DOI: 10.11817/j.ysxb.1004.0609.2020-37544
粉末冶金制备Fe28Ni28Mn28Cr8Cu8与Fe28Ni28Mn28Cr8Al8高熵合金的微观组织与力学性能
夏泽邦,陈维平,蒋珍飞,付志强
(华南理工大学 广东省金属新材料制备与成形重点实验室,广州 510640)
摘 要:
采用“机械合金化(MA)+放电等离子烧结(SPS)”的方法制备出Fe28Ni28Mn28Cr8Cu8和Fe28Ni28Mn28Cr8Al8两种高熵合金块体,并研究其微观组织和力学性能。结果表明:两种高熵合金均在机械合金化后形成了FCC+BCC相的合金粉末。与Fe28Ni28Mn28Cr8Cu8合金相比较,当Cu元素被Al元素取代之后,Fe28Ni28Mn28Cr8Al8高熵合金粉末中BCC结构的固溶体相含量明显增加。经SPS烧结后,Fe28Ni28Mn28Cr8Cu8由单相FCC和少量富Cr相组成;Fe28Ni28Mn28Cr8Al8则形成双相FCC结构。同时,两种高熵合金在室温下均表现出良好的压缩性能;相较于Fe28Ni28Mn28Cr8Cu8,Fe28Ni28Mn28Cr8Al8的屈服强度由716 MPa提高到1181 MPa,抗压强度由1908 MPa提高为2111 MPa,硬度也由267 HV上升到482 HV,但塑性却由38.6%降至26.1%。
关键词:
文章编号:1004-0609(2020)-05-1049-08 中图分类号:TG113 文献标志码:A
人们对于金属材料的使用可以追溯到数千年以前,随着时间的发展,合金的种类也越来越多,例如铁合金、铝合金、镍合金、钛合金等,并在人类的日常活动中得到了广泛应用[1]。近百年来,随着科技的日新月异,传统的合金在某些领域已经很难满足人们对金属材料性能的要求。
2004年,台湾的叶均蔚教授打破传统合金设计理念的束缚,首次提出高熵合金这一全新的合金设计思路[2]。高熵合金最初的定义是指由五种或五种以上的元素,以介于5%到35%之间的摩尔分数组合而形成的合金系统。研究发现,高熵合金中的多主元特性引起的高混合熵效应、晶格畸变效应、迟缓扩散效应等赋予了高熵合金具有独特的固溶体相,促使高熵合金兼具结构材料和功能材料所需的众多独特的性能,然而这些性能在传统合金中通常难以实现。例如,高的强度和硬度、良好的耐磨性、优异的低温断裂韧性、耐蚀性以及抗高温软化和特殊的磁性能[5-13]等。因此,高熵合金被视为一种潜力巨大的新型结构材料与功能材料,在航空航天、汽车工业、机械制造以及冶金、化工等领域作为高强、耐磨、耐高温、耐蚀等材料具有广泛的应用前景,是近年来金属材料领域主要研究热点之一。但与传统合金类似,高强度与高韧塑性不可兼得,这是制约该材料工程应用的关键。
目前的研究表明面心立方固溶体 (FCC)结构的高熵合金具有良好的塑性;但是,该类高熵合金在强度通常较低(屈服强度≤400 MPa);通过合理的成分设计和采用适当的制备工艺对FCC高熵合金的微观组织结构进行调控,则可以获得综合力学性能优异的高熵合金结构材料[14-17]。文献[18-20]表明相较于传统的铸造法,采用机械合金化和放电等离子烧结工艺制备出的高熵合金晶粒更为细小,有利于大幅度提高FCC高熵合金的强度。另外,为降低合金的制造成本,选择了不含Co的FeNiMn系FCC高熵合金为基体。研究表明FeNiCoCuCr高熵合金为单相FCC结构,且具有优异的塑性[21]。因此,本研究选择Cr、Cu两种高熵合金中的常用元素添加到FeNiMn合金中从而设计出Fe28Ni28Mn28Cr8Cu8高熵合金。为降低合金的密度,选择将合金中的Cu元素用Al元素替代;另外,文献[22]表明在高熵合金中加入适量的Al元素,有助于提高合金的强度,但Al含量过高将会导致高熵合金中BCC相的形成,从而降低合金的塑性。因此,为避免在合金中生成BCC相,制备出综合性能良好的FCC系高熵合金,设计出成分为Fe28Ni28Mn28Cr8Al8高熵合金块体。
综上所述,本研究设计出两种低成本FCC高熵合金,采用“机械合金化+放电等离子烧结”制备相应的FCC块体合金,并研究了Al和Cu对该类FCC高熵合金的微观组织与力学性能的影响。
1 实验
