CVD金刚石膜反应器内气相化学的理论研究进展
安希忠1,李长兴1,刘国权2,沈峰满1,李友清1
(1. 东北大学 材料与冶金学院,辽宁 沈阳,110004;
2. 北京科技大学 材料科学与工程学院,北京,100083)
摘 要:
摘 要:综述CVD金刚石膜沉积过程中反应器内气相化学的理论研究进展,阐述不同条件下反应器内的气相化学反应模型、反应机理及各种数值仿真方法,总结这些气相反应的选取及所对应的动力学机理。研究结果表明:CVD金刚石膜反应器内的气相化学是一个十分复杂的过程,与双碳组元相比,单碳组元对膜沉积的贡献较大,在组元C2H2,C2,CH3,C和CH中,决定膜生长的组元由具体操作条件而定。对CVD金刚石膜反应器内气相化学的研究结果不但可以为探讨膜生长机理的表面化学提供准确输入,还可为高效、优质膜的获得提供理论依据。
关键词:
中图分类号:TB34;TN304;O484 文献标志码:A 文章编号:1672-7207(2010)03-0896-10
Theoretical study progress of gas phase chemistry in
CVD diamond film reactor
AN Xi-zhong1, LI Chang-xing1, LIU Guo-quan2, SHEN Feng-man1, LI You-qing1
(1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, China)
Abstract: The theoretical progress of gas phase chemistry in chemical vapor deposition (CVD) diamond film growth reactor was reviewed. The gas phase reaction model, reaction mechanism and all kinds of numerical simulation methods in different types of reactors and conditions were presented. Meanwhile, the choice of these gas reactions and the corresponding reaction kinetics were summarized as well. The results indicate that the gas phase chemistry in CVD diamond film reactor is a rather complicated process, compared with double-carbon species, single-carbon species create larger contributions on film growth. However, species such as C2H2, C2, CH3, C and CH play a dominant role on film growth depending on detailed processing conditions. On the whole, the research on gas phase chemistry in CVD diamond film reactor can not only provide accurate input for the surface chemistry of film growth mechanism, but also provide theoretical reference for the efficient acquisition of CVD diamond film with high quality.
Key words: diamond film; chemical vapor deposition (CVD); gas phase chemistry
化学气相沉积(CVD)金刚石膜在物理、化学、电学、热学和光学等方面[1-2]均具有优异的性能。自20世纪80年代以来,人们对优质CVD金刚石膜的合成进行了大量研究,并取得了很大进展[1-6]。其中,膜在热力学介稳定环境中的形核以及生长机理成为研究热点[1, 7]。对不同取向膜生长的表面机理,人们进行了大量的理论和实验研究[8-21],主要有:以甲基(CH3)为 主要生长组元的表面生长机理;以甲基(CH3)和乙炔(C2H2)为主要生长组元的生长机理;还有以C,C2或CH为主要生长组元的机理[22-23]。由于各种不同生长机理的提出,加上膜实际生长过程中的不确定性以及实验研究的难度, 人们对金刚石膜的生长机理至今没达成共识。
为了探讨CVD金刚石膜的生长机理,从原子尺度建立一套能够真实反映膜生长的表面反应动力学十分重要[24],在CVD金刚石膜生长过程中,反应器内的气相反应具有重要作用。因为在膜生长过程中,氢原子与其他气相组元和衬底表面处于动态平衡,表面碳氢基团的形成会引发膜的生长[25]。若能够准确表征反应器内所发生的气相反应及动力学,则可解释膜的生长机理。然而,气相中每个反应的速率很难通过实验原位确定,因为化学动力学实验通常费用较高且很难操作,而且反应的中间产物存在时间短、浓度低,这使它们难以用标准的解析方法来检测[26]。为此,Goodwin等[27]用一组从气相碳氢组元中的反应模拟整个生长过程,衬底上方气相成分可为探究膜生长的动力学机理提供依据。多年来,许多科学工作者致力于CVD金刚石膜反应器内气相化学的研究[3, 8, 28-33],试图为实际膜沉积过程中的表面反应提供准确、详实的数据。CVD金刚石膜反应器内气相化学的确定对于从原子尺度合理解释膜生长的表面反应机理、从微观尺度研究沉积膜显微组织变化以及从宏观上确定反应气氛对膜沉积速度和质量的影响均具有重要的理论和现实意义。鉴于此,本文作者对近20年来CVD金刚石膜沉积过程中反应器内气相化学的理论研究进行综述,重点对不同条件下反应器内的气相反应模型及机理进行总结。
