硅锌矿在(NH4)2SO4-NH3-H2O体系中高液固比下的浸出动力学
刘志宏,曹志阎,刘智勇,李启厚,李玉虎
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:
锌矿在(NH4)2SO4-NH3-H2O体系中高液固比下的浸出动力学。采用粒径为96~109 μm的纯硅锌矿样品,在液固质量比为200时,考察搅拌速度、反应温度及总氨浓度对锌浸出率的影响。研究结果表明:提高温度和总氨浓度能显著提高浸出速率,而当搅拌速度大于250 r/min时,其对浸出速率的影响较小;浸出过程遵循孔隙扩散控制粒子模型,扩散与化学反应并非仅发生于颗粒外表面,而是发生在整个外表面及孔隙内部,浸出速度受孔隙扩散控制;浸出过程的表观活化能与反应级数分别为71.35 kJ/mol和4.27。
关键词:
硅锌矿;(NH4)2SO4-NH3-H2O体系;高液固比;浸出动力学;粒子模型;
中图分类号:TF803.21;TF813 文献标志码:A 文章编号:1672-7207(2012)02-0418-06
Leaching kinetics of willemite in (NH4)2SO4-NH3-H2Osystem athigher mass ratio of liquid to solid
LIU Zhi-hong, CAO Zhi-yan, LIU Zhi-yong, LI Qi-hou, LI Yu-hu
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Leaching kinetics of willemite in (NH4)2SO4-NH3-H2O system were investigated by using pure mineral of willemite as raw materials when mass ratio of liquid to solid was 200. The effects of factors on the leaching rate, such as stirring speed, temperature and total ammonium concentration, were studied experimentally. The results show that increasing temperature and total ammonium concentration can enhance the leaching rate remarkably, while when the speed is larger than 250 r/min, the stirring has no obvious effect on the leach. The leach process obeys the grain model with porous diffusion control, indicating that the diffusion and chemical reaction take place, not only on the outer surface of the particles, but also on the out surface together with the cavities of the porous particles. Kinetic studies demonstrate that the leach process is controlled by the porous diffusion, determining the apparent active energy and reaction order to be 71.35 kJ/mol and 4.27, respectively.
Key words: willemite; (NH4)2SO4-NH3-H2O system; high ratio of liquid to solid; leaching kinetics; grain model
