Trans. Nonferrous Met. Soc. China 24(2014) 3702-3709
Effect of sulfur impurity on coke reactivity and its mechanism
Jin XIAO, Song-yun DENG, Qi-fan ZHONG, Shao-long YE
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 28 April 2014; accepted 25 August 2014
Abstract:
Effect of sulfur impurity on coke reactivity was investigated by simulating petroleum coke with low-impurity pitch coke and impurities doping. And its mechanism was discussed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive spectrometer (EDS). The results show that sulfur has strong catalysis on both air and CO2 reactivity of coke in the case of no other impurity interference. Its catalysis is probably realized by triggering organic sulfur→H2S→SO2→COS and elemental sulfur (Sx)→SO2 and organic sulfur→H2S→COS→Sx→C2S→COS reaction systems during coke-O2 and coke-CO2 reactions, respectively, which are partly circular with functions of increasing carbon consumption and enlarging coke specific surface area.
Key words:
coke; reactivity; sulfur impurity; catalysis;
1 Introduction
Petroleum coke is both the chief component and the main impurity source of carbon anode used in aluminum industry. Therefore, its quality has a significant influence on the anode quality and the economic and technical indexes of aluminum production. In recent ten years, with the worsening of crude oil quality and the increasing of crude oil refining degree of refiner, a quality problem of petroleum coke in increasing of impurities, such as sulfur, vanadium, calcium and sodium, spreads all over the world [1]. It has been identified that impurities of vanadium, calcium and sodium all have a strong catalysis on air and CO2 reactivity of coke [2,3]. However, sulfur is the impurity whose content increases most evidently in petroleum coke, but the effect of sulfur on coke reactivity is still not fully understood until now. HOUSTON and OYE [4] reviewed the date of actual aluminum production and reported that the effect of sulfur on anode reactivity was difficult to define because it changed as the anode impurities situation changed remarkably. HARDIN and BEILHARZ [5] and TRAN et al [6] reported that air reactivity of petroleum coke increased while its CO2 reactivity decreased with increasing the sulfur content. But TRAN et al [6] also emphasized that the experiment results were probably interfered by other impurities in the coke to some extent. SORLIE [7] reported that the air reactivity of carbon anode increased first and then decreased with the increase of sulfur content, while the CO2 reactivity of anode decreased constantly. FRANCA et al [8] carried out an industry scale experiment using high-sulfur coke to produce carbon anode and reported that both the air and CO2 reactivity of anodes decreased obviously with sulfur content increasing. HUME et al [9] indicated that the effect of sulfur on inhibiting the CO2 reactivity of carbon anode was probably because sulfur weakened the catalysis of sodium by forming a stable nonmobile complex with sodium. EIDET et al [10] and ZHOU et al [11] found that sulfur could also inhibit the catalysis of iron and cause a reduction of the air reactivity of cokes and carbon anodes by forming iron sulfides. Using the characters similarity between pitch coke and petroleum coke, ENGVOLL [12] studied the reactivity changes of pitch coke doped with dibenzothiophene (DBT) and calcium species, and reported that sulfur could remarkably inhibit the catalytic effect of calcium on the coke-CO2 reaction.
It is the trend of the future that sulfur content of petroleum coke increases year by year. In such a situation, identifying the effect of sulfur on the reactivity of petroleum coke has become very important for the efficient utilization of high-sulfur coke in aluminum industry now. In this work, effect of sulfur impurity on coke reactivity without other impurity interference was investigated by simulating petroleum coke with low-impurity pitch coke and sulfur contained species doping. The mechanism was then discussed by the XRD, SEM and EDS methods.
2 Experimental
2.1 Materials
A kind of coal par pitch with low impurity was used to prepare coke samples in this study. Characteristic of coke sample with no dopant is listed in Table 1.
Table 1 Characteristics of pitch coke without any dopant
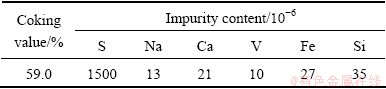
Sulfur in petroleum coke is presented in both organic and inorganic form, in which thiophenes, pyrite and sulfate species mainly exist, respectively. Thus, referring to the method of ENGVOLL [12], DBT and ammonium sulfate ((NH4)2SO4), and dilute sulfuric acid (30% H2SO4, mass fraction) were chosen as the organic and inorganic sulfur dopant in the experiment, respectively.