本实验以质量分数≥99.9%、粉末粒度≤45μm的Fe、Ni、Mn、Cr、Cu和Al粉末为原料。按摩尔比配制Fe28Ni28Mn28Cr8Cu8和Fe28Ni28Mn28Cr8Al8高熵合金的粉末。为方便阅读,下文将Fe28Ni28Mn28Cr8Cu8简称为HEA1,Fe28Ni28Mn28Cr8Al8简称为HEA2。随后,将混合均匀的合金粉末进行球磨。球磨采用湿磨工艺,球料比为10:1,转速设定为300 r/min,整个过程在QM-3SP2 型行星式球磨机上进行。球磨过程中,使用环己烷作为过程控制剂,并且用氩气作为保护气体。分别对球磨0 h、20 h、40 h的粉末进行XRD测试。将完成机械合金化的合金粉末在真空中充分干燥,随后在Dr. Sinter825放电等离子设备上烧结成合金块体。整个SPS烧结过程大致为:首先,加热4 min将温度由室温升至600 ℃;接着,再经过4 min将温度升高到900 ℃;最后再用2 min加热到1000 ℃。并且在1000 ℃下保温8 min,整个烧结过程中保持恒压,压强为30 MPa,最终获得的合金块体尺寸为d 20 mm× 8 mm。后续将试样切割至所需尺寸,并打磨、抛光得到试样进行后续的测试。
不同球磨时间合金粉末以及烧结后的合金块体的XRD采用X射线衍射仪(XRD,Bruker-D8 Advance, Germany)进行检测。采用扫描电子显微镜(SEM, NOVA NANOSEM 430, USA)对合金块体的微观组织形貌进行观察,并利用能谱分析对合金中各区域、组织进行定性定量检测。合金块体维氏硬度的测定则采用HVS-1000型显微硬度计完成。维氏硬度的测试参数设置为:在载荷2.94 N下保压15 s,每个样品分别取10个点进行试验,去掉最大值、最小值后取平均值作为合金的硬度。将尺寸为d 3 mm×4.5 mm的压缩试样放置在万能力学试验机(AG-100NX, Shimadzu, Japan)上对合金的室温压缩性能进行测试。测试时压头下压的速率为0.27 mm/min,各成分合金分别测试三次,断后即停。
2 结果与分析
2.1 XRD谱分析
图1所示为球磨过程中不同阶段粉末的XRD谱。图1(a)所示为HEA1粉末的XRD谱。初始状态(0 h),HEA1中所有元素的XRD衍射峰均可以观察到。当球磨时间到达20 h时,可以看到元素峰的强度明显减弱,表明高熵合金粉末中各元素已部分合金化;球磨时间延长至40 h时,机械合金化完成,最终形成了FCC+ BCC 结构的高熵合金粉体,最终粉末衍射峰的宽度增加,表明球磨过后合金粉末的晶粒减小,晶格应变增大[23]。

图1 两种高熵合金元素粉末球磨不同时间的XRD谱
Fig. 1 XRD patterns of two high entropy alloys with different milling times
图1(b)所示为HEA2粉末的XRD谱。与HEA1的XRD谱类似,初始状态(0 h)时,各元素的衍射峰清晰可见。球磨20 h后,元素粉的衍射峰强度明显下降,甚至消失;湿磨40 h后,XRD衍射峰未发生变化,表明此时粉末已经完成机械合金化,最终形成了FCC+BCC相的高熵合金粉末。与HEA1不同的是,HEA2中BCC相粉末的比例较大,表明:当Cu元素被Al元素替代后,Al将会促进粉末中BCC相的形成。这主要是因为Al元素的原子半径要大于合金中其他元素的原子半径,造成较大的晶格畸变,而BCC相的致密度要低于FCC,有利于畸变能的释放[24];Cu则容易固溶于基体中,形成FCC相。因此,球磨之后HEA2中BCC相含量要高于HEA1中BCC相含量。
图2所示为经SPS烧结后HEA1和HEA2高熵合金块体的XRD图谱;其中HEA1形成了FCC+Cr7C3的组织, 而HEA2则为单一的FCC结构。显然两种高熵合金粉末在SPS烧结后都发生了相变,造成烧结后相变的主要原因是因为粉末的机械合金化是一个非平衡状态下的工艺过程,在球磨机的高速运转下,各元素粉末持续处于破碎和结合的循环中,这样剧烈的变形促进了粉末的合金化,同时形成了亚稳态的过饱和固溶体。此外,在球磨的过程中产生了大量的纳米晶,导致晶界体积分数增大,而在晶界处储存了大量的畸变能,降低了相变所需的自由能。最终,高熵合金粉末在SPS烧结成形的过程中,由于高温以及磁场、电场、等离子体场等多场效应的耦合作用下,使得亚稳态的BCC相的晶格发生重组,最终形成相对更加稳定的FCC结构。随后,采用排水法测得HEA1和HEA2块体的致密度分别为96.4%和97.3%。可见,两种高熵合金均达到了较高的致密度。
