.
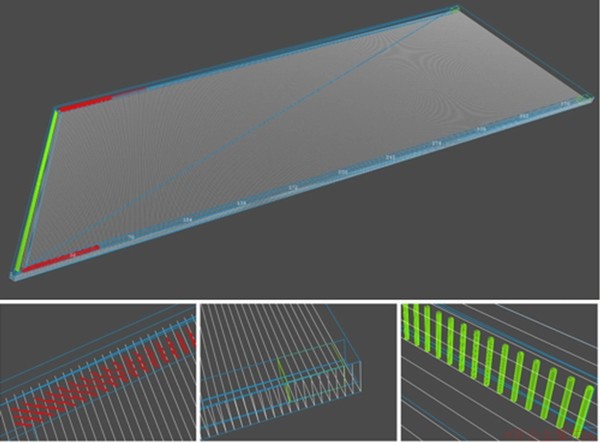
Figure 6 Detailed construction diagrams
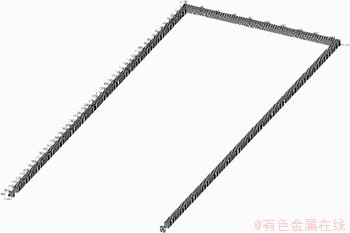
Figure 7 Mesh model of coal mine tunnel
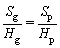 (2)
(2)
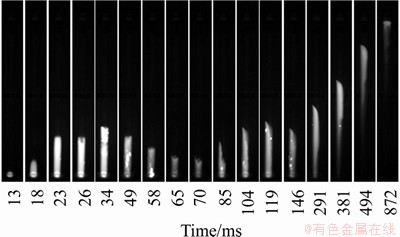
Figure 8 Process of flame propagation of 9.5% methane explosion in pipeline
 (3)
(3)
where Sg is the displacement of the flame front in the pipeline, Sp is the displacement of the flame front in the picture, Hg represents the length of the pipe in the picture, Hp represents the size of the picture, V is the velocity of flame front and ΔT is the time interval of two pictures.
The relationship between the flame front velocities and time is shown in Figure 9. With the process of methane explosion going on, the amount of heat given out continued to accumulate and turbulence intensity was increased by the effect of the pipe wall. Meanwhile, the flame front was deformed and the velocity of flame front increased to the first peak quickly. As the top of reaction pipe is closed, the reflection and superposition happened when the pressure propagated to the top of the pipe. Then, the unburned gas was compressed and the flame propagated to the maximum promptly with the effect of explosion vent. Additionally, the flame front velocity obtained by calculation fluctuated on the time axis until the flame front speed was reduced to 0 m/s. Compared with 7.5% and 11.0% methane, 9.5% is the theoretical equivalent concentration of methane combustion in the air in which methane reacts with air completely. The explosive power is the largest and the reaction rate is the fastest at that concentration. That is why the flame front velocity of 9.5% methane is larger than the other two concentrations no matter forward or back propagation.
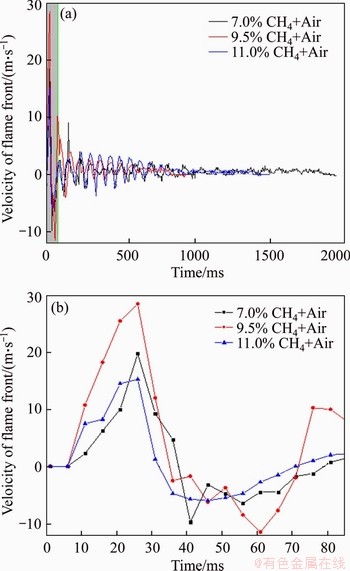
Figure 9 Velocities of flame front for methane-air mixtures
4.2 Comparative analysis of numerical simulation and experiment results
The explosion of methane at the specified conditions which was similar to the experimental conditions was simulated by FLACS software. Figures 10 and 11 illustrate the change of product concentration field and flame front velocity of methane explosion respectively. The flame structure can be expressed by the product concentration field during the explosion in a way. The product concentration field with ellipse initially propagated to the end of closed pipe in Figure 10. Then it started to sag because of pressure release. With the flame propagation, the product concentration field showed “tulip”. Finally, the “tulip” disappeared gradually and the front of product concentration field presented triangle. That is essentially in agreement with the flame propagation of methane explosion in Figure 8. The velocity of flame front for 9.5% methane simulated by CFD in Figure 11 was compared with experimental results (Figure 9). The velocity of flame front increased gradually in the positive direction and reached a peak. Then it dropped to 0 and started to peak in the negative direction. Finally, it fluctuated and reduced to 0. Compared with experimental results, the velocity of flame front simulated by CFD is roughly consistent. The error is caused by the condition where energy does not suffer a loss in the ideal state. Therefore, the flame propagation time by simulation is significantly shorter than that in the experiment and there is no obvious fluctuation in the flame propagation velocity later. In summary, the results obtained by simulation agree with experimental results essentially.