2.2 Sample preparation
The coal tar pitch of 100 g was melted by oil bath method at 200 °C. A certain amount of dopant was added into the melt pitch and mixed well. The pitch container with melt pitch was moved together into a furnace reactor at 550 °C and the pitch was carbonized for 1 h to make the precursors of samples. The spongy parts of 5 mm above and below the precursors were cut off, respectively, and the rest precursors were crushed into particles. The precursor particles were calcined with calcined coke covering at 1100 °C for 1 h, and the coke samples were gotten.
2.3 Sample analysis
Reactivity measurement of coke samples was conducted using a reactor (see Fig. 1) under isothermal condition in air or CO2 flow of 50 L/h. For each experiment, 5 g of sample (particle size 1.0-1.4 mm) was put into the reactor at 600 °C (air reactivity test) or 1000 °C (CO2 reactivity test) for 1 h. The reactivity of samples was characterized by the mass loss rate during the reaction test. The higher the mass loss rate was, the higher the reactivity was.
Sulfur content measurement of samples was conducted with the sulfur detector (HDS3000) produced by Huade Company of Hunan province, China.
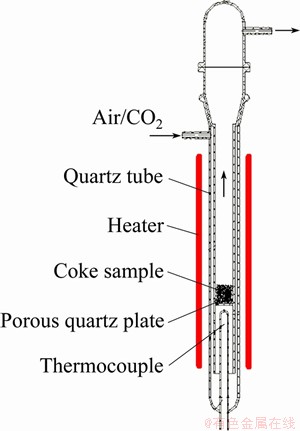
Fig. 1 Structure of reactivity test device
XRD analysis was conducted with Rigaku D/Max 2500 diffractometer with Cu Kα line radiation. XRD data were obtained at 2θ from 5° to 60° with a scan speed of 4 (°)/min. SEM and EDS analyses were conducted with FEI Quanta 200 scanning electron microscope, equipped with an energy dispersive spectrometer. The accelerating voltage was 20 kV.
3 Results and discussion
3.1 Effect of sulfur on coke reactivity
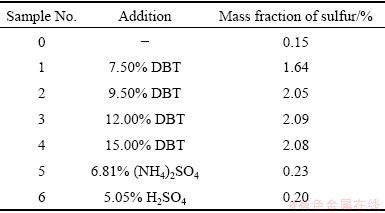
As listed in Table 2, several pitch coke samples were prepared by adding different amounts of DBT, (NH4)2SO4, or H2SO4 into the low-impurity pitch. The masses of sulfur in samples 2, 5 and 6 were the same. It is found that DBT can bring a certain amount of sulfur impurity into cokes. But probably because some of the DBT volatilized during the carbonization process, the sulfur content of the cokes reached the peak at only about 2% (mass fraction). By contrast, (NH4)2SO4 and H2SO4 cannot increase the sulfur content of coke. It is speculated that the sulfur brought by them had been turned into sulfur-containing gas, such as SO2 or H2S, during the carbonization process.
Table 2 Characteristics of coke samples
The air and CO2 reactivity test results of samples 0-6 are shown in Fig. 2. It is found that both the air and CO2 reactivities of coke increase obviously with the increase of sulfur content while the increasing trend of CO2 reactivity is more evident. The mass loss rates of coke during air and CO2 reactivity test increase from 18.9% and 7.1% to 26.5% and 35.0%, respectively, when the sulfur content of coke increases from 0.15% to 2.05%. Moreover, the sulfur contents of cokes before and after reactivity test were measured. It is found that the sulfur content changes little through the process. Obviously, except a small amount of sulfur freedom from carbon chain with the consumption of coke during the reaction process, most of the sulfur is still tied up in the carbon backbone of the coke.