2.2 块体的相组成和微观组织
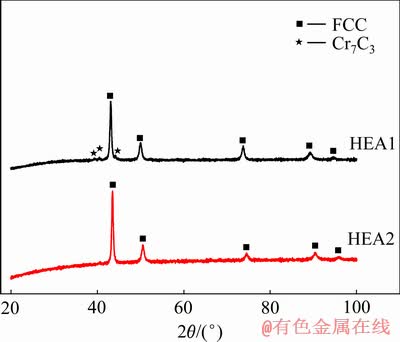
图2 两种高熵合金块体的XRD谱
Fig. 2 XRD patterns of two bulk high entropy alloys
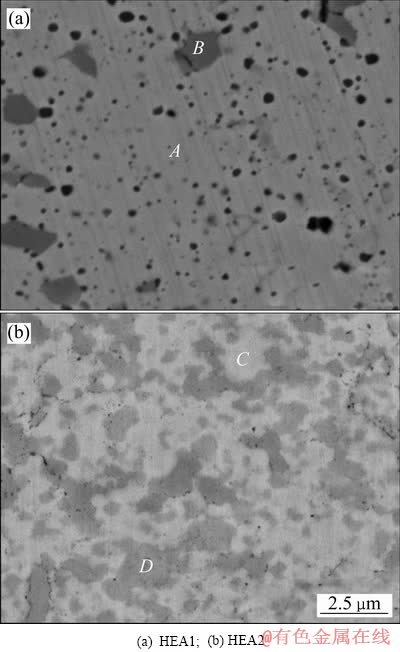
图3 两种高熵合金的BSE像
Fig. 3 BSE images of two high entropy alloys
两种高熵合金的背散射电子(BSE)照片如图3所示。从图3(a)中可以看到HEA1组织中元素的分布情况主要由大块的浅灰色区域A和分散的形状不规则的深灰色区域B组成。图3(b)所示为HEA2的高倍背散射电子图像,可以看到,与HEA1合金相似,HEA2的微观组织中元素的分布也大致分为两块区域,即浅色区域C和灰色区域D。与HEA1高熵合金不同的是,区域C和区域D都分布地较为均匀,即形成了双相结构。另外,可以看到两种高熵合金均形成了细小的晶粒,并且HEA2晶粒的粒径要明显小于HEA1的。两种高熵合金块体的能谱分析(EDS)结果列于表1中。另外,为方便理解,将两种高熵合金中各金属元素以及C元素两两间的混合焓均列于表2[25]。
可以看到在区域A中,除了Cr的含量较名义成分较低外,其它元素的比例均达到了预期,说明SPS烧结后形成了成分比较均匀的组织;在区域B中,Cr的含量明显高于名义成分,而在Cr富集的区域C元素的含量也明显增大,这说明在烧结过程在Cr、C两个元素发生了反应。产生这种富Cr相的原因主要是 因为Cr的熔点要高于HEA1中的其他元素,自扩散系数较低,在机械合金化过程中难以固溶到新相中,造成了Cr元素的偏聚;此外,在HEA1中,Fe、Ni、Mn三种元素等原子比时能够能形成FCC固溶体相[26],而Cu与Ni之间能够很好地发生固溶,根据表2,可知Cu与Cr之间的混合焓为较大的正值,使得Cr元素更加难以固溶,导致部分区域的Cr元素的摩尔分数要远高于名义成分,并与过程控制剂(环己烷)在高温下的分解产生的C元素反应。根据文献[27]可以知道在Fe、Ni、Mn、Cr、Cu各元素中形成Cr7C3所需的自由能最低,因此,Cr7C3的形成具有最大热力学动力。
表1 两种高熵合金EDS/BSE能谱分析结果
Table 1 EDS/BSE analysis results of two high entropy alloys

表2 各元素原子对的混合焓[25]
Table 2 Chemical mixing enthalpy of binary equiatomic alloys[25]

HEA2的能谱分析结果为:颜色较深的区域D中,Cr元素的含量较高,而Fe、Ni、Al这三种元素的摩尔分数要低于名义成分;在区域C中的元素的分布情况恰好与区域D相反。这主要是因为当Cu元素被Al元素替代后,由于Al和Cr之间的混合焓较负,结合能力较强,减少了Cr元素的偏聚,因此,降低了部分局域中Cr元素与C元素反应的可能,使得HEA2中只形成了FCC结构的相,并未发现Cr7C3的存在。同时,根据表2可以看到Mn元素与其他元素之间的混合焓都比较接近零,因此,Mn能与合金中的其它元素形成固溶体。
图4所示为两种高熵合金的TEM明场像以及衍射花样。图4(a)所示为HEA1合金的TEM明场像,图4(a′)所示为晶粒A沿[011]轴的衍射花样。可以确定晶粒A的晶体结构为FCC,计算得到其晶格常数为0.364 nm,与根据XRD谱计算得到的0.363 nm十分接近。HEA1合金的EDS/TEM结果如表3所列,结果表明在晶粒A中除Cr元素外,其他元素的含量均接近于名义成分;此外,在明场像照片中还观察到了少量的深色晶粒(图4(a)中B)以及微量的亮白色小颗粒(晶粒C)的存在,根据两者的EDS/TEM结果,可以确定晶粒B和晶粒C分别为Cr7C3和MnO。因此,HEA1合金中相组成为:主相为FCC相、存在少量的Cr7C3相以及微量的MnO,三种相的晶粒尺寸分别约为2.5 μm、1.5 μm和300 nm。图4(b)所示为HEA2高熵合金的TEM明场相照片及其衍射花样,可以看出HEA2合金的晶粒较HEA1更为细小,根据表3中HEA2合金的EDS/TEM的结果可以发现HEA2高熵合金中主要存在两种化学成分不同的相(FCC1和FCC2),结合明场相照片可以确定FCC1的晶粒尺寸约为500 nm,而FCC2的晶粒尺寸约为300 nm。两相的衍射花样分别如图4(b′)和(b″)所示。可以确定这两种相的晶体结构均为FCC。通过计算发现FCC1相和FCC2相的晶格常数分别为0.361 nm和0.360 nm,该结果与根据XRD计算HEA2中FCC相的晶格常数的结果(0.360 nm)十分接近。此外,如图4(b)中箭头所示,发现了微量亮白色的小颗粒,其EDS/TEM结果表明这些小颗粒为Al2O3。