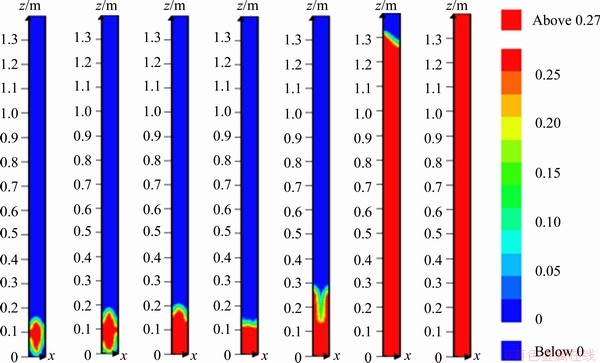
Figure 10 Distribution of product concentration field
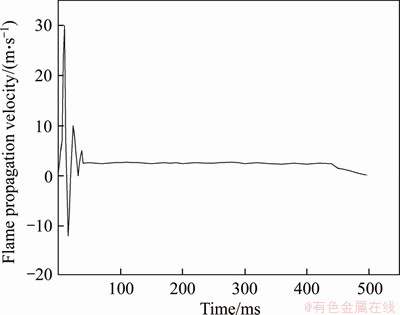
Figure 11 Velocity of flame front for 9.5% methane-air
4.3 Vented methane explosion in pipeline
Figure 12 shows the variation of methane explosion pressure at the center of the pipeline with time under three vented modes. Under the condition where ignition and vent were at the same end of the pipeline, the central pressure of pipeline hit the highest quickly, then fluctuated continuously until decreased to 0 m/s. When ignition and vent were at each end of the pipeline, the pressure rose initially to the maximum and then dropped to the negative pressure. However, the pressure continued to increase to the peak in the pipeline without a vent. Compared with three vented condition, the maximum explosion pressure in the closed pipeline without a vent is 793.77 kPa, the largest one, followed by the condition where ignition and vent are at each end, 74.54 kPa. The smallest one, only 7.79 kPa, equivalent to about 1% of the largest one, is the pipeline with ignition and vent at the same end. On the whole, the explosion pressure of methane (9.5%, 10.0%, 11.0%) reached the peak quickly while that of methane (7.0%, 8.0%) had obvious delay to hit the maximum.
The variation of the central temperature of pipeline with time under three vented modes is shown in Figure 13 in which the change trends are roughly identical. When ignition and vent were at the same end of the pipeline, the central temperature of pipeline heaved up in great swells at about 0.2 s, then remained unchanged. Under the condition where ignition and vent were at each end of the pipeline, the central temperature of pipeline reached the peak more quickly, then had a fluctuation within a narrow range and dropped to a certain value. Without vent in the pipeline, the temperature rose rapidly at first and then had a slight increase. Moreover, 7.0% and 8.0% methane lagged behind the other three concentrations in terms of time, which was similar to the change of methane explosion pressure.