近年来,随着我国锌产量的高速增长,作为主要炼锌原料的硫化锌矿资源已趋于短缺,开发利用氧化锌矿资源的重要性日益凸显。我国西南、西北等地氧化锌矿资源丰富,但其品位低、难选、硅和碱性脉石含量高,且矿物组成复杂,含锌矿物主要有硅锌矿(Zn2SiO4)、异极矿(Zn4(Si2O7)(OH)2·H2O)、菱锌矿(ZnCO3)和水锌矿(Zn5(CO3)2(OH)6)等,属典型的难冶资源。采用火法工艺处理,可以实现锌与Fe,Si,Ca和Mg等杂质的分离[1],但金属回收率低,能耗高,环境污染严重,因此,酸浸、苛性碱浸和氨浸等湿法工艺成为研究的热点。浸出时硅胶的大量生成导致液固分离困难,是酸浸处理氧化锌矿的技术难点。为此, 已开发出若干种工艺技术[2-5],主要分为2类:第1类是浸出后采用中和絮凝法得到易于分离的矿浆,如中和絮凝法和老山工艺;第2类是在浸出过程中抑制硅的溶解,主要有Redina法、微波辅助法和高压酸浸法等。这些工艺仅适合处理低碱性脉石的高品位氧化锌矿。与酸浸工艺相比,苛性碱浸出工艺选择性较 好[6-8],但也存在一些难点:浸出液中硅、铝、铅含量较高,除杂困难;浸出液难以进行有效电积,或电积时只能产出树枝状锌粉。该工艺目前尚处于实验室研究阶段。氨法浸锌工艺简单、高效、原料适应性广、选择性高,并且终端产品灵活,既可生产活性氧化锌,也可电积获得阴极锌。与其他工艺相比,更适合处理高硅高碱性脉石的低品位氧化锌矿。但研究表明,矿石中锌的物相对其浸出率具有决定性的影响,当矿石中硅锌矿等硅酸盐类含锌矿物含量较高时,锌浸出率偏低[9-11]。人们对氧化锌矿氨浸动力学进行了研究,表明浸出速率控制步骤以及表观活化能、反应级数和速率常数等浸出动力学参数,因矿石品位及含锌物相而异[12-16]。由于这些研究所用原料均为天然氧化锌矿石,其矿物组成既包括游离氧化锌,又含有一定量的硅酸盐类锌矿物,因此,并未揭示硅酸盐类锌矿物在氨浸条件下难以浸出的原因。刘智勇等[17]对硅锌矿在(NH4)2SO4-NH3-H2O体系中的浸出机理进行了系统研究,确定其浸出反应为:
Zn2SiO4+(2i-2)NH3(aq)+4NH4+=2[Zn(NH3)i]2++(NH4)2SiO3(aq)+H2O (1)
(NH4)2SiO3(aq)+H2O=2NH3(aq)+H4SiO4(aq) (2)
H4SiO4(aq)=SiO2(amorph)+2H2O (3)
总反应为:
Zn2SiO4+(2i-4)NH3(aq)+4NH4+=2[Zn(NH3)i]2++SiO2(amorph)+H2O (4)
式中:i为1,2,3或4。在浸出过程中,Zn2+与NH3形成配合物进入溶液;硅酸根首先形成(NH4)2SiO3进入溶液;(NH4)2SiO3不稳定,发生水解生成NH3与H4SiO4(aq),当溶液中SiO2浓度达到饱和后会转化为无定形SiO2沉淀。在低液固质量比下浸出,浸出液中H4SiO4很快达到饱和,速率过程受反应(3)制约,甚至趋于停滞。而在很高的液固比下,硅锌矿浸出缓慢仍是其难以浸出的内在因素。本文作者对硅锌矿在高液固质量比下的浸出动力学进行研究;采用受孔隙扩散控制的粒子模型,对浸出实验数据进行处理,表征浸出过程的控制步骤、表观活化能和反应级数等动力学特性。
1 实验
1.1 实验原料
硅锌矿样品系以云南兰坪的异极矿为原料,于 1 100 ℃下煅烧3 h制备得到。煅烧反应方程式为:
Zn4Si2O7(OH)2·H2O=2Zn2SiO4+2H2O (5)
异极矿及由其制备的硅锌矿样品XRD图谱如图1所示。硅锌矿样品破碎、筛分后备用,如无特别说明,浸出实验样品粒度为96~109 μm。表1所示为该样品的主要成分、比表面积、孔隙率及平均孔径等,图2所示为其SEM照片。
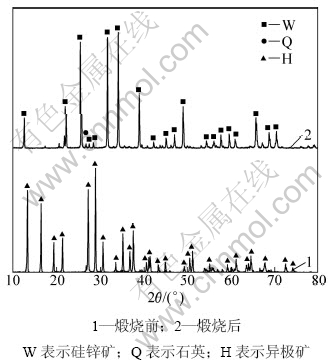
图1 样品的XRD图谱
Fig.1 XRD patterns of ore samples
表1 硅锌矿物理化学性质
Table 1 Physicochemical properties of willemite sample

由图1和表1可知:异极矿样品煅烧后,已完全脱水转化为硅锌矿,杂质含量很低,基本为硅锌矿纯矿物,其XRD图谱及化学成分与文献[6,18]中的一致。由图2可见:硅锌矿样品形貌为类球形,颗粒粒径均匀,疏松多孔,是由粒度为1 μm左右的微细粒子组成的多孔团聚体。
实验试剂为氨水(AR,湖南株洲石英化玻有限公司生产)、硫酸铵(AR,上海国药集团生产)、硅酸钠(AR,广东汕头西陇化工有限公司生产);水为去离 子水。