1 CVD金刚石膜反应器内气相化学的研究现状
由于物理实验及检测存在难度,人们对CVD金刚石膜反应器内气相化学的研究大多采用计算机进行数值模拟或仿真。最早用具体的化学动力学模拟来分析碳沉积过程气相化学的可能是Kline等[34]。他们计算(并从实验上确定)了在甲烷等离子体反应器内C2Hx碳氢基团的浓度比CH3和其他碳氢基团的浓度高得多。尽管他们表明CH3是“最可能的膜沉积组元”,但其结果清楚地显示C2H2应该是甲烷等离子体中的主要组元。
Goodwin等[27]开发了一个一维模型模拟金刚石生长的气相环境,其中包括对速度、温度及组元浓度分布的计算。他们使用该模型模拟了Celii等[7-8]在热丝(HF)反应器内所进行的金刚石膜化学气相沉积的物理实验,所计算的组元浓度与实验测得的浓度一致。然而,对气相环境模型需要求解的不只是速度、温度及浓度场,还要包括对流、扩散传输以及具体的反应机制。为了模拟当前所用的反应器,至少需要采用二维模型[35],在大多情况下采用三维模型,而用一维模型会存在很多制约因素。
Frenklach[36]模拟了理想热丝反应器内金刚石膜在氢和氩稀释的甲烷混合物中生长的气相化学。模型中假定对于不同反应温度,反应器内的压力和流动速率不变。在原有的112个反应和39个组元的基础上,又增加了19个反应和6个新组元,描述了甲烷高温分解形成CH3和CH2基团的初始阶段,及随后反应生成C2H6,C2H5,C2H4和C2H3,C2H4和C2H3成为形成C2H2的前驱气体。在基本条件(即摩尔分数0.3% CH4-H2的混合气体,压力为2.666 kPa,初始反应温度为2 600 K,该温度以1 400 K/s的速率随时间呈直线下降)下的模拟结果表明:在金刚石的沉积温度下,C2H2是主要的碳氢组元。然而,如果温度下降速率从1.4×103 K/s变为1.4×106 K/s,CH4和CH3就成为主要的生长组元,如图1所示[36]。
为了研究CVD金刚石膜的生长机理,Frenklach等[3]又进一步研究了CH4-H2,CH4-Ar以及CH4-O2-H2气体混合物在热丝反应器内的气相化学。所用的描述气相动力学的化学反应机理是基于他们在实验室中用于燃烧和火焰环境的2种机理。前者是包含125个反应和31个组元的气相化学,主要用于甲烷的高温分解和氧化,并被优化来定量再现各种点火和火焰实验;后者的机理包含342个反应和70个组元,主要是定量描述多环芳烃在乙炔和乙烯火焰中的形成和生长。结合这2种机理,除去给定条件下对膜沉积作用效果不明显的反应,Frenklach等[3]获得了158个反应、50个组元的气相化学机理,结果表明:在膜沉积区域气相环境中,定量预测的关键依赖于气相的历史,这不但包括气相在后灯丝区域,还包括在前及近灯丝区域。
前面的研究大多集中在热丝系统,这是由于热丝的实验结果很丰富而直流电弧的结果较少。Coltrin 等[37]理论研究了低压直流电弧等离子体喷射反应器内CVD金刚石膜的生长,将反应器内气相化学动力学与流体流动相耦合来进行模拟,表面化学包含有关气相组元浓度的边界条件。在该反应器内等离子枪的轴向垂直于衬底表面[12],由等离子枪喷出的高速气流在撞击衬底时产生轴向对称的流场。图2所示为等离子枪/衬底及所考虑的反应器几何示意图[37]。其中:图2(a)描述了所考虑的等离子枪反应器的类型,尽管从喷嘴喷出的气流直径较小,但通过扩展产生的压力降会导致喷射气体迅速蔓延。他们假定离子枪出口处流场近似在很大的直径范围内,且轴向速度分量是均匀的,并忽略了任何等离子体的影响。研究中的气相反应机理包含34个反应,见文献[37]中的表1,其中,反应的温度依赖性由改进的Arrhenius公式表示:ki=AiTβiexp[-Ei/(RT)](其中:ki为反应i的反应速率常数;Ai为指前因子;βi为温度指数;Ei为激活能;R为摩尔气体常数;T为热力学温度)。模型中的每个反应都是可逆的,通过反应平衡常数可以获得逆反应速率。反应机理中考虑了多个反应的速率常数对压力依赖性的关系。所有的速率表达式、平衡常数以及热力学性能等都用CHEMKIN程序包估算[38]。
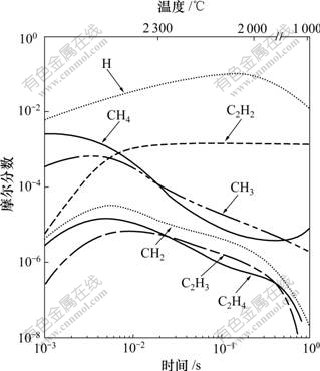
基本条件为:摩尔分数0.3% CH4-H2混和气体, 压力为2 666 Pa, 初始反应温度为2 600 K,该温度以1 400 K/s的速率线性下降
图1 CVD金刚石膜在基本条件下沉积时反应器内主要反应组元的浓度分布[36]
Fig.1 Concentration distributions of major reaction species for CVD diamond film under base condition
他们在计算中考虑了等离子体枪内氢气分解度对气相成分及金刚石膜生长速率的影响,计算中所用的入口条件为:温度T=2 500 K,CH4的摩尔分数x0(CH4)= 0.005。考虑氢原子4种不同的摩尔分数,分别为0.05,0.30,0.60和0.95,入口处的平衡相为氢气。图3所示为入口处氢原子的浓度对气相组元分布的影响[37]。由图3可见:由于纯CH4在入口处的喷入,在反应器内实际上存在2个浓度边界层,一个在入口处,另一个在衬底。而在沉积的表面存在3个边界层即:动量、热及浓度边界层。碳氢组元的分布主要依赖于入口处氢原子的浓度x0(H)。当取最小值x0(H)=0.05时,氢原子迅速通过反应CH4+H![]() CH3+H2从CH4上萃取1个氢原子,直到产生足够CH3使反应达到局部平衡。随后没有明显的氢原子的萃取,只有少量CH和C生成。当氢原子的浓度x0(H)=0.30时,氢萃取反应得到了足够的驱动,在大部分的计算区域内C是主要组元。当入口处的x0(H)达到0.95时,C的摩尔分数比其最近的单碳组元的摩尔分数高4个数量级,并且比C2H2高1个数量级。图3及文献[39-40]表明:C是可能的生长组元,因此,C被考虑到表面反应机理中去。人们还对CH2,CH和C2H基团也进行了研究,但是,这些基团的摩尔分数与C,C2H2或CH3的摩尔分数相比低2个数量级。因此,在Suzuki等[39-40]的研究中,其他基团通常被忽略,不能作为生长组元。结果表明:当等离子体枪内只有较少的H2分解时,CH3为主要的生长组元,但在较高的分解水平时,C成为主要的生长组元。当进给气体中的CH4含量小于1%时,在这些条件下的金刚石生长过程中作为第3种生长组元的C2H2不起作用。