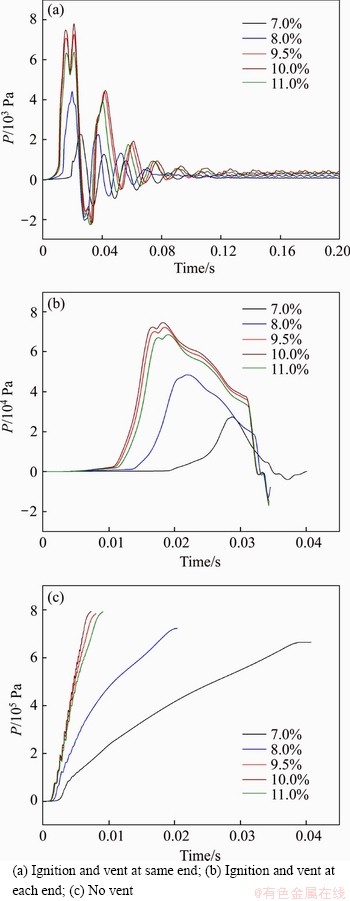
Figure 12 Variation of central pressure of pipeline:
The change of product concentration at the center of the pipeline is as shown in Figure 14 where the variations are basically similar under three vented modes. The product concentration rapidly increased at the beginning, and then kept the maximum value unchanged. The order of time to reach the maximum product concentration is t10%<>9.5%<>11%<>8%<>7%. Furthermore, compared with the other three concentrations, 7.0% and 8.0% methane had a significant delay to reach the peak. The time to reach the maximum in the pipeline with ignition and vent at each end or without vent is shorter than that under the other condition. However, the vented condition has no obvious influence on the final concentration of product in the process of methane explosion.

Figure 13 Variation of central temperature of pipeline:
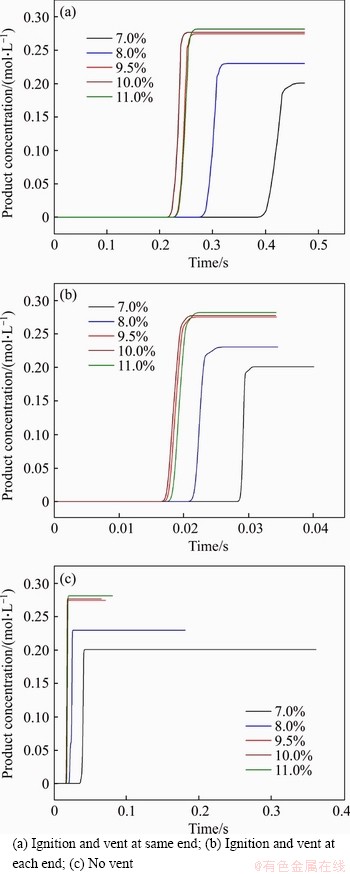
Figure 14 Variation of central product concentration of pipeline:
4.4 Vented methane explosion in coal mine tunnel
Figure 15 shows the variation of the maximum explosion pressure at the airproof wall where the symmetrical branch roadways (3×4×20) in intake and return airways are moved from goaf to airproof wall, with spacing 50 m. It is noted that the pressure corresponding to 0 m means the pressure of sensor when intake and return airways are without branch roadway. As illustrated in Figure 15, with the increase of the distance from 50 to 400 m, the maximum explosion pressure in return airway increased to 2.241 MPa gradually, then decreased to 1.818 MPa with faster rate. Compared to the pressure of return airway without branch roadway, it shows a relatively lower pressure when the distance between goaf and branch roadway is 50 m and the pressure decreases by 29.4%. However, the pressure in intake airway presents a small fluctuation trend, only to rise to 0.705 MPa at 400 m. Therefore, it is obvious to decrease the maximum explosion pressure in return airway with the branch roadway at 50 m from goaf while that in intake airway did not change significantly.
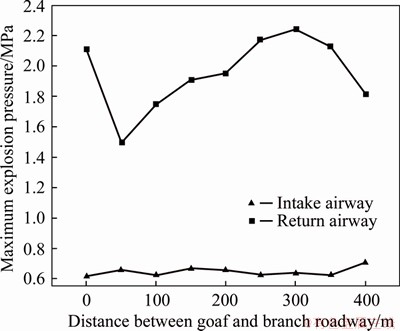
Figure 15 Pressures with different distances
Figure 16 displays the change of pressures at the airproof wall with different sectional dimensions of the branch roadway (20 m) which is 50 m away from goaf. Compared with the condition without branch roadway, the pressure peak of airproof wall with the branch roadway had a significant decline in return airway, while it had a slight rise in intake airway. In addition, explosion propagation time was longer in the tunnel with branch roadway than that without branch roadway (0*0 represents the tunnel without branch roadway). And there were more pressure peaks appearing in the tunnel with branch roadway, contrasting with only one peak in the tunnel without branch roadway. But the secondary pressure peak was smaller than that of the first peak. This can be explained that part of explosion propagation is shared by the branch roadway in which the decreasing overpressure propagates to the tunnel conversely. By comparing different sectional dimensions of the branch roadway, the larger section of the branch roadway indicated the lower peak pressure to some extent.