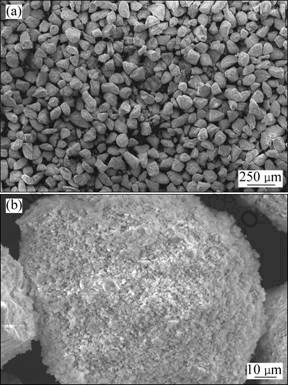
图2 硅锌矿样品SEM分析结果
Fig.2 SEM photographs of willemite sample with particle size of 96-109 μm
1.2 浸出实验
浸出液根据设定的总氨浓度、NH4+与NH3摩尔比,由(NH4)2SO4,NH3·H2O和去离子水配制而成。浸出实验在2 L三口瓶中进行,瓶上3个开口分别装设温度计、机械搅拌器(2片半径为50 mm桨叶)。三口瓶置于恒温水浴中,水浴温度在-1~1 ℃波动。每次配制1 L浸出液,置于三口瓶中,预热到实验温度,加入5 g样品开始浸出。每隔一定时间,取10 mL矿浆离心分离,分别取浸出液及渣样进行分析检测。
1.3 分析检测
用EDTA络合滴定法测定浸出液中锌浓度,据此计算锌浸出率;采用日本RIGAKU-TTRⅢ型X 线衍射仪(Cu靶,波长λ=0.154 06 nm)对样品进行物相分析;用日本JSM-6360LV 型扫描电镜(SEM)观察样品形貌;采用低温氮吸附法分析矿石样品比表面积和孔隙结构,仪器为美国ASAP2010型比表面积及孔径测定仪。
2 结果与讨论
2.1 搅拌速度对浸出率的影响
在总氨浓度为7 mol/L,NH4+与NH3摩尔比为1,温度为323 K,液固质量比为200的条件下,考察搅拌速度对锌浸出率影响,结果如图3所示。由图3可见:当搅拌速度低于250 r/mim时,浸出速率随搅拌强度增大而增加;当搅拌速度大于250 r/mim后,浸出速率的增长趋于平稳,说明在该搅拌强度下,已足以消除外扩散对浸出速率的影响。在随后的实验中,搅拌速度固定为350 r/min。
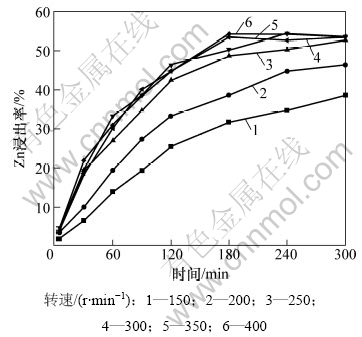
图3 搅拌速度对浸出率的影响
Fig.3 Effect of stirring speed on zinc leaching efficiency
2.2 温度对浸出率的影响
在总氨浓度5 mol/L,NH4+与NH3摩尔比为1,液固质量比为200和搅拌速率350 r/min的条件下,考察温度对锌浸出率的影响,结果如图4所示。图4表明:反应温度对锌浸出率有显著影响。在上述实验条件下浸出300 min,当浸出温度从303 K上升到333 K时,浸出率从12.4%提高到46.3%。
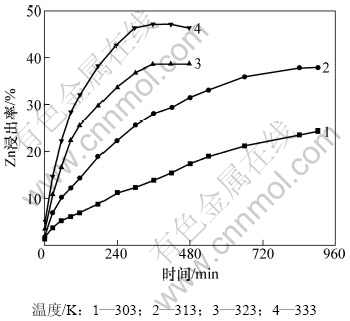
图4 温度对Zn浸出率的影响
Fig.4 Effect of temperature on zinc leaching efficiency
2.3 总氨浓度对浸出率的影响
在NH4+与NH3摩尔比为1、温度为323 K、液固质量比为200、搅拌速率为350 r/min的条件下,考察总氨浓度对锌浸出率的影响,结果如图5所示。由图5可见:总氨浓度对锌浸出率的影响非常显著。在总氨浓度为7 mol/L时,锌浸出率随着浸出时间的延长快速增大,经180 min浸出,Zn的浸出率达到53.31%,之后停止增大;而当总氨浓度降低到4 mol/L时,锌的浸出率随浸出时间的延长变化缓慢,浸出300 min后,锌浸出率只有22.79%。
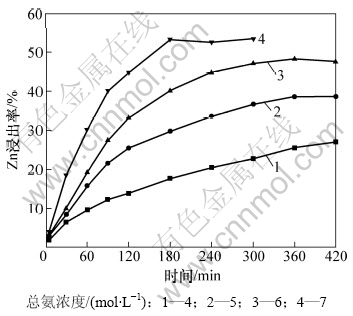
图5 总氨浓度对Zn浸出率的影响
Fig.5 Effect of total ammonium concentration on zinc leaching efficiency
2.4 结果分析
2.4.1 动力学模型
实验所用硅锌矿样品为多孔球形颗粒(见图2、表1),液相反应物会扩散到固体内部,扩散与化学反应不是仅仅发生在颗粒外表面,而是同时发生在整个扩散区域内,在动力学数据处理中,不可简单地使用收缩未反应核模型。目前,多孔颗粒非催化反应动力学模型主要有均匀模型[19]、随机分布模型[20-22]和粒子模型[23-25]。分析结果表明:均匀模型及随机分布模型与样品孔隙结构及实验数据难以吻合,不适用于对本研究所得实验数据进行处理。
粒子模型最初用于描述多孔氧化物气体还原过程。该模型假设矿石颗粒是一些性质相同的微小粒子集合体,这些粒子致密且形状单一,在浸出过程中皆遵循收缩未反应核模型。液相反应物通过粒子间的孔隙向颗粒中心扩散,并与粒子反应;固体反应物含量随着反应进行而减少。粒子模型示意图如图6所示。研究表明:该模型也适用于多孔颗粒湿法浸出过 程[18, 26]。