CH3+H2从CH4上萃取1个氢原子,直到产生足够CH3使反应达到局部平衡。随后没有明显的氢原子的萃取,只有少量CH和C生成。当氢原子的浓度x0(H)=0.30时,氢萃取反应得到了足够的驱动,在大部分的计算区域内C是主要组元。当入口处的x0(H)达到0.95时,C的摩尔分数比其最近的单碳组元的摩尔分数高4个数量级,并且比C2H2高1个数量级。图3及文献[39-40]表明:C是可能的生长组元,因此,C被考虑到表面反应机理中去。人们还对CH2,CH和C2H基团也进行了研究,但是,这些基团的摩尔分数与C,C2H2或CH3的摩尔分数相比低2个数量级。因此,在Suzuki等[39-40]的研究中,其他基团通常被忽略,不能作为生长组元。结果表明:当等离子体枪内只有较少的H2分解时,CH3为主要的生长组元,但在较高的分解水平时,C成为主要的生长组元。当进给气体中的CH4含量小于1%时,在这些条件下的金刚石生长过程中作为第3种生长组元的C2H2不起作用。
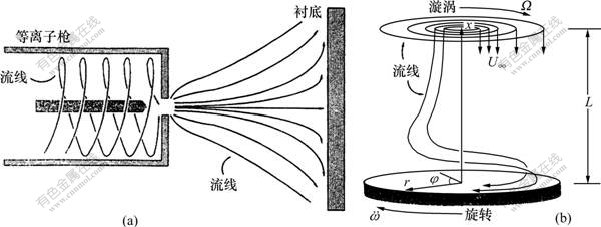
(a) 等离子枪/衬底的侧视图;(b) 反应器几何示意图(图中的系统用圆柱坐标来描述(r, φ, x))
图2 直流电弧等离子体反应器及其几何示意图[37]
Fig.2 DC arc plasma jet reactor and its geometry
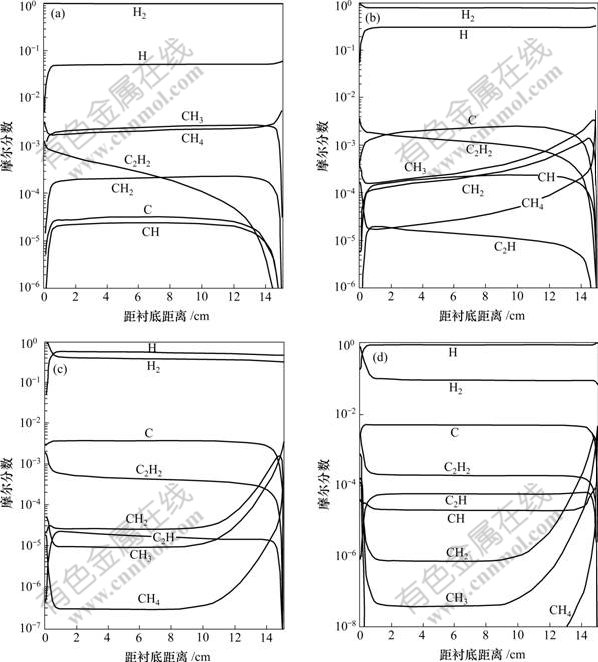
入口处4种不同氢原子的摩尔分数:(a) 0.05; (b) 0.30; (c) 0.60; (d) 0.95
图3 入口温度为2 500 K、入口处甲烷浓度为0.5%及衬底温度为1 200 K时的气相组元分布[37]
Fig.3 Gas-phase species distributions for inlet temperature 2 500 K, and inlet CH4 concentration 0.5%,
and substrate temperature 1 200 K[37]
Mankelevich等[35]开发了用于CVD金刚石膜反应器内流体流动、热传递及气相化学自一致计算的二维模型,选用的是其实验室中的直流电弧放电反应器。所使用的CHER程序包含气相动力学模块、化学动力学模块以及非平衡电子动力学电力学模块,采用质量、动量、能量及化学组元守恒方程对圆柱形几何进行数值求解。气相反应机理包括采用78个反应和15个组元,通过对文献[37, 41]中所用的气体温度敏感性及H2的分解水平而获得。通过自一致计算获得了化学组元的空间分布和流量以及气体的流动参数,并将其中的一些参数与实验进行了对比,结果发现:在衬底上的对流传输被抑制,化学组元的传输主要是通过扩散过程来进行。然而,必须考虑反应器内的对流传质。对于气相反应机理,气相反应温度分布十分关键。模拟最高温度出现在放电中心区,并沿径向和电极方向降低。由于热扩散(在放电区中心)和表面反应(衬底附近),所选的组元沿轴向的分布规律是总的含碳量沿轴向下降。
Schmidt等[42]对热丝CVD金刚石膜生长过程中的气相成分进行了研究。输入的气体为H2,CH4及O2的混合物,但只改变O2的含量。在给定流动条件下,热丝反应器内气相浓度的变化采用四极质谱仪(QMS)确定。由灯丝反应、扩散及流动过程造成的气相成分的变化可以通过QMS相对于灯丝在垂直方向上的位置来测定。对于QMS不能测量的基团浓度,可以通过CHEMKIN软件包来获得。在不加氧的条件下,他们的实验结果与理论计算具有可比性。对H2-CH4-O2系统,Frenklach等[3]提出了相对简单的气相化学反应机制,该机制包含31个反应和18个组元[42],他们计算了在灯丝位置附近具有最大值及最小值的主要生长组元的特征浓度分布,这些组元包括CH4,CH3及C2H2。结果表明[3, 43]:在衬底附近,CH3的摩尔分数非常低,这表明用于生长过程的CHx基团必须在表面处形成;此外,氧使乙炔浓度的降低程度比使甲烷浓度的降低程度更大,这导致生长速率下降和膜质量提高。在HFCVD中CH3是最重要的生长组元,而C2H2可导致石墨夹杂的形成;O2与CHx反应形成稳定的CO和CO2,C原子可能参与金刚石的沉积过程,导致随着O2浓度的增加,膜的生长速率降低。在衬底位置处CH2信号强度与金刚石的生长速率成正比。他们指出:衬底表面附近的反应CH4+H→CH3+H2非常重要。
Goodwin等[43]研究了金刚石薄膜在低压、富碳 氢/氧燃烧火焰中的合成。在氧炔焰的数值模拟中,他们使用了Miller等[44]提出的富火焰机理(218个反应)。对于丙烯和丙烷火焰,他们又添加了16个反应[43]来描述C3H8和C3H6的氧化。这些反应来自文献[45],但他们对速率常数进行了更新,并将数值仿真结果与物理实验进行了对比,发现有很好的一致性。
结合Coltrin[46]和Goodwin等[27]的研究,Kassmann等[47]开发了一个用于金刚石膜CVD的理论模型。该模型是基于稳态下反应气体流动的质量、动量及能量平衡。同时,使用了与Evans等[48]相似的变换将模型方程转化为一组普通的差分方程。文献[47]中的表1给出了构成模型方程所选的一组合适的无量纲变量。膜形成的气相反应取自Coltrin等[37]的直流电弧喷射反应器模型。额外的反应被用于描述氯对金刚石生长的影响。文献[47]中的表2列出了所包含的41个气相反应,其中用于描述氯反应动力学的参数见文献[47]中的表3和表4。通过对Sandia 国家实验室的Spin代码[49]进行改进,在IBM逻辑RISC/6000工作站上数值解析了模型方程。对有无衬底弹出的情形,都给出了对热丝、直流电弧喷射及氯激活系统的求解。结果表明:通过原子氯化学分解氢气将会明显降低能耗;从衬底喷入甲烷可以使甲基在最佳位置形成,即刚好在衬底上方形成,这不仅提高了对碳的捕捉能力,还导致生长速率更高;通过使用氯激活和在衬底喷入甲烷而优化的反应器能够以1 200 mm/h的速率生长出多孔金刚石膜,且所用的能量也比传统的直流电弧等离子体喷射小1个数量级。