图4 两种高熵合金的TEM像
Fig. 4 TEM images of two HEAs: Bright field image(a) of HEA1 and SAED pattern(a′) of grain A along [011] zone axis; Bright field image(b) of HEA2 and SAED patterns of FCC1 phase(b′) and FCC2 phase(b″) along [011] zone axis
表3 两种高熵合金EDS/TEM能谱分析结果
Table 3 EDS/TEM analysis results of two high entropy alloys

2.3 高熵合金的力学性能
对HEA1和HEA2两种高熵合金的室温压缩性能进行测试并测定两种高熵合金块体的维氏硬度。图5所示为两种高熵合金的室温压缩应力-应变曲线。从图5中可以看到,HEA1高熵合金的室温压缩屈服强度、断裂强度、断裂应变以及硬度各项力学性能参数分别为:716 MPa,1908 MPa,38.6%,267 HV;而HEA2高熵合金对应的各项性能指标分别为:1181 MPa,2111 MPa,26.1%,482 HV。将其他典型的FCC系高熵合金的室温压缩性能列在表4中。整体看来,HEA1与HEA2高熵合金均具有较高的强度和良好的塑性,这主要是由于两种都主要由FCC相组成,因此具有一定的塑性,同时,由于合金中的晶粒的尺寸较为细小,因此,两种高熵合金都具有较高的屈服强度。同时,相较于HEA1合金,HEA2高熵合金具有更高的强度,特别是屈服强度增加明显,但塑性略有降低。结合透射照片分析可知,造成这两种合金性能差别的最主要原因是由于晶粒的大小不同,HEA1主相的晶粒大小约为2.5 μm;而HEA2主相的平均晶粒大小不足500 nm(FCC1的粒径约为500 nm,而FCC2的粒径约为300 nm),从而使得HEA2晶界强化更加明显,最终HEA2的屈服强度较高且塑性略低。
表4 高熵合金室温下的力学性能
Table 4 Mechanical properties of HEAs at room temperature

图5 两种高熵合金的室温压缩应力-应变曲线
Fig. 5 Compressive strain-stress curves of two high entropy alloys at room temperature
3 结论
1) HEA1与HEA2高熵合金粉末均在球磨40 h后完成了合金化,并且都形成了FCC结构和BCC结构的固溶体相。但根据XRD谱,在含有Al元素的HEA2合金粉末中,BCC结构固溶体相的比例要明显高于HEA1中的BCC相比例。
2) 在经过1000 ℃的SPS烧结后,HEA1与HEA2合金粉末中亚稳态的BCC相均发生了相变转变为更为稳定的FCC相。其中HEA1形成了FCC+Cr7C3相结构,而HEA2高熵合金的微观组织则为明显的两相结构,分别为富FeNiAl相和富Cr相。
3) 根据微观组织的照片可以观察到两种合金的晶粒都较为细小,细晶强化效果显著,使得HEA1和HEA2都表现出高的压缩强度和良好的压缩塑性。此外,由于HEA2的晶粒较HEA1的更为细小,并且Cu元素被原子半径较大的Al元素所代替,具有更明显的细晶强化和固溶强化作用,从而使得HEA2合金的强度要远高于HEA1的,但塑性却有所下降。
REFERENCES
[1] 安继儒, 田龙刚. 金属材料手册[M]. 北京: 化学工业出版社, 2008: 46-936.
AN Ji-ru, TIAN Long-gang. Handbook of metal materials[M]. Beijing: Chemical Industry Press, 2008: 46-936.