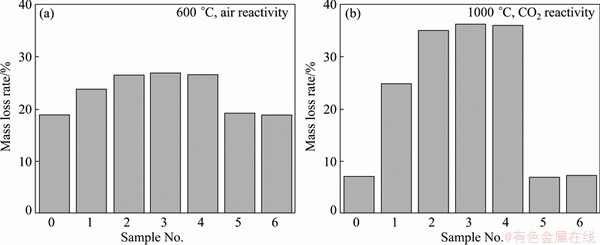
Fig. 2 Air (a) and CO2 (b) reactivity test results of samples
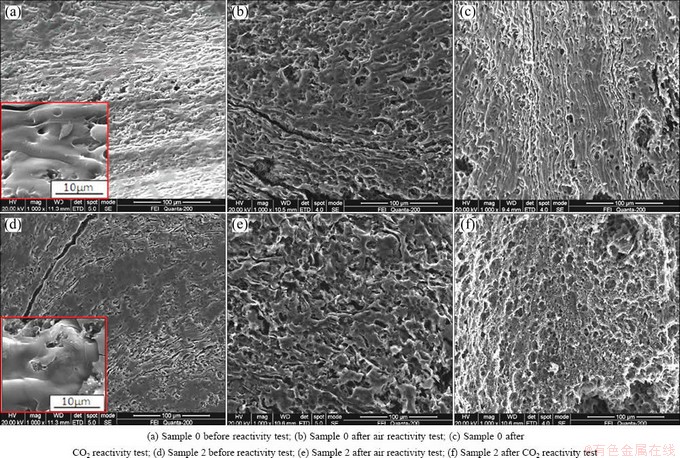
Fig. 3 SEM images of samples 0 and 2
The SEM images of samples 0 and 2 are shown in Fig. 3. It is found that the original appearance characteristics of the two samples are very similar. But after the reactivity test, some obvious differences appear. Compared with sample 0, there are more corrosion marks like grooves on the surface of sample 2 after air reactivity test, as shown in Figs. 3(b) and (e); and there are many tiny round etch holes on the surface of sample 2 after CO2 reactivity test, as shown in Figs. 3(c) and (f). TRAN et al [6] speculated that it was because sulfur weakened the bonding of adjacent carbons in the ring structure and lowered the activation energy that the oxidation resistance of coke decreased with increasing sulfur content. But obviously, considering the possible distribution of sulfur in coke, this theory cannot explain the appearance of the deep etch holes in Fig. 3(f).
3.2 Effect of sulfur on XRD structural parameters of coke
The XRD analysis results of samples with different sulfur contents are shown in Fig. 4. And the corresponding calculated coherent stacking heights (Lc) and the distance between the grapheme layers (d002) of these samples are listed in Table 3. It is found that sulfur content has little effect on the microstructure of coke. There is no obvious correlation between the XRD structural parameters and the sulfur content of the coke. According to Ref. [12], such a little wave of lattice parameters of coke cannot cause an obvious change in air or CO2 reactivity of coke.
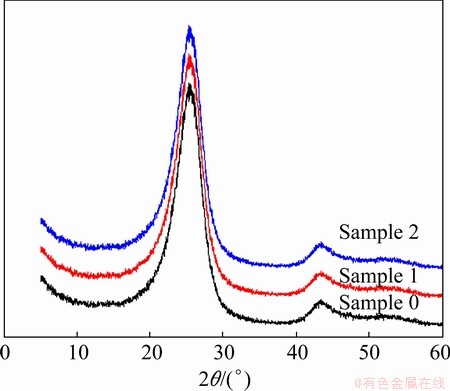
Fig. 4 XRD images of samples with different sulfur content
Table 3 d002 and Lc of samples with different sulfur contents
3.3 Catalytic mechanism of sulfur discussion
3.3.1 Catalytic mechanism of sulfur in coke-O2 reaction
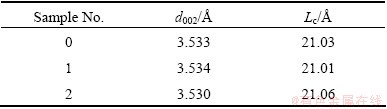
SEM and EDS analyses were conducted on various corrosion areas of sample 2 after air reactivity test, and the results are shown in Fig. 5. It shows the typical corrosion morphology with grooved corrosion character on the sample surface. EDS analysis indicates that, there is usually a sulfur-enrichment phenomenon in the areas (e.g. area A) of deep corrosion groove, as shown in Fig. 5(c) (the complex background is caused by the sunken surface structure). By contrast, only carbon and a small amount of oxygen could be detected in the other surface areas (e.g. area B) of the coke, as shown in Fig. 5(d). Figure 5(b) shows a kind of special corrosion morphology with huge corrosion pits character on the sample surface. Only two regions with such a character were found by SEM. EDS analysis indicates that the sulfur-enrichment phenomenon was also found in the areas (e.g. area C) of the huge corrosion pits, as shown in Fig. 5(e). Moreover, a white fine substance was found by high magnification observation in the huge corrosion pits. As shown in Fig. 5(f), there is a clear characteristic peak of sulfur shown in the EDS analysis result of this substance. The sulfur content in the area D reaches 11% (mole fraction) with the interference of carbon base. Considering that there is no stable solid compounds composed of carbon and sulfur at room temperature, the substance is supposed to be Sx only.