Mankelevich等[50]开发了热丝CVD反应器的三维模型,研究金刚石生长的气相和表面过程。在数值求解的传输方程中,考虑了气相和表面反应机理、分子扩散和热扩散、灯丝处的催化氢分解以及衬底处的表面动力学。由3个模块组成的模型能够描述气相过程(传热和传质、化学动力学)、衬底处的气相-表面过程(金刚石的生长机理)以及反应混合物激活的过程(灯丝处的气体加热及氢的催化分解)。在第1个模块中,数值求解了一组关于质量、动量、能量和组元浓度的守恒方程,在直角坐标系中,类似方程的具体数学形式见文献[51]。对气相化学和热化学的模拟中使用的是用于C-H-O混合物的GRI-Mech1.2具体的反应机 理[52],第2个模块考虑了碳氢组元、原子和分子氢与固相的反应。对热丝反应器内混合物激活的主要影响假定为灯丝处的气体加热及H2的非均匀分解。气相的温度分布主要由灯丝的热传递决定。采用Wahl等[53]的物理实验结果对Mnakelevich 等[51]所提出的三维模型进行了验证,主要是比较CH3的浓度,结果发现两者有较好的一致性。
Mankelevich等[54]报道了在热丝激活的的CH4-N2- H2和CH4-NH3-H2气体混合物中表面反应和气相化学的理论和实验研究,目的是解释在热丝反应器内通过控制氮的加入量(以N2和NH3的形式加入)对CH4-H2气体混合物的影响。完整的模型包括以下3个方面:(1) 反应混合物的激活(即气体的加热及在热丝表面催化氢原子的生成);(2) 气相过程(传热和传质及化学动力学);(3) 在衬底处的气相表面过程。由于在模拟过程中没有衬底,所以,最后一个过程没有被考虑。研究结果表明:N2是非反应性的,而加入NH3对气相化学和成分产生主要影响,尤其是引出了一系列的H替换反应,这些反应参与到将CH3基团转化成HCN的不可逆反应中去,因此,降低了参与金刚石生长的自由碳氢组元的数量,与文献[55]中的结果一致。
为研究CVD金刚石膜生长过程中近表面处的气相化学,Larson等[56]开发了用于大气压下射频(RF)等离子体反应器的数值模型。模拟中气相反应机理的 核心部分是用于甲烷燃烧的GRI机理中的碳氢反 应[52],并有几个包含原子碳的反应。因为在4 000 K,1×105 Pa及输入甲烷条件下,质量分数为90%的碳以原子碳的形式存在。研究中所用的气相反应机理及反应速率来源于文献[56]中的表2,其中包含46个气相反应。由于这些计算中的峰值温度是4 000 K,所 以,许多反应的速率常数是通过在较低温度下的测量或计算来外推得到,这增加了反应速率的不确定 性。然而,该系统中的许多反应在反应器内的大部分区域都接近平衡状态,尤其是在温度最高的位置,速率常数通常都比较稳定,反应平衡通过来自CHEMKIN热力学数据库的热化学参数来确定[38]。该模型预测了CH4,C2H2,C2H4和C2H6等组元的摩尔分数在很宽范围内都与用色谱法的测量结果保持一致。同时,该模型也预测了H,CH3,C2H2及C在金刚石膜生长表面处的浓度,结果发现:在RF等离子体反应器内,CH3和C2H2及原子C都将对膜的沉积起重要作用。对大气压RF等离子体金刚石CVD反应器内近表面处的气相化学环境与几个其他的CVD金刚石的环境进行对比发现:预测得到的热等离子体导致表面有更高的H-CH3与C2H2-CH3的浓度比。
使用CHEMKIN程序包[11],Tsang等[57]数值模拟了CVD金刚石膜生长过程中热丝反应器内出现的不同碳氢组元的浓度分布,将所计算的值与使用分子束质谱直接原位测量得到的结果进行对比。将不同的碳氢气体(CH4,C2H2,C2H4,及C2H6)作为输入气体混合物的碳源,确保C与H2的物质的量比保持在1%。当C2H6用作反应源气时,达到热平衡后,通过计算发现从未饱和的C2组元向C1组元的转变不是直接的气相反应过程,即在反应器内有1个或多个中间反应发生,而这些反应在标准的碳氢反应模型中并没有出现。他们认为:这些被忽略的反应可能会包含表面催化的氢化作用,在这种情况下,它很容易发生在灯丝的表面。有关这方面的问题还有待于进一步研究。除了数值模拟稳定的碳氢组元外,Tsang等[58]使用同样的方法还对典型CVD过程中甲基CH3和氢原子的浓度作为灯丝温度的函数进行了模拟,并将之与物理实验的结果进行了对比。
Riccardi等[59]数值研究了用于沉积金刚石和类金刚石碳膜的典型射频反应器内、低压CH4-Ar等离子体的气相化学,所用的数值模拟方法可以通过发生在等离子体中的复杂化学动力学来确定基团组元。研究结果表明:在纯甲烷放电的情况下,CH3是最充足的含碳基团,而碳的二聚物C2在CH4的放电中被高度稀释。因此,他们认为:在CH4等离子体中,膜生长的气相反应源气为CH3;而在CH4-Ar等离子体中,C2作为膜生长的气相反应源气。
Li等[60]提出了描述金刚石膜生长的气相化学模型,该模型中包含了10个碳氢组元及8个反应。基于该模型,假定由从头计算分子轨道理论预测得到的分子和过渡态数据以及由统计力学计算得到的热力学量是热力学平衡的,他们计算了化学组元的气相成分。尽管该模型预测的绝对成分与文献中获得的实验数据较符合,但该模型只是定性地解释了实验中所观察到的各种气相组元的浓度随进给气体中甲烷浓度增加而发生的变化。
Dong等[61]使用蒙特卡罗(MC)方法模拟了CH4/H2气相混合物在电子辅助化学气相沉积(EACVD)中的分解过程,给出了电子速度分布及H2分解的变化,同时还研究了甲烷的分解随反应源气中甲烷浓度的变化。研究结果表明:主要的金刚石生长组元为CH3和CH3+。