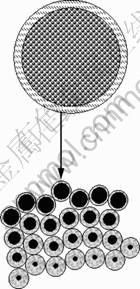
图6 粒子模型
Fig.6 Grain model
当孔隙扩散不可忽略时,浸出过程类似于受内扩散控制的致密颗粒收缩核反应。在这种情况下,对于球体或类球体矿石颗粒的反应,粒子模型数学表达式如下:
![]() (6)
(6)
![]() (7)
(7)
式中:c为总氨浓度,mol/L;R0 为颗粒的起始半径,cm;ρw 为矿石的密度,mol/cm3;ε0为矿石颗粒初始孔隙率,%;Deff 为扩散系数,cm2/s;b为化学计 量数。
将图4和图5 所示数据转化为锌浸出率的函数1-2/3η-(1-η)2/3与浸出时间t的关系图,分别见图7和图8。
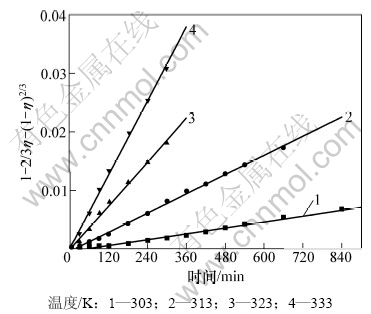
图7 不同温度下1-2/3η-(1-η)2/3与时间关系图
Fig.7 Relationship between 1-2/3η-(1-η)2/3 and time at various temperatures
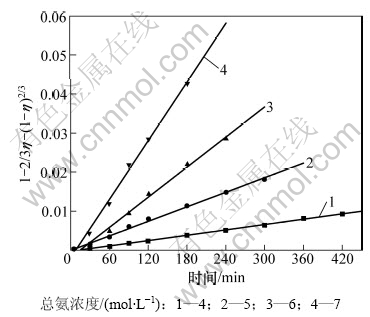
图8 不同总氨浓度下1-2/3η-(1-η)2/3与时间关系图
Fig.8 Relationship between 1-2/3η-(1-η)2/3 and time at various total ammonium concentrations
由图7和图8可见:1-2/3η-(1-η)2/3与时间t呈很好的线性关系,在浸出实验条件下式(6)均成立。表明浸出过程符合孔隙扩散控制的粒子模型。
2.4.2 表观活化能与表观反应级数
将图7中各温度下1-2/3η-(1-η)2/3与t之间的关系进行线性回归,所得直线斜率即为不同浓度下的k。以ln k 对1/T作图,如图9所示。根据阿累尼乌斯方程,由图9中所得直线斜率,可得实验条件下,浸出反应表观活化能为71.35 kJ/mol。
同理,将图8中不同总氨浓度下1-2/3η-(1-η)2/3与t之间的关系进行线性回归,所得直线斜率即为不同总氨浓度下的k。以ln k对ln c作图,如图10所示。由图10所得直线斜率,可得实验条件下,浸出反应表观级数为4.27。
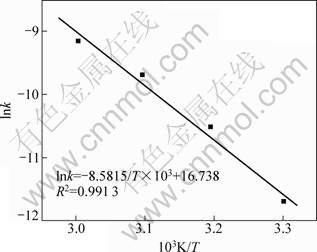
图9 硅锌矿浸出的阿累尼乌斯图
Fig.9 Arrhenius plot for leaching of willemite
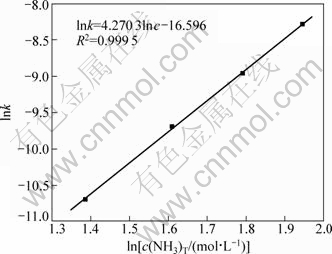
图10 总氨浓度与k关系图
Fig.10 Relationship between ln k and total ammonium concentration
须指出的是:由于受孔隙扩散的影响,多孔颗粒反应动力学会不适用,实验得到的仅为活化能和反应级数的表观值。当受孔隙扩散影响时,浸出反应的表观活化能普遍较大。而反应级数大于3的化学反应亦仅在理论上存在,在自然界中尚未发现[18]。
3 结论
(1) 通过实验研究了多孔硅锌矿样品在(NH4)2SO4-NH3-H2O体系中液固质量比为200时的浸出。当搅拌速度大于250 r/min时,外扩散对浸出过程的影响已基本消除;温度和总氨浓度对浸出速率影响显著。
(2) 浸出过程符合孔隙扩散控制的粒子模型,表明反应不只是发生在颗粒外表面,而是发生在包含孔隙内部的整个扩散区域。反应表观反应活化能为71.35 kJ/mol,表观反应级数为4.27。
参考文献:
[1] Matthew I G, Elsner D. The processing of zinc silicate ores: A review[J]. Metallurgical and Materials Transactions B, 1977, 8(1): 85-91.
[2] Matthew I G, Elsner D. The hydrometallurgical treatment of zinc silicate ores[J]. Metallurgical and Materials Transactions B, 1977, 8(1): 73-83.