[2] YEHJ W, CHEN S K, LIN S J, CHIN T S, SHUN T T, TSAU C H, CHANG S Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes[J]. Advanced Engineering Materials, 2004, 6(5): 299-303.
[3] CANTOR B, CHANG I T H, KNIGHT P, VINCENT A J B. Microstructural development in equiatomic multicomponent alloys[J]. Materials Science and Engineering A, 2004, 375/377: 213-218.
[4] CHEN M R, LIN S J, YEH J W, CHEN S K, HUANG Y S, TU C P. Microstructure and properties of Al0.5CoCrCuFeNiTix (x=0-2.0) high-entropy alloys[J]. Materials Transactions, 2006, 47(5): 1395-1401.
[5] DAN S G, BHATTACHARJEE P P, TSAI C W, YEH J W. Effect of heavy cryo-rolling on the evolution of microstructure and texture during annealing of equiatomic CoCrFeMnNi high entropy alloy[J]. Intermetallics, 2016, 69: 1-9.
[6] LI Z M, PRADEEP K G, DENG Y, RAABE D, TASAN C C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off[J]. Nature, 2016, 534: 227-230.
[7] GLUDOVATZ B, HOHENWARTER A, CATOOR D, CHANG E H, GEORGE E P, RITCHIE R O. A fracture-resistant high-entropy alloy for cryogenic applications[J]. Science, 2014, 345: 1153-1158.
[8] YANG T, ZHAO Y L, TONG Y, JIAO Z B, WEI J, CAI J X, HAN X D, CHEN D, HU A. Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys[J]. Science, 2018, 362: 933-937.
[9] QIU X W. Corrosion behavior of Al2CrFeCoxCuNiTi high-entropy alloy coating inalkaline solution and salt solution[J]. Results in Physics, 2019, 12: 1737-1741.
[10] YAO Y G, HUANG Z N, XIE P F, STEVEN D L, ROHIT J J. Carbothermal shock synthesis of high-entropy-alloy nanoparticles[J]. Science, 2018, 359: 1489-1494.
[11] FU Z Q, CHEN W P, WEN H M, ZHANG D L, CHEN Z, ZHENG B L, ZHOU Y Z, LAVERNIA E J. Microstructure and strengthening mechanisms in an FCC structured single-phase nanocrystalline Co25Ni25Fe25Al7.5Cu17.5 high-entropy alloy[J]. Acta Mater, 2016, 107: 59-71.