使用GRI-Mech3.0[52]数据库,戚学贵等[62]对各沉积方法中CVD金刚石膜反应器内C-H-O和C-H-N体系中的气氛气相化学进行了计算,计算中均不考虑可能的电子碰撞分解和离子反应,而将气源转变为活性基团的过程处理为热化学过程,且与压力有关的反应速率取压力较低时的速度。假设整个气相体系在反应过程中是恒温恒压且均匀的,并以气相平衡浓度作为激活温度下的气相组成。结果表明:甲基是CVD金刚石膜的主要生长组元,乙炔导致非金刚石碳沉积,氢原子刻蚀非金刚石碳。加氧的一个重要作用是通过气相反应改变这些组元的浓度,而加氮在改变这些组元浓度的同时,CN等含氮组元还强烈地参与了膜成核和生长的表面过程。
在直流电弧等离子体反应器内,Huang等[63]采用与物理实验相耦合的方法数值模拟了金刚石膜生长过程中出现的C,C2H2,C2H及C2组元的摩尔分数,模拟中使用的是流体力学和热力学模型。计算中考虑了3种气体,即CH4,H2和Ar。气体的初始速度根据气体的流速及入口直径来计算。所用的模型中进行了如下假设:(1) CH4的分解与能量转变无关,因为CH4在整个气体中的含量较低;(2) 初始气体的温度定为50 ℃;(3) 等离子体处于局部热力学平衡,这意味着区域内具有动力学和化学平衡;(4) 气体的流动状态是稳定的。计算结果表明:C,C2,C2H及C2H2组元的摩尔分数沿着衬底的径向距离发生变化,这主要是因为在该距离上不同的等离子体温度下,等离子体组元的分解和再结合的速率不同。通常是随着衬底径向距离的增加,C2和原子C的摩尔分数降低,C2H2的摩尔分数增大,而C2H的摩尔分数则在一个中间距离上出现峰值。对于直流电弧等离子体喷射CVD反应器内CH,C2及H等主要组元的存在,在Chen等[64]的物理实验中也得到了证实。
Petherbridge等[65]提出了在x%H2S-1%CH4-H2 (x为0~1)和1%CS2-H2气体混合物CVD金刚石系统中C-S耦合的机理及气相组元反应化学的数值计算,计算中所用的是SENKIN代码,它是CHEMKIN软件包的一部分[38]。对包含于计算中的所有含碳氢组元的反应及依赖于温度的速率常数都来自于GRI-Mech3.0反应机理数据库[62]。含硫组元的反应数据从有关文献中获得;对于速率,如果文献中没有,则用含氧组元的数据来近似。气相中的化学反应见文献[65]中的表1,其计算结果与微波等离子体及热丝激活CVD环境有一定的一致性,因此,可以证明以前提出的机理[66]。另外,他们所忽略的反应(例如那些在金刚石生长表面所发生的反应)似乎会对硫结合到多晶CVD金刚石膜的程度及属性产生更大的影响。随后,Rennick等[67] 利用二维模型模拟了直流电弧放电CVD反应器内的气相反应化学,所用的热化学数据及简化的化学反应机理均取自文献[68],其中包含23个组元和76个可逆反应。该模型的输出包括气体温度的空间分布、流场以及作为诸如H2和CH4流动速率及输入功率等参数函数的各种组元的数量密度。
近年来,人们对热丝[69]、直流电弧喷射[70]及微波等离子体反应器内金刚石膜微晶、纳米晶及超纳米晶生长的气相化学进行了研究。结果发现:在热丝反应器内(气体混合物为Ar-CH4-H2),当灯丝温度较高时(如Tfil为2 700 K),在膜生长表面附近其他C1组元的浓度尤其是C原子浓度较大,它们会与膜生长表面处的悬挂键发生反应,促进膜的生长[69];在直流电弧喷射反应器内,Mankelevich等[70-71]采用二维计算机模型模拟了反应器内25个中性组元及14个带电组元的气相化学及其对反应器内操作条件如H2和CH4的流动及输入功率的依赖性。结果表明:实验中观察到的较大且不均匀的生长速率主要由C原子和CH组元的分布(在大功率Bristol反应器内)及CHy(y=0~3)组元的分布(在小功率的SRI反应器内)所决定[70];在微波等离子体反应器内,超纳米晶的生长主要是由C加入到单个基团位置决定,CH3组元的加入对膜生长的贡献较小。尽管来自单基团位置的贡献很大,纳米晶的生长仍然主要受CH3向双基团的位置的生长速率所支配,因此,其生长速率比超纳米晶的生长速率高得多。
2 结论及展望
采用数值仿真的方法对CVD金刚石膜反应器内的气相化学的理论研究进展进行了综述。气相化学的研究结果无论是对获得理论上膜生长的表面反应机理,还是获得实际高效、优质膜均具有重要的理论和实际意义。换言之,掌握实际膜生长反应器内的气相化学是至关重要的。由于每种反应器内的气相化学模型都存在着很多假设,即很多参数的获得是通过估算或完全假定来实现的,同时,即使对相同反应器,实现CVD金刚石膜生长的气相化学也不相同,因此,要真实地再现CVD金刚石膜反应器内的气相化学,必须进行精准的物理实验,它可以为气相化学反应提供所需的动力学信息。随着实验技术的不断发展,这些实验结果必将为反应器内气相化学的理论研究提供强有力的依据,从而为探讨CVD金刚石膜生长的表面化学反应机制(膜的生长机理)打下坚实的基础。
参考文献:
[1] Lee S T, Lin Z D, Jiang X. CVD diamond films: Nucleation and growth[J]. Materials Science and Engineering: R: Reports, 1999, 25: 123-154.
[2] Gicquel A, Hassouni K, Silva F, et al. CVD diamond films: From growth to applications[J]. Current Applied Physics, 2001, 1(6): 479-496.
[3] Frenklach M, Wang H. Detailed surface and gas-phase chemical kinetics of diamond deposition[J]. Phys Rev B, 1991, 43(2): 1520-1545.
[4] 肖剑荣, 徐慧, 王焕友, 等. 退火对氟化类金刚石膜结构及电学性能的影响[J]. 中南大学学报: 自然科学版, 2007, 38(4): 669-673.
XIAO Jian-rong, XU Hui, WANG Huan-you, et al. Effects of annealing on structural and electric property of fluorinated diamond-like carbon thin films[J]. Journal of Central South University: Science and Technology, 2007, 38(4): 669-673.