[3] Bodas M G. Hydrometallurgical treatment of zinc silicate ore from Thailand[J]. Hydrometallurgy, 1996, 40: 37-49.
[4] Hua Y, Lin Z, Yah Z. Application of microwave irradiation to quick leach of zinc silicate ore[J]. Minerals Engineering, 2002, 15: 451-456.
[5] 李存兄, 魏昶, 樊刚, 等. 高硅氧化锌矿加压酸浸处理[J]. 中国有色金属学报, 2009, 19(9): 1678-1683.
LI Cun-xiong, WEI Chang, FAN Gang, et al. Pressure acid leaching of high silicon zinc oxide ore[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(9): 1678-1683.
[6] Santos F M F, Pina P S, Porcaro R, et al. The kinetics of zinc silicate leaching in sodium hydroxide[J]. Hydrometallurgy, 2010, 102: 43-49.
[7] CHEN Ai-liang, ZHAO Zhong-wei, JIA Xi-jun, et al. Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore[J]. Hydrometallurgy, 2009, 97: 228-232.
[8] ZHAO Zhong-wei, LONG Shuang, CHEN Ai-liang, et al. Mechanochemical leaching of refractory zinc silicate (hemimorphite) in alkaline solution[J]. Hydrometallurgy, 2009, 99: 255-258.
[9] Harvey T G. The hydrometallurgical extraction of zinc by ammonium carbonate: A review of the Schnabel process[J]. Mineral Processing and Extractive Metallurgy Review, 2006, 27: 231-279.
[10] 唐谟堂, 欧阳民. 硫铵法制取等级氧化锌[J]. 中国有色金属学报, 1998, 8(1): 118-121.
TANG Mo-tang, OUYANG Min. Preparation of grade zinc oxide by using ammonium sulfate and ammonia complex leaching process[J]. The Chinese Journal of Nonferrous Metals, 1998, 8(1): 118-121.
[11] 张保平, 唐谟堂, 杨声海. 氨法处理氧化锌矿制取电锌[J]. 中南工业大学学报:自然科学版, 2003, 34(6): 619-623.
ZHANG Bao-ping, TANG Mo-tang, YANG Sheng-hai. Treating zinc oxide ores using ammonia-ammonium chloride to produce electrolysis zinc[J]. Journal of Central South University of Technology: Natural Science, 2003, 34(6): 619-623.
[12] FENG Lin-yong, YANG Xian-wan, SHEN Qing-feng, et al. Pelletizing and alkaline leaching of powdery low grade zinc oxide ores[J]. Hydrometallurgy, 2007, 89: 305-310.