Figure 16 Pressures with different sectional dimensions of branch roadway:
Figure 17 displays the change of pressures at the airproof wall with different lengths of the branch roadway. The branch roadway with the section of 3×4 is 50 m from the goaf. And the branch roadway is perpendicular to return and intake airways. With the increase of the branch roadway from 20 to 60 m, the pressure of the first peak reduced gradually while others were opposite, particularly in intake airway. It is obvious that the presence of branch roadway decreases the pressure peak in return airway in a large part while the length of branch roadway has a small impact on it in return and intake airways.
5 Conclusions
1) CFD simulation and the related experiment are carried out to determine the characteristics of vented methane explosion. It is shown that the velocity of flame front of 9.5% methane is larger than that of 7.0% and 11.0% methane no matter forward or back propagation. Additionally, the picture of flame propagation captured is basically consistent with the distribution of product concentration field obtained by CFD simulation. Moreover, the velocity of flame front of 9.5% methane explosion acquired by experiment is also in accordance with simulation results, which indicates that the simulation results is reliable to be applied to practice.
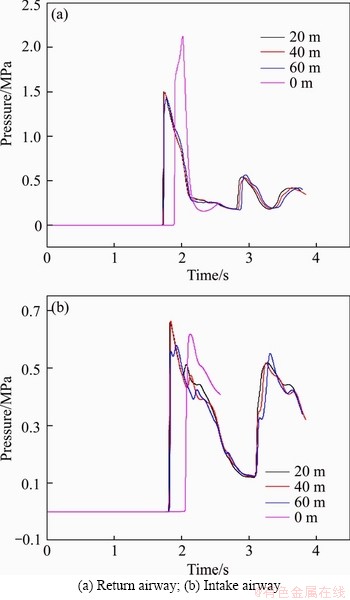
Figure 17 Pressures with different lengths of branch roadway:
2) With regard to the methane explosion in pipeline, the explosion pressure of methane (9.5%, 10.0%, 11.0%) reaches the peak quickly while that of methane (7.0%, 8.0%) has obvious delay to hit the maximum. Besides, the maximum explosion pressure of methane (9.5%, 10.0%, 11.0%) is much higher than the other two concentrations. Compared with three vented conditions, the maximum explosion pressure in the closed pipeline without a vent is the largest one, followed by the condition where ignition and vent are at each end and the condition where ignition and vent are at the same end, respectively. Therefore, it is effective to install pressure relief valves at the same end of ignition in pipeline for the prevention of methane explosion.
3) In terms of the explosion in coal mine tunnel, the branch roadway has significant effects on the reduction of the maximum explosion pressure of airproof wall, especially in return airway. According to CFD simulation, the section and the length of the branch roadway cause small influence on the maximum explosion pressure while the distance from the goaf affects it obviously. Considering economic cost, the best optimum is selected as follows. The branch roadway with the section of 2×4 and the length of 20 m is 50 m away from goaf. That can significantly reduce the explosion pressure in coal mine tunnel and effectively prevent the occurrence of larger damage.
References
[1] LUO Z, SU B, LI Q, WANG T, KANG X, CHENG F, GAO S, LIU L. Micromechanism of the initiation of a multiple flammable gas explosion [J]. Energy & Fuels, 2019, 33: 7738-7748. DOI: 10.1021/acs.energyfuels. 9b00480.
[2] SU B, LUO Z, WANG T, XIE C, CHENG F. Chemical kinetic behaviors at the chain initiation stage of CH4/H2/air mixture [J]. Journal of Hazardous Materials, 2020. DOI: 10.1016/ j.jhazmat.2020.123680.
[3] SU B, LUO Z, WANG T, YAN K, CHENG F, DENG J. Coupling analysis of the flame emission spectra and explosion characteristics of CH4/C2H6/Air mixtures [J]. Energy & Fuels, 2020, 34: 920-928. DOI: 10.1021/acs. energyfuels.9b03242.