[5] MA Ying, WANG Lin-jun, LIU Jian-min, et al. Characterization of (100)-orientated diamond film grown by HFCVD method with a positive DC bias voltage[J]. Trans Nonferrous Mer Soc China, 2006, 16(s1): s313-s316.
[6] May P W. Materials science: the new diamond age?[J]. Science, 2008, 319(5869): 1490-1491.
[7] Celii F G, Pehrsson P E, Wang H T, et al. Infrared detection of gaseous species during the filament-assisted growth of diamond[J]. Appl Phys Lett, 1988, 52(24): 2043-2045.
[8] Harris S J, Weiner A M, Perry T A. Measurement of stable species present during filament-assisted diamond growth[J]. Appl Phys Lett, 1988, 53(17): 1605-1607.
[9] Harris S J. Mechanism for diamond growth from methyl radicals[J]. Appl Phys Lett, 1990, 56(23): 2298-2300.
[10] Chu C J, D’Evelyn M P, Hague R H, et al. Mechanism of diamond growth by chemical vapor deposition on diamond (100), (111), and (110) surfaces: Carbon-13 studies[J]. J Appl Phys, 1991, 70(3): 1695-1705.
[11] Matsui Y, Yabe H, Hirose Y. The growth mechanism of diamond crystals in acetylene flames[J]. Jpn J Appl Phys, 1990, 29(8): 1552-1560.
[12] Goodwin D G. Simulations of high-rate diamond synthesis: Methyl as growth species[J]. Appl Phys Lett, 1991, 59(3): 277-284.
[13] Loh K P, Foord J S, Jackman R B. Reactive chemistry of C2Hx species on CVD diamond[J]. Diamond and Relat Mater, 1998, 7(2/5): 243-246.
[14] Hsu W L. Mole fractions of H, CH3, and other species during filament-assisted diamond growth[J]. Appl Phys Lett, 1991, 59(12): 1427-1429.
[15] Frenklach M. Monte Carlo simulation of diamond growth by methyl and acetylene reactions[J]. J Chem Phys, 1992, 97(8): 5794-5802.
[16] Battaile C C, Srolovitz D J, Butler J E. Molecular view of diamond CVD growth[J]. Journal of Electronic Materials, 1997, 26(9): 960-965.
[17] Okkerse M, De Croon M H J M, Kleijn C R, et al. A surface and a gas-phase mechanism for the description of growth on the diamond(100) surface in an oxy-acetylene torch reactor[J]. J Appl Phys, 1998, 84(11): 6387-6398.
[18] Grujicic M, Lai S G. Atomistic simulation of chemical vapor deposition of (111)-oriented diamond film using a kinetic Monte Carlo method[J]. Journal of Materials Science, 1999, 34(1): 7-20.
[19] 安希忠, 张禹, 刘国权, 等. CVD金刚石膜{100}取向在改进化学反应模型下生长的原子尺度模拟[J]. 稀有金属材料与工程, 2002, 31(5): 349-352.
AN Xi-zhong, ZHANG Yu, LIU Guo-quan, et al. An atomic scale simulation on {100} oriented CVD diamond film grown under modified chemical reaction model[J]. Rare Metal Materials and Engineering, 2002, 31(5): 349-352.