The analysis above indicates that the catalytic effect of sulfur is acted on the region around the original position of the sulfur. Since there is no obvious sign of desulfurization, the effect is probably caused by a small amount of sulfur freedom from the bond of carbon chain with the consumption of coke during the coke-O2 reaction. Thus, according to Refs. [13-17], it is speculated that the catalytic effect of sulfur on the coke-O2 reaction is probably caused by the reactions as follows:
Organic sulfur→H2S(g) (1)
Organic sulfur→SO2(g) (2)
H2S(g)+3/2O2(g)=SO2(g)+H2O(g) (3)
xSO2(g)+xC=Sx(g)+xCO2(g) (x=2,4,6,8) (4)
SO2(g)+2C+1/2O2(g)=COS(g)+CO2 (g) (5)
COS(g)+O2(g)=CO2(g)+1/2SO2(g) (6)
Sx(g)+xO2(g)=xSO2 (g) (x=2,4,6,8) (7)
As to Reactions (1)-(3), it is indicated that there are two species, H2S (major) and SO2 (minor), which can be transformed directly from the organic sulfur in coke or coal under non-oxidizing and heating atmosphere, such as N2, CO and H2 [13,14]. But H2S is easy to be oxidized in air at high temperature and converted to SO2 since its ignition point is only 260 °C.
GEORGE and RECHARD [15] indicated that SO2 reacted with carbon easily through Reaction (4) at high temperature, and this principle has been used widely to get Sx from SO2-containing tail gas. BEJARANO et al [16] used recycle Sx from SO2 gas successfully with coke as carbon source, which was prepared by pitch. CHEN et al [17] indicated that both Reactions (4) and (5) occurred during the carbothermal reduction process of SO2 under the condition of sufficient carbon material. And the total conversion rate of SO2 to COS and gaseous Sx can reach 98% in a short time at 600 °C.
As to Reactions (6) and (7), COS and Sx are flammable and easy to be converted to SO2 in air at 600 °C.
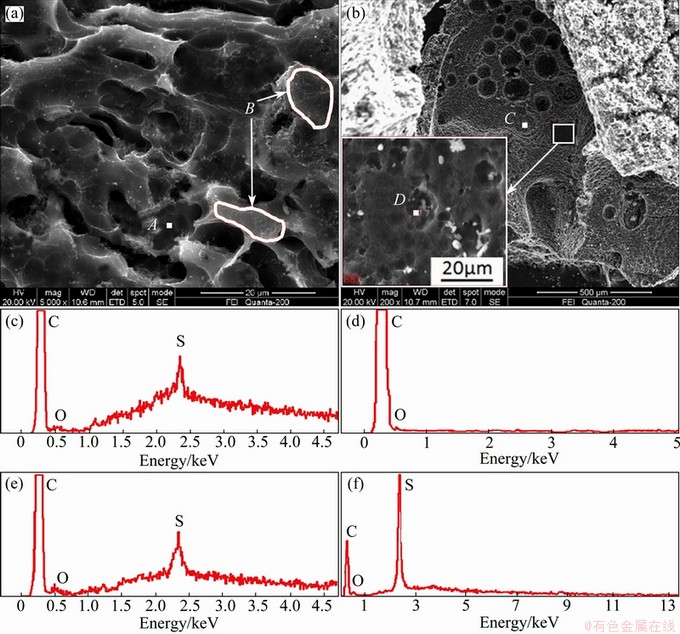
Fig. 5 SEM images of typical corrosion morphology (a) and special corrosion morphology (b) of sample 2 after air reactivity test, and EDS analyses of areas A (c), B (d), C (e) and D (f)
Thermodynamics parameter of Reactions (1) and (2) cannot be evaluated because the exact component of organic sulfur is unknown. The △G and △H of Reactions (3)-(7) in the temperature range of 400-700 °C are calculated by HCS chemistry software and listed in Table 4 (x was set as 2). The results show that the △G and △H of Reactions (3)-(7) in the temperature range are all negative and, hence, it is feasible that all of these reactions take place with heat releasing during the coke-O2 reaction process.