[4] LUO Z, HAO Q, WANG T, LI R, CHENG F, DENG J. Experimental study on the deflagration characteristics of methane-ethane mixtures in a closed duct [J]. Fuel, 2020, 259: 1-10. DOI: 10.1016/j.fuel.2019.116295.
[5] SU B, LUO Z, WANG T, ZHANG J, CHENG F. Experimental and principal component analysis studies on minimum oxygen concentration of methane explosion [J]. International Journal of Hydrogen Energy, 2020, 45(21): 12225-12235. DOI: 10.1016/j.ijhydene.2020.02. 133.
[6] LUO Z, LI D, SU B, ZHANG S, DENG J. On the time coupling analysis of explosion pressure and intermediate generation for multiple flammable gases [J]. Energy, 2020, 198: 1-10. DOI: 10.1016/j.energy.2020.117329.
[7] FERRARA G, BENEDETTO A, SALZANO E, SALZANO E, RUSSO G. CFD analysis of gas explosions vented through relief pipes [J]. Journal of Hazardous Materials, 2006, 137(2): 654-665. DOI: 10.1016/ j.jhazmat.2006.03.037.
[8] PROUST C, LEPRETTE E. The dynamics of vented gas explosions [J]. Process Safety Progress, 2010, 29(3): 231-235. DOI: 10.1002/prs.10368.
[9] BAUWENS C R, CHAFFEE J, DOROFEEV S B. Vented explosion overpressures from combustion of hydrogen and hydrocarbon mixtures [J]. International Journal of Hydrogen Energy, 2011, 36(3): 2329-2336. DOI: 10.1016/j.ijhydene. 2010.04.005.
[10] WANG Z, PAN M, WANG S, SUN D. Effects on external pressures caused by vented explosion of methane-air mixtures in single and connected vessels [J]. Process Safety Progress, 2015, 33(4): 385-391. DOI: 10.1002/prs.11668.
[11] GUO J, LI Q, CHEN D, HU K, SHAO K, GUO C, WANG C. Effect of burst pressure on vented hydrogen-air explosion in a cylindrical vessel [J]. International Journal of Hydrogen Energy, 2015, 40(19): 6478-6486. DOI: 10.1016/j.ijhydene. 2015.03.059.
[12] KUNDU S, ZANGANEH J, ESCHEBACH D, MAHINPEY N, MOGHTADERI B. Explosion characteristics of methane– air mixtures in a spherical vessel connected with a duct [J]. Process Safety & Environmental Protection, 2017, 111: 85-93. DOI: 10.1016/j.psep.2017.06.014.
[13] CAO Y, LI B, GAO K. Pressure characteristics during vented explosion of ethylene-air mixtures in a square vessel [J]. Energy, 2018, 151: 26-32. DOI: 10.1016/j.energy.2018. 03.012.
[14] SUN S, QIU Y, XING H, WANG M. Effects of concentration and initial turbulence on the vented explosion characteristics of methane-air mixtures [J]. Fuel, 2020, 267: 1-9. DOI: 10.1016/j.fuel.2020.117103.
[15] MOKHTAR K, KASMANI R, HASSAN C, HAMID M, EMAMI S, NOR M. Reliability and applicability of empirical equations in predicting the reduced explosion pressure of vented gas explosions [J]. Journal of Loss Prevention in the Process Industries, 2020, 63: 1-10. DOI: 10.1016/j.jlp.2019.104023.
[16] ZHANG Y, JIAO F, HUANG Q, CAO W, SHI L, ZHAO M, YU C, NIE B, CAO X. Experimental and numerical studies on the closed and vented explosion behaviors of premixed methane-hydrogen/air mixtures [J]. Applied Thermal Engineering, 2019, 159: 1-11. DOI: 10.1016/ j.applthermaleng.2019.113907.
[17] WAN S, YU M, ZHENG K, WANG C, YUAN Z, YANG X. Effect of side vent size on a methane/air explosion in an end-vented duct containing an obstacle [J]. Experimental Thermal and Fluid Science, 2019, 101: 141-150. DOI: 10.1016/j.expthermflusci.2018.10.004.
[18] PHYLAKTOU H, ANDREWS G E. Gas explosions in long closed vessels [J]. Combustion Science & Technology, 1991, 77(1-3): 27-39. DOI: 10.1080/00102209108951718.