[20] AN Xi-zhong, ZHANG Yu, LIU Guo-quan, et al. Atomic-scale kinetic monte carlo simulation of {100}-oriented diamond film growth in C-H and C-H-Cl systems by chemical vapor deposition[J]. Chin Phys Lett, 2002, 19(7): 1019-1020.
[21] AN Xi-zhong, LIU Guo-quan, WANG Fu-zhong, et al. Electronic structure study of growth species adsorption and reaction on cluster models for the diamond surface using LDA method[J]. Diamond and Relat Mater, 2003, 12(12): 2169-2174.
[22] Gomez-Aleixandre C, Sanchez O, Castro A, et al. Optical emission characterization of CH4+H2 discharges for diamond deposition[J]. J Appl Phys, 1993, 74(6): 3752-3757.
[23] Gruen D M, Zuiker C D, Krauss A R, et al. Carbon dimer, C2, as a growth species for diamond films from methane/hydrogen/ argon microwave plasmas[J]. J Vac Sci Technol A, 1995, 13(3): 1628-1632.
[24] Brenner D W. Empirical potential for hydrocarbons for use in simulating the chemical vapor deposition of diamond films[J]. Phys Rev B, 1990, 42(15): 9458-9471.
[25] Dawnkaski E J, Srivastava D, Garrison B J. Time dependent monte carlo simulations of H reactions on the diamond {001}(2?1) surface under chemical vapor deposition conditions[J]. J Chem Phys, 1995, 102(23): 9401-9411.
[26] Jensen K F, Rodgers S T, Venkataramani R. Multiscale modeling of thin film growth[J]. Current Opinion in Solid State and Materials Science, 1998, 3(6): 562-569.
[27] Goodwin D G, Gavillet G G. Numerical modeling of the filament-assisted diamond growth environment[J]. J Appl Phys, 1990, 68(12): 6393-6490.
[28] Frenklach M. Monte Carlo simulation of hydrogen reactions with the diamond surface[J]. Phys Rev B, 1992, 45: 9455-9461.
[29] Zhao X G, Carmer C S, Weiner B, et al. Molecular dynamics with the AM1 potential: Reactions on diamond surfaces[J]. J Phys Chem, 1993, 97(8): 1639-1648.
[30] Harris S J. Gas-phase kinetics during diamond growth: CH4 as-growth species[J]. J Appl Phys, 1989, 65(8): 3044-3048.
[31] Harris S J, Weiner A M. Methyl radical and H-atom concentrations during diamond growth[J]. J Appl Phys, 1990, 67(10): 6520-6526.
[32] Harris S J, Weiner A M, Perry T A. Filament-assisted diamond growth kinetics[J]. J Appl Phys, 1991, 70(3): 1385-1391.
[33] Bigelow L K, D’Evelyn M P. Role of surface and interface science in chemical vapor deposition diamond technology[J]. Surface Science, 2002, 500(1/3): 986-1004.
[34] Kline L E, Partlow W D, Bies W E. Electron and chemical kinetics in methane RF glow-discharge deposition plasmas[J]. J Appl Phys, 1989, 65(1): 70-77.
[35] Mankelevich Y A, Rakhimov A T, Suetin N V. Two-dimensional model of reactive gas flow in a diamond film CVD reactor[J]. Diamond and Relat Mater, 1995, 4(8): 1065-1068.
[36] Frenklach M. The role of hydrogen in vapor deposition of diamond[J]. J Appl Phys, 1989, 65(12): 5142-5149.
[37] Coltrin M E, Dandy D S. Analysis of diamond growth in subatmospheric DC plasma-gun reactors[J]. J Appl Phys, 1993, 74 (9): 5803-5820.
[38] Kee R J, Rupley F M, Miller J A. Sandia National Laboratories Report[R]. New Mexico: Sandia National Laboratories, 1989.
[39] Suzuki K, Sawabe A, Inuzuka T. Growth of diamond thin films by dc plasma chemical vapor deposition and characteristics of the plasma[J]. Jpn J Appl Phys, 1990, 29(1): 153-157.
[40] Suzuki K, Sawabe A, Inuzuka T. Characterizations of the dc discharge plasma during chemical vapor deposition for diamond growth[J]. Appl Phys Lett, 1988, 53(19): 1818-1819.
[41] Yu B W, Girshick S L. Atomic carbon vapor as a diamond growth precursor in thermal plasmas[J]. J Appl Phys, 1994, 75(8): 3914-3923.
[42] Schmidt I, Benndorf C, Joeris P. Gas phase composition and film properties of hot filament diamond synthesis from CH4-H2-O2 gas mixtures[J]. Diamond and Relat Mater, 1995, 4(5/6): 725-729.
[43] Goodwin D G, Glumac N G, Shin H S. Diamond thin film deposition in low-pressure premixed flames[C]//Proceedings of Twenty-Sixth Symposium (International) on Combustion. Pittsburgh, PA: The Combustion Institute, 1996: 1817-1824.
[44] Miller J A, Mellius C F. Kinetic and thermodynamic issues in the formation of aromatic compounds in flames of aliphatic fuels[J]. Combust Flame, 1992, 91(1): 21-39.
[45] Warnatz J. The structure of laminar alkane-, alkene-, and acetylene flames[C]//Proceedings of Eighteenth Symposium (International) on Combustion. Pittsburgh, PA: The Combustion Institute, 1981: 369-384.
[46] Coltrin M E, Kee R J, Evans G H. A mathematical model of the fluid mechanics and gas-phase chemistry in a rotating disk chemical vapor deposition reactor[J]. J Electrochem Soc, 1989, 136(3): 819-829.
[47] Kassmann D E, Badgwell T A. Modeling diamond chemical vapor deposition in a rotating disk reactor[J]. Diamond and Relat Mater, 1996, 5(3/5): 221-225.
[48] Evans G H, Greif R, Forced flow near a heated rotating disk: A similarity solution[J]. Numerical Heat Transfer, 1988, 14(3): 373-387.