Based on the analysis above, the catalytic process of sulfur on the coke-O2 reaction is speculated, as shown in Fig. 6.
Sulfur, exposed to the air with the consumption of coke, is converted to SO2 through Reactions (1)-(3). Small corrosion pits are formed on the coke surface. A cyclic reaction system with calytic effect on coke-O2 reaction is formed by Reactions (4)-(7). The reaction system can increase the air reactivity of coke by increasing the consumption of coke directly and releasing heating. During this process, the corrosion pits on coke surface are enlarged constantly. The larger corrosion pits increase the specific surface area of coke and provide better places for the concentration of Sx, COS and SO2 gas, which creates excellent environment for the cyclic reaction system and, hence, causes the deepening and enlarging of the corrosion pits on coke surface further. After the coke-O2 reaction, the coke surface, with an even distribution of sulfur previously, presents the typical corrosion morphology, as shown in Fig. 5 (a), while the one with sulfur-concentration shows the special corrosion morphology, as shown in Fig. 5(b). Most of the sulfur-containing gas escapes from the coke surface and a small amount of Sx condenses in the corrosion pits.
3.3.2 Catalytic mechanism of sulfur in coke-CO2 reaction
SEM and EDS analyses were conducted on various corrosion areas of sample 2 after CO2 reactivity test, as shown in Fig. 7. It is found that, there is also a sulfur- enrichment phenomenon in the deep corrosion hole areas (e.g. area E) of the coke surface, as shown in Fig. 7(b). By contrast, there are only carbon and a small amount of oxygen detected in the other areas (e.g. area F) of the coke surface. It is tried to search the condensed solid Sx in the areas of sulfur-enrichment, but there is no special finding probably because the corrosion holes are too deep to be observed.
Table 4 △G and △H of Reactions (3)-(7) in temperature of 400-700 °C (kJ/mol)
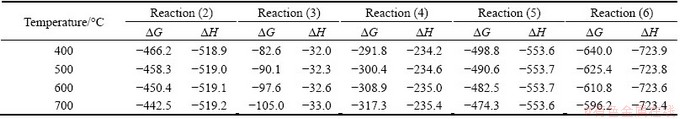
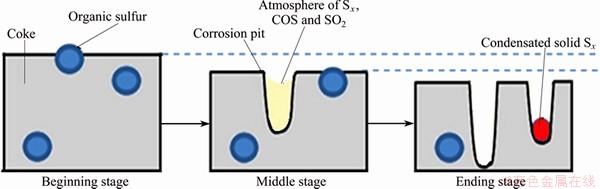
Fig. 6 Catalytic process of sulfur on the coke-O2 reaction
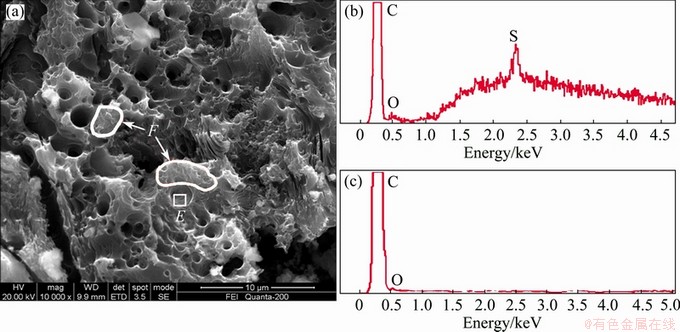
Fig. 7 SEM image (a) of sample 2 after CO2 reactivity test, and EDS analyses of areas E (b) and F (c)
The analysis above indicates that the corrosion holes on the coke surface should be formed by the effect of sulfur, and they are probably the direct reason causing the increase of mass loss rate of coke during the coke-CO2 reaction. WANG [18] indicated that besides SO2 and H2S, there was also a certain amount of COS and CS2 detected during the gasification process of sulfur-containing coke. DUAN et al [19] and CALKINS [20] studied the conversion of organic sulfur of coal in CO2 atmosphere and indicated that the organic sulfur can be converted to four kinds of species of H2S, SO2, COS and CS2. LU et al [14] had a further study on the conversion process later and reported that the conversion rate of organic sulfur to COS increased with the increase of CO2 concentration in the atmosphere. According to Refs. [18-22], reactions probably occurring during the coke-CO2 reaction process are summarized as follows:
Organic sulfur→H2S (8)