[13] 朱云, 胡汉, 苏云生, 等. 难选氧化锌矿氨浸动力学[J]. 过程工程学报, 2002, 2(1): 81-85.
ZHU Yun, HU Han, SU Yun-sheng, et al. Kinetics of leaching poorly-floated zinc-oxide-ore with ammonia[J]. The Chinese Journal of Process Engineering, 2002, 2(1): 81-85.
[14] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai, et al. Dissolution kinetics of smithsonite ore in ammonium chloride solution[J]. Hydrometallurgy, 2005, 80: 67-74.
[15] WANG Rui-xiang, TANG Mo-tang, YANG Sheng-hai, et al. Leaching kinetics of low grade zinc oxide ore in NH3-NH4Cl-H2O system[J]. Journal of Central South University of Technology, 2008, 15(5): 679-683.
[16] 刘晓丹, 张元福. 铵盐浸出氧化锌矿动力学的研究[J]. 贵州工业大学学报: 自然科学版, 2004, 33(2): 82-84.
LIU Xiao-dan, ZHANG Yuan-fu. Study on kinetics of ammonium salt leaching on znicite[J]. Journal of Guizhou University of Technology: Natural Science Edition. 2004, 33(2): 82-84.
[17] 刘智勇, 刘志宏, 曹志阎, 等. 硅锌矿在(NH4)2SO4-NH3-H2O体系中的浸出机理[J]. 中国有色金属学报, 2011, 21(11): 2929-2935.
LIU Zhi-yong, LIU Zhi-hong, CAO Zhi-yan, et al. Leaching mechanism of willemite in (NH4)2SO4-NH3-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(11): 2929-2935.
[18] Souza A D, Pina P S, Lima E V O, et al. Kinetic of sulphuric acid leaching of a zinc silicate calcine[J]. Hydrometallurgy, 2007, 89: 337-345.
[19] Ishida M, Wen C Y. Comparison of kinetic and diffusional models for solid-gas reactions[J]. AICHE Journal, 1968, 14(2): 311-317.
[20] Bhatia S K, Perlmutter D D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control[J]. AICHE Journal, 1980, 26(3): 379-386.
[21] Bhatia S K, Perlmutter D D. A random pore model for fluid-solid reactions: II. Diffusion and transport effects[J]. AICHE Journal, 1981, 27(2): 247-254.
[22] Petersen E E. Reaction of porous solids[J]. AICHE Journal, 1957, 3(4): 443-448.
[23] Szekely J, Evans J W. Studies in gas-solid reactions: Part I. A structural model for the reaction of porous oxides with a reducing gas[J]. Metallurgical and Materials Transactions B, 1971, 2(6): 1691-1698.
[24] Szekely J, Evans J W. Studies in gas-solid reactions: Part II. An experimental study of nickel oxide reduction with hydrogen[J]. Metallurgical and Materials Transactions B, 1971, 2(6): 1699-1710.
[25] Evans J W, Song S. A model for the design of gas-solid reactors[J]. Metallurgical and Materials Transactions B, 1973, 4(7): 1701-1707.
[26] Georgiou D, Papangelakis V G. Sulphuric acid pressure leaching of a limonitic laterite: chemistry and kinetics[J]. Hydrometallurgy, 1998, 49: 23-46.
收稿日期:2011-01-28;修回日期:2011-04-01
基金项目:国家重点基础研究发展计划(“973”计划)项目(2007CB613604)
通信作者:刘智勇(1981-),男,内蒙古赤峰人,博士研究生,从事冶金复杂物料处理及功能粉体材料制备的研究;电话:0731-88830478;E-mail:csuliuzhiyong@163.com
摘要:通过实验研究硅锌矿在(NH4)2SO4-NH3-H2O体系中高液固比下的浸出动力学。采用粒径为96~109 μm的纯硅锌矿样品,在液固质量比为200时,考察搅拌速度、反应温度及总氨浓度对锌浸出率的影响。研究结果表明:提高温度和总氨浓度能显著提高浸出速率,而当搅拌速度大于250 r/min时,其对浸出速率的影响较小;浸出过程遵循孔隙扩散控制粒子模型,扩散与化学反应并非仅发生于颗粒外表面,而是发生在整个外表面及孔隙内部,浸出速度受孔隙扩散控制;浸出过程的表观活化能与反应级数分别为71.35 kJ/mol和4.27。