[49] Coltrin M E, Kee R J, Evans G H, et al. Spin (version 3.83): A fortran program for modeling one-dimensional rotating-disk/ Stagnation-flow chemical vapor deposition reactors[R]. Livermore, California: Sandia National Laboratories, 1991: 1-25.
[50] Mankelevich Y A, Rakhimov A T, Suetin N V. Three- dimensional simulation of a HFCVD reactor[J]. Diamond and Relat Mater, 1998, 7(8): 1133-1137.
[51] Mankelevich Y A, Rakhimov A T, Suetin N V. Two-dimensional simulation of a hot-filament chemical vapor deposition reactor[J]. Diamond and Relat Mater, 1996, 5(9): 888-894.
[52] Bowman C T, Hanson R K, Davidson D F, et al. Reaction rate coefficients and thermodynamic data[EB/OL]. [1999]. http://www.me.berkeley.edu/gri_mech/.
[53] Wahl E H, Owano T G, Kruger C H, et al. Measurement of absolute CH3 concentration in a hot-filament reactor using cavity ring-down spectroscopy[J]. Diamond and Relat Mater, 1996, 5(3/5): 373-377.
[54] Mankelevich Y A, Suetin N V, Smith J A, et al. Investigations of the gas phase chemistry in a hot filament CVD reactor operating with CH4/N2/H2 and CH4/NH3/H2 gas mixtures[J]. Diamond and Relat Mater, 2002, 11(3/6): 567-572.
[55] May P W, Burridge P R, Rego C A, et al. Investigation of the addition of nitrogen-containing gases to a hot filament diamond chemical vapor deposition reactor[J]. Diamond and Relat Mater, 1996, 5(3/5): 354-358.
[56] Larson J M, Swihart M T, Girshick S L. Characterization of the near-surface gas-phase chemical environment in atmospheric- pressure plasma chemical vapor deposition of diamond[J]. Diamond and Relat Mater, 1999, 8(10): 1863-1874.
[57] Tsang R S, May P W, Ashfold M N R. Modeling of the gas phase chemistry during diamond CVD: the role of different hydrocarbon species[J]. Diamond and Relat Mater, 1999, 8(2/5): 242-245.
[58] Tsang R S, May P W, Cole J, et al. Simulations of the hot-filament diamond CVD gas-phase environment: Direct comparison with experimental measurements[J]. Diamond and Relat Mater, 1999, 8(8/9): 1388-1392.
[59] Riccardi C, Barni R, Sindoni E, et al. Gaseous precursors of diamond-like carbon films: chemical composition of CH4/Ar plasmas[J]. Vacuum, 2001, 61: 211-215.
[60] Li Y X, Brenner D W, Dong X L, et al. First principles prediction of gas-phase composition and substrate temperature for diamond film growth[J]. Molecular Simulation, 2000, 25 (1/2): 41-51.
[61] Dong L F, Chen J Y, Li X W, et al. Dissociation process of CH4/H2 gas mixture during EACVD[J]. Thin Solid Films, 2001, 390(1/2): 93-97.
[62] 戚学贵, 陈则韶, 王冠中, 等. C-H-O和C-H-N体系生长金刚石膜的气相化学模拟[J]. 无机材料学报, 2004, 19(2): 404-410.
QI Xue-gui, CHEN Ze-shao, WANG Guan-zhong, et al. Simulation of gas phase chemistry in C-H-O and C-H-N systems for chemical vapor deposition diamond films[J]. Journal of Inorganic Materials, 2004, 19(2): 404-410.
[63] Huang T B, Tang W Z, Lü F X, et al. Influence of plasma power over growth rate and grain size during diamond deposition using DC arc plasma jet CVD[J]. Thin Solid Films, 2003, 429(1/2): 108-113.
[64] Chen G C, Li B, Lan H, et al. Gas phase study and oriented self-standing diamond film fabrication in high power DC arc plasma jet CVD[J]. Diamond and Relat Mater, 2007, 16(3): 477-480.
[65] Petherbridge J R, May P W, Shallcross D E, et al. Simulation of H-C-S containing gas mixtures relevant to diamond chemical vapor deposition[J]. Diamond and Relat Mater, 2003, 12(12): 2178-2185.
[66] Petherbridge J R, May P W, Fuge G M, et al. Sulfur doping of diamond films: spectroscopic, electronic, and gas-phase studies[J]. J Appl Phys, 2002, 91(6): 3605-3613.
[67] Rennick C J, Smith A G, Smith J A, et al. Improved characterization of C2 and CH radical number density distributions in a DC arc jet used for diamond chemical vapor deposition[J]. Diamond and Relat Mater, 2004, 13(4/8): 561-568.
[68] Manklevich Y A, Suetin N V, Ashfold M N R, et al. Chemical kinetics in carbon depositing D. C. arc jet CVD reactors[J]. Diamond and Relat Mater, 2003, 12(3/7): 383-390.
[69] May P W, Mankelevich Y A. The mechanism for ultrananocrystalline diamond growth: Experimental and theoretical studies[J]. Mater Res Soc Symp Proc, 2007, 956: 127-134.
[70] Mankelevich Y A, Ashfold M N, Orr-Ewing A J. Measurement and modeling of Ar/H2/CH4 arc jet discharge chemical vapor deposition reactors Ⅱ: Modeling of the spatial dependence of expanded plasma parameters and species number densities[J]. J Appl Phys, 2007, 102(6): 063310-1-11.
[71] May P W, Mankelevich Y A. From ultrananocrystalline diamond to single crystal diamond growth in hot filament and microwave plasma-enhanced CVD reactors: a unified model for growth rates and grain sizes[J]. J Phys Chem C, 2008, 112(32): 12432-12441.
收稿日期:2009-06-05;修回日期:2009-08-19
基金项目:国家自然科学基金资助项目(59872003)
通信作者:安希忠(1973-),男,辽宁盖州人,博士,副教授,从事粉体和颗粒及薄膜材料的数值仿真及物理实验研究;电话:024-83686465;E-mail: anxz@mail.neu.edu.cn