Organic sulfur→SO2 (9)
H2S(g)+CO2(g)=COS(g)+H2O(g) (10)
H2S(g)+C+2CO2(g)=COS(g)+2CO(g)+H2O(g) (11)
SO2(g)+2C=CO(g)+COS(g) (12)
SO2(g)+C=(1/x)Sx(g)+CO2(g), x=2,4,6,8 (13)
SO2(g)+2CO(g)=2CO2(g)+(1/x)Sx(g), x=2,4,6,8 (14)
Sx(g)+ (x/2)C=(x/2)CS2(g), x=2,4,6,8 (15)
COS(g)=CO(g)+(1/x)Sx(g), x=2,4,6,8 (16)
SO2(g)+2H2S(g)=(3/x)Sx(g)+2H2O(g), x=2,4,6,8 (17)
SO2(g)+CS2(g)=(2/x)Sx(g)+CO2(g), x=2,4,6,8 (18)
SO2(g)+2COS(g)=(3/x)Sx(g)+2CO2(g), x=2,4,6,8 (19)
CS2(g)+CO2(g)=2COS(g) (20)
Reactions (8) and (9) present the conversion of organic sulfur to H2S and SO2. SHI et al [21] indicated that in the reductive environment of coke-CO2 reaction, H2S and SO2 can react with carbon, CO2 or CO and be converted to COS and Sx according to Reactions (10)- (14) under certain temperature conditions. According to known preparation methods of CS2, during the coke- CO2 reaction, CS2 can be produced by Reaction (15) only. The reactants Sx of Reaction (15) can be produced by Reactions (13), (14) and (16)-(19) [21]. CS2 can be converted to COS through Reaction (20) [22]. The △G of Reactions (10)-(20) in the temperature range of 800- 1100 °C was calculated by HCS chemistry software, as listed in Table 5 (x was set as 2).
As listed in Table 5, except the △G of Reaction (10), △G of Reactions (11)-(20) are all negative in the temperature range of 800-1100 °C. Therefore, Reactions (11)-(20) are able to occur during the coke-CO2 reaction process. However, considering the sulfur content of coke and the strong effect of sulfur on the coke-CO2 reaction, it is very likely that the reactions caused by sulfur create a circular reaction system, similar to Reactions (4)-(7), with acceleration effect on the consumption rate of coke. Thus, based on the analysis above, the catalytic process of sulfur on the coke-CO2 reaction is speculated, as shown in Fig. 8.
Sulfur, exposed to the air with the consumption of coke, is converted to H2S and SO2 through Reactions (8) and (9). Small corrosion pits are formed on the coke surface. H2S and SO2 are converted to COS through Reactions (11) and (12). Both of the reactions contribute to the consumption increasing of the coke directly. In the meantime, parts of SO2 are probably converted to Sx by Reactions (13), (14) and (17)-(19). 3) Reactions (15), (16) and (20) create a cyclic reaction system with a circulation conversion among COS, Sx and CS2. During the conversion process, Reaction (15) increases the consumption of coke constantly with the process similar to Fig.6, making the corrosion pits deeper and larger. The corrosion pits provide better places for the concentration of COS, Sx and CS2, promoting the circular reactions system of Reactions (15), (16) and (20) further. During this process, the deep corrosion pits are formed because of the strong catalytic effect of the cyclic reactions system on the coke-CO2 reaction. At the ending stage of the coke-CO2 reaction, most of the surfur-containing gas escapes from the coke surface. There is probably only a small amount of Sx condensing and leaving at the bottom of the deep corrosion hole finally, because the ending temperature of the coke-CO2 reaction is about 1000 °C, which is much higher than that of air reactivity test.