[19] INDRACKI J, KOBIERA A, RARATA G, WOLANSKI P. Influence of ignition position and obstacles on explosion development in methane–air mixture in closed vessels [J]. Journal of Loss Prevention in the Process Industries, 2007, 20(4-6): 551-561. DOI: 10.1016/j.jlp.2007.05.010.
[20] WILLACY S K, PHYLAKTOU H N, ANDREWS G E, FERRARA G. Stratified propane–air explosions in a duct vented geometry: Effect of concentration, ignition and injection position [J]. Process Safety & Environmental Protection, 2007, 85(2): 153-161. DOI: 10.1205/psep06020.
[21] GUO J, SUN X, RUI S, CAO Y, HU K, WANG C. Effect of ignition position on vented hydrogen–air explosions [J]. International Journal of Hydrogen Energy, 2016, 40(45): 15780-15788. DOI: 10.1016/j.ijhydene.2015.09.038.
[22] TOMLIN G, JOHNSON D M, CRONIN P, PHYLAKTOU H, ANDREWS G. The effect of vent size and congestion in large-scale vented natural gas/air explosions [J]. Journal of Loss Prevention in the Process Industries, 2015, 35: 169-181. DOI: 10.1016/ j.jlp.2015.04.014.
[23] QI S, DU Y, WANG S, ZHOU Y, LI G. The effect of vent size and concentration in vented gasoline-air explosions [J]. Journal of Loss Prevention in the Process Industries, 2016, 44: 88-94. DOI: 10.1016/j.jlp.2016.08.005.
[24] BATRAEV I S, VASIL’EV A A, UL’YANITSKII V Y, SHTERTSER A A, RYBIN D K. Investigation of gas detonation in over-rich mixtures of hydrocarbons with oxygen [J]. Combustion Explosion & Shock Waves, 2018, 54(2): 207-215. DOI: 10.1134/ S0010508218020107.
[25] ZHANG K, WANG Z, CHEN Z, JIANG F, WANG S. Influential factors of vented explosion position on maximum explosion overpressure of methane-air mixture explosion in single spherical container and linked vessels [J]. Process Safety Progress, 2018, 37(2): 248-255. DOI: 10.1002/ prs.11940.
[26] SINHA A, WEN J. A simple model for calculating peak pressure in vented explosions of hydrogen and hydrocarbons [J]. International Journal of Hydrogen Energy, 2019, 44(40): 22719-22732. DOI: 10.1016/j.ijhydene.2019.02.213.
[27] NIE B, HE X, WANG C, LU H, XUE F. Computational method of the propagation velocity of methane explosion flame based on correlation coefficient of images [J]. Combustion Science and Technology, 2015. DOI: 10.1080/ 00102202.2015.1019 621.
[28] AMYOTTE P R, PATIL S, PEGG M J. Confined and vented ethylene/air deflagrations at initially elevated pressures and turbulence levels [J]. Process Safety & Environmental Protection, 2002, 80(2): 71-77. DOI: 10.1205/0957582027 53553185.
[29] ZHANG K, WANG Z, GONG J, LIU M, DOU Z, JIANG J. Experimental study of effects of ignition position, initial pressure and pipe length on H2-air explosion in linked vessels [J]. Journal of Loss Prevention in the Process Industries, 2017, 50: 1-6. DOI: 10.1016/j.jlp.2017.09.0.
[30] JIANG B, LIN B, SHI S, ZHU C, LIU Q, ZHAI C. A numerical simulation of the influence initial temperature has on the propagation characteristics of, and safe distance from, a gas explosion [J]. International Journal of Mining Science and Technology, 2012, 22(3): 307-310. DOI: 10.1016/ j.ijmst.2012.04.004.
[31] WEI H, XU Z, ZHOU L, GAO D, ZHAO J. Effect of initial pressure on flame-shock interaction of hydrogen-air premixed flames [J]. International Journal of Hydrogen Energy, 2017, 42(17): 12657-12668. DOI: 10.1016/ j.ijhydene.2017.03.099.
[32] WEN X, YU M, JI W, YUE M, CHEN J. Methane–air explosion characteristics with different obstacle configurations [J]. International Journal of Mining Science and Technology, 2015, 25(2): 213-218. DOI: 10.1016/ j.ijmst.2015.02.008.