[12] LEI Z F, LIU X J, WU Y, WANG H, JIANG S H, WANG S D, HUI X D. Enhanced strength and ductility in a high-entropy alloy via order edoxygen complexes[J]. Nature, 2018, 563: 546-550.
[13] BHARAT G, STEPHANE G, DEEP C, ZHENG Yu-feng, RAJIV S M, RAJARSHI B. Tensile yield strength of a single bulk Al0.3CoCrFeNi high entropy alloy can be tuned from 160 MPa to 1800 MPa[J]. Scripta Materialia, 2019, 162:18-23.
[14] STEPANOV N D, SHAYSULTANOV D G, CHERNICHENKO R S, TIKHONOVSKY M A, ZHEREBTSOV S V. Effect of Al on structure and mechanical properties of Fe-Mn-Cr-Ni-Al non-equiatomic high entropy alloys with high Fe content[J]. Journal of Alloys and Compounds, 2019, 770: 194-203.
[15] PRAVEEN S, MURTY B S, KOTTADA R S. Alloying behavior in multi-component AlCoCrCuFe and NiCoCrCuFe high entropy alloys[J]. Materials Science and Engineering A, 2012, 534: 83-89.
[16] KILMAMETOV A, KULAGIN R, MAZILKINA, SEILS S, BOLL T, HEILMAIER M, HAHN H. High-pressure torsion driven mechanical alloying of CoCrFeMnNi high entropy alloy[J]. Scripta Materialia, 2019, 158: 29-33.
[17] CHENG Hu, XIE Yan-chong, TANG Qun-hua, RAO Cong, DAI Pin-qiang. Microstructure and mechanical properties of FeCoCrNiMn high-entropy alloy produced by mechanical alloying and vacuum hot pressing sintering[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(7): 1360-1367.
[18] FU Zhi-qiang, CHEN Wei-ping, WEN Hai-ming, Sam Morgan, CHEN Fei, ZHENG Bao-long, ZHOU Yi-zhang, ZHANG Lian-meng, Enrique JLavernia. Microstructure and mechanical behavior of a novel Co20Ni20Fe20Al20Ti20 alloy fabricated by mechanical alloying and spark plasma sintering[J]. Materials Science and Engineering A, 2015, 644: 10-16.
[19] RIZALDY M P, BHARAT G, JUNHO L, TALUKDER A, HWANG J Y, HO J R, RAJARSHI B, SOON H H. Microstructures and mechanical properties of mechanically alloyed and spark plasma sintered Al0.3CoCrFeMnNi high entropy alloy[J]. Materials Chemistry and Physics, 2018, 210: 62-70.
[20] VAIDYA M, ANIRUDHA K, MARSHAL A, PRADEEP K G, MURTY B S. Phase evolution and stability of nanocrystalline CoCrFeNi and CoCrFeMnNi high entropy alloys[J]. Journal of Alloys and Compounds, 2019, 770: 1004-1015.
[21] WANG P, CAI H N, CHENG X W. Effect of Ni/Cr ratio on phase, microstructure and mechanical properties of NixCoCuFeCr2-x (x=1.0, 1.2, 1.5, 1.8 mol) high entropy alloys[J]. Journal of Alloys and Compounds, 2015, 662: 20-31.
[22] WANG W R, WANG W L, WANG S C, TSAI Y C, LAI C H, YEH J W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys[J]. Intermetallics, 2012, 26: 44-51.
[23] SCHUH B, MENDEZ M F, VOLKER B, GEORGE E P, CLEMENS H, PIPPAN R, HOHENWARTER A. Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation[J]. Acta Materialia, 2015, 96: 258-268.
[24] WANG F J. Atomic packing efficiency and phase transition in a high entropy alloy[J]. Journal of Alloys and Compounds, 2009, 478: 321-324.
[25] TAKEUCHI A, INOUE A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element[J]. Materials Transactions, 2005, 46 (12): 2817-2829.
[26] WU Z, BEI H, OTTO F, PHARR G M, GEORGE E P. Recovery, recrystallization, grain growth and phase stability of a family of FCC-structured multi-component equiatomic solid solution alloys[J]. Intermetallics, 2014, 46: 131-140.