Table 5 △G of Reactions (10)-(20) in temperature range of 800-1100 °C
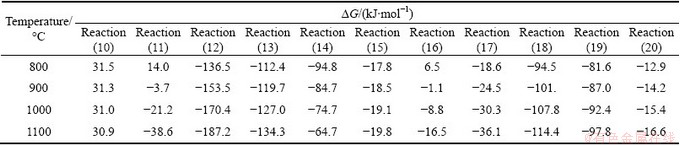
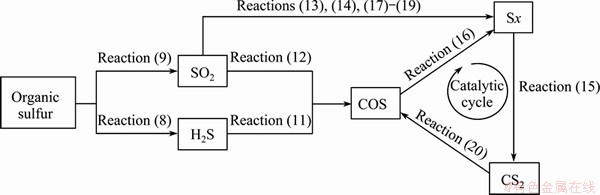
Fig. 8 Catalytic process of sulfur on coke-CO2 reaction
4 Conclusions
1) Sulfur has strong catalysis on both the air and CO2 reactivity of coke in the case of no other impurity interference. The mass loss rates of coke during the coke-air and coke-CO2 reaction increase from 18.9% and 7.1% to 26.5% and 35.0%, respectively, when the sulfur content of coke increases from 0.15% to 2.05%.
2) Sulfur has little effect on the crystalline structure of coke. It is speculated that the catalytic effect of sulfur on the air and CO2 reactivity of coke is realized based on the mechanism. Sulfur, freed from the bond of carbon chain with the consumption of coke, triggers reaction system of organic sulfur→H2S→SO2→COS and Sx→SO2 and organic sulfur→H2S→COS→ Sx→C2S →COS during the coke-O2 and coke-CO2 reaction, respectively. Both of the reaction systems are partly circular with functions of increasing carbon consumption directly and enlarging coke specific surface area, which finally cause the effective increase of air and CO2 reactivity of the coke.
References
[1] EDWARDS L C, NEYREY K J, LOSSIUS L P. A review of coke and anode desulfurization [J]. Light Metals, 2007: 895-900.
[2] ZHANG Jin-bin. Research on petroleum coke calcining process and pitch anti-oxidation improvement [D].Changsha: Central South University, 2013. (in Chinese)
[3] BELITSKUS D L, SIMON H A, BART E F. A review and assessment of effects of sodium in Hall-Heroult cell anodes [J]. Light Metals, 1999: 517-522.
[4] HOUSTON G J, OYE H A. Consumption of anode carbon during aluminium electrolysis (II) [J]. Aluminium, 1985, 61: 346-347.
[5] HARDIN E H, BEILHARZ C L. Correlations of laboratory and commercially calcined petroleum coke properties [J]. Light Metals, 1991: 565-574.
[6] TRAN K N, BERKOVICH A J, TOMSEER A, BHATIA S K. Influence of sulfur and metal microconstituents on the reactivity of carbon anodes [J]. Energy & Fuels, 2009, 23(4): 1909-1924.
[7] SORLIE M. Effect of sulphur on anode reactivity and electrolytic consumption [J]. Light Metals, 1994: 659-665.
[8] FRANCA G, MESQUITA C, EDWARDS L, VOGT F. Anode quality improvements at the Valesul smelter [J]. Light Metals, 2003: 535-540.
[9] HUME S M, FISCHER W K, PERRUCHOUD R C, METSON J B, BAKER R T. Influence of petroleum coke sulphur content on the sodium sensitivity of carbon anodes [J]. Light Metals, 1993: 535-535.
[10] EIDET T, SOERLIE M, THONSTAD J. Effects of iron and sulphur on the air and CO2 reactivity of cokes [J]. Light Metals, 1997: 511-520.
[11] ZHOU Zhi-jie, HU Qi-jing, LIU Xin, YU Guang-suo, WANG Fu-chen. Effect of iron species and calcium hydroxide on high-sulfur petroleum coke CO2 gasification [J]. Energy & Fuels, 2012, 26(3): 1489-1495.
[12] ENGVOLL M A. Reactivity of anode raw materials and anodes for production of aluminium [D]. Trondheim: Norwegian University of Science and Technology, 2002.