[33] LI H, GUO J, TANG Z, LI J, HUANG P, ZHANG S. Effects of ignition, obstacle, and side vent locations on vented hydrogen-air explosions in an obstructed duct [J]. International Journal of Hydrogen Energy, 2019, 44(36): 20598-20605. DOI: 10.1016/j.ijhydene.2019.06.029.
[34] VYAZMINA E, JALLAIS S. Validation and recommendations for FLACS CFD and engineering approaches to model hydrogen vented explosions: Effects of concentration, obstruction vent area and ignition position [J]. International Journal of Hydrogen Energy, 2016, 41(33): 15101-15109. DOI: 10.1016/j.ijhydene.2016.05.189.
[35] BAO Q, FANG Q, ZHANG Y, CHEN L, YANG S, LI Z. Effects of gas concentration and venting pressure on overpressure transients during vented explosion of methane– air mixtures [J]. Fuel, 2016, 175: 40-48. DOI: 10.1016/ j.fuel.2016.01.084.
(Edited by FANG Jing-hua)
中文导读
甲烷-空气泄爆特性的实验与数值模拟
摘要:为研究甲烷-空气混合物在管道、煤矿巷道等封闭空间内的通风爆炸特性,进行了相关实验和数值模拟。本文利用爆炸实验系统,结合FLACS软件,研究了不同泄爆条件下甲烷在管道和煤矿巷道中的爆炸特性和火焰传播特性。数值模拟与物理实验结果基本一致,说明甲烷爆炸的数值模拟是可靠的,且可应用于实际。研究结果表明,五种体积分数下甲烷的爆炸参数(压力、温度和产物浓度)变化趋势相同,但10.0%甲烷的爆炸强度最大,9.5%甲烷,11.0%甲烷,8.0%甲烷,7.0%甲烷的爆炸强度依次减弱。在不同泄爆条件下,甲烷爆炸压力和温度在无泄压条件的管道中最高,其次是在异端点火和泄压的管道,在同端点火和泄压的管道中最小。然而,三种泄爆条件对甲烷爆炸的最终产物浓度没有明显影响。对于煤矿巷道,距采空区50 m处的支巷使回风巷道的最大爆炸压力明显减小,而进风回风巷道的最大爆炸压力变化不大。此外,当支巷道较长或支巷截面较大时,密闭墙处峰值压力略有降低。
关键词:甲烷-空气;火焰传播;CFD模拟;泄爆;点火位置;压力峰值
Foundation item: Project(51674193) supported by the National Natural Science Foundation of China; Project(2019-JLM-9) supported by the Natural Science Foundation of Shaanxi Province, China; Project(2019-M-663780) supported by the Postdoctoral Science Foundation, China
Received date: 2019-06-10; Accepted date: 2020-04-09
Corresponding author: LUO Zhen-min, PhD, Professor; Tel: +86-13186055293; E-mail: zmluo@xust.edu.cn; ORCID: https://orcid.org/ 0000-0001-5247-9656
Abstract: To research the characteristics of vented explosion of methane-air mixture in the pipeline, coal mine tunnel or other closed space, the experiments and numerical simulations were carried out. In this work, explosion characteristics and flame propagation characteristics of methane in pipeline and coal mine tunnel are studied by using an explosion test system, combined with FLACS software, under different vented conditions. The numerical simulation results of methane explosion are basically consistent with the physical experiment results, which indicates that the numerical simulation for methane explosion is reliable to be applied to the practice. The results show that explosion parameters (pressure, temperature and product concentration) of methane at five volume fractions have the same change trend. Nevertheless, the explosion intension of 10.0% methane is the largest and that of 9.5% methane is relatively weak, followed by 11.0% methane, 8.0% methane and 7.0% methane respectively. Under different vented conditions, the pressure and temperature of methane explosion are the highest in the pipeline without a vent, followed by the pipeline where ignition or vent position is in each end, and those are the lowest in the pipeline with ignition and vent at the same end. There is no significant effect on final product concentration of methane explosion under three vented conditions. For coal mine tunnel, it is indicated that the maximum explosion pressure at the airproof wall in return airway with the branch roadway at 50 m from goaf is significantly decreased while that in intake airway does not change overwhelmingly. In addition, when the branch roadway is longer or its section is larger, the peak pressure of airproof wall reduces slightly.