[27] VAIDYA M, ANIRUDHA K, MARSHAL A, PRADEEP K G, MURTY B S. Phase evolution and stability of nanocrystalline CoCrFeNi and CoCrFeMnNi high entropy alloys[J]. Journal of Alloys and Compounds, 2019, 770: 1004-1015.
[28] WU B Y, CHEN W P, JIANG Z F, CHEN Z, FU Z Q. Influence of Ti addition on microstructure and mechanical behavior of a FCC-based Fe30Ni30Co30Mn10 alloy[J]. Materials Science and Engineering A, 2016, 676: 492-500.
[29] SHUN T, CHANG L, SHIU M. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys[J]. Materials Characterization, 2012: 63-67.
[30] WANG X F, ZHANG Y, QIAO Y, CHEN G L. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys[J]. Intermetallics, 2007, 15: 357-362.
Microstructure and mechanical properties of Fe28Ni28Mn28Cr8Cu8 and Fe28Ni28Mn28Cr8Al8 high entropy alloys prepared by powder metallurgy
XIA Ze-bang, CHEN Wei-ping, JIANG Zhen-fei, FU Zhi-qiang
(Guangdong Key Laboratory for Advanced Metallic Materials Processing, South China University of Technology, Guangzhou 510640, China)
Abstract: Fe28Ni28Mn28Cr8Cu8 and Fe28Ni28Mn28Cr8Al8 high-entropy alloys (HEAs) were prepared by mechanical alloying (MA) and spark plasma sintering (SPS), and their microstructure and mechanical properties were studied in detail. The results show that a combination of FCC+BCC structure phases forms after MA in both HEAs. Compared with Fe28Ni28Mn28Cr8Cu8, the content of BCC-structured solid solution phase increases evidently in Al-containing Fe28Ni28Mn28Cr8Al8. After SPS sintering, one FCC and a small amount of Cr-rich phase can be observed in the bulk Fe28Ni28Mn28Cr8Cu8 HEA, while Fe28Ni28Mn28Cr8Al8 has two FCC phases. Additionally, both HEAs exhibit good compressive properties at room temperature. However, compared with Fe28Ni28Mn28Cr8Cu8, the Al-containing Fe28Ni28Mn28Cr8Al8 shows a higher strength with a lower plasticity. Compared with Fe28Ni28Mn28Cr8Cu8, the yield strength of Fe28Ni28Mn28Cr8Al8 increases from 716 MPa to 1181 MPa, the compressive strength increases from 1908 MPa to 2111 MPa, and the hardness increases from 267 HV to 482 HV, respectively, but the plasticity decreases from 38.6% to 26.1%.
Key words: high entropy alloy; mechanical alloying; spark plasma sintering; microstructure; mechanical property
Foundation item: Project(GJ201601) supported by Guangdong Key Laboratory for Advanced Metallic Materials Processing, China
Received date: 2019-03-26; Accepted date: 2020-04-28
Corresponding author: CHEN Wei-ping; Tel: +86-20-87113832; E-mail: mewpchen@scut.edu.cn
(编辑 王 超)
基金项目:广东省金属新材料制备与成形重点实验室开放课题(GJ201601)
收稿日期:2019-03-26;修订日期:2020-04-28
通信作者:陈维平,教授,博士;电话:020-87113832;E-mail:mewpchen@scut.edu.cn
摘 要:采用“机械合金化(MA)+放电等离子烧结(SPS)”的方法制备出Fe28Ni28Mn28Cr8Cu8和Fe28Ni28Mn28Cr8Al8两种高熵合金块体,并研究其微观组织和力学性能。结果表明:两种高熵合金均在机械合金化后形成了FCC+BCC相的合金粉末。与Fe28Ni28Mn28Cr8Cu8合金相比较,当Cu元素被Al元素取代之后,Fe28Ni28Mn28Cr8Al8高熵合金粉末中BCC结构的固溶体相含量明显增加。经SPS烧结后,Fe28Ni28Mn28Cr8Cu8由单相FCC和少量富Cr相组成;Fe28Ni28Mn28Cr8Al8则形成双相FCC结构。同时,两种高熵合金在室温下均表现出良好的压缩性能;相较于Fe28Ni28Mn28Cr8Cu8,Fe28Ni28Mn28Cr8Al8的屈服强度由716 MPa提高到1181 MPa,抗压强度由1908 MPa提高为2111 MPa,硬度也由267 HV上升到482 HV,但塑性却由38.6%降至26.1%。