[13] MAKARYTCHEV S V, CEN K F, LUO Z Y. Staged desulphurization by direct sorbent injection in pulverized-coal boilers [J]. Energy, 1994, 19(9): 947-956.
[14] LU Dang-zhen, XU Ming-hou, YAO Hong, LIU Xiao-wei, GU Yin, JIANG Wei. SOx emission during the oxy-fuel combustion of density separated coal fractions [J]. Journal of Engineering Thermophysis. 2009, 30(8): 1427-1430. (in Chinese)
[15] GEORGE E K, RECHARD J W. Equilibrium studies of direct reduction of SO2 by coal [J]. Fuel, 1984, 63(10): 1450-1454.
[16] BEJARANO C A, JIA C Q, CHUNG K H. A study on carbothermal reduction of sulfur dioxide to elemental sulfur using oilsands fluid coke [J]. Environmental Science & Technology, 2001, 35(4): 800-804.
[17] CHEN Yin, WANG Le-fu, LI Xue-hui. The investigation of direct catalytic reduction of dioxide sulfur to element sulfur [J]. Natural Gas Chemical Industry, 2003, 28(1): 21-24. (in Chinese)
[18] WANG Xiao-peng. The preparation of catalysts and research of kinetics for carbon disulfide hydrolysis under moderate temperature [D]. Taiyuan: Taiyuan University of Technology, 2007. (in Chinese)
[19] DUAN Lun-bo, ZHAO Chang-sui, ZHOU Wu, QU Cheng-rui, CHEN Xiao-ping. Investigation on coal pyrolysis in CO2 atmosphere [J]. Energy & Fuels, 2009, 23(7): 3826-3830.
[20] CALKINS W H. Investigation of organic sulfur-containing structures in coal by flash pyrolysis experiments [J]. Energy & Fuels, 1987, 1(1): 59-64.
[21] SHI Ji-hui, ZENG Kang-mei, YI Hua-qiang, YANG Ji-cheng. Techniques for removal of SO2 from flue gas by reduction [J]. Techniques and Equipment for Environmental Pollution Control, 2004, 5(5): 75-80. (in Chinese)
[22] FENG Jiang-hua, LI Hui-min, ZHANG Ji-ming, YI Qing-jun. Improvement of H2S and HCN removal from coke oven gas process [J]. Guangdong Chemical Industry, 2011, 38(12): 52. (in Chinese).
硫杂质对焦反应性的影响及其机理
肖 劲,邓松云,仲奇凡,叶绍龙
中南大学 冶金与环境学院,长沙 410083
摘 要:采用以低杂质沥青焦模拟石油焦和外掺杂的方式,研究硫杂质元素对焦反应性的影响,并通过XRD、SEM和EDS等检测手段探讨其作用机理。结果表明:在无其他杂质元素干扰的情况下,硫实际上是一种对焦的空气和CO2反应性都具有明显催化性的杂质元素。其催化作用可能是通过在焦的空气和CO2反应过程中分别引发有机硫→H2S→SO2→COS和单质硫(Sx)→SO2和有机硫→H2S→COS→Sx→C2S→COS两组可部分循环并具有增加碳耗和增大焦比表面积作用的反应体系来实现的。
关键词:焦;反应性;硫杂质;催化作用
(Edited by Chao WANG)
Foundation item: Project (51374253) supported by the National Natural Science Foundation of China
Corresponding author: Song-yun DENG; Tel: +86-731-88876454; E-mail: dengsongyun1@163.com
DOI: 10.1016/S1003-6326(14)63518-4
Abstract: Effect of sulfur impurity on coke reactivity was investigated by simulating petroleum coke with low-impurity pitch coke and impurities doping. And its mechanism was discussed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive spectrometer (EDS). The results show that sulfur has strong catalysis on both air and CO2 reactivity of coke in the case of no other impurity interference. Its catalysis is probably realized by triggering organic sulfur→H2S→SO2→COS and elemental sulfur (Sx)→SO2 and organic sulfur→H2S→COS→Sx→C2S→COS reaction systems during coke-O2 and coke-CO2 reactions, respectively, which are partly circular with functions of increasing carbon consumption and enlarging coke specific surface area.


