Trans. Nonferrous Met. Soc. China 29(2019) 2141-2150
Preparation and properties of C/C-ZrB2-SiC composites by high-solid-loading slurry impregnation and polymer infiltration and pyrolysis (PIP)
Li-ping RAN, Fei RAO, Ke PENG, Huan YIN, Mao-zhong YI
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 5 September 2018; accepted 1 July 2019
Abstract:
Ultrahigh-temperature ceramics were added to C/C composites to meet their application requirement in a high-temperature oxidizing environment. C/C-ZrB2-SiC composites were fabricated by high-solid-loading slurry impregnation with polymer infiltration and pyrolysis. The dispersion and rheological behavior of ZrB2 slurry and the microstructural, mechanical, and ablation properties of the C/C-ZrB2-SiC composites were investigated. Results indicated that a well-dispersed and low-viscosity ZrB2 slurry was obtained using 0.40 wt.% polyethyleneimine as a dispersant at pH 5. Ceramics were uniformly distributed in the short-cut fiber layer and needle-punched area. The flexural strength of the C/C-ZrB2-SiC composites was 309.30 MPa. The composites exhibited satisfactory ablation resistance under the oxyacetylene flame of 2500 °C, and the mass and linear ablation rates were 0.40 mg/s and 0.91 μm/s, respectively. A continuous and compact ZrO2 layer, which could effectively reduce the diffusion rate of oxygen and protect the composites from being ablated, was formed.
Key words:
C/C-ZrB2-SiC composites; microstructure; mechanical properties; ablation properties;
1 Introduction
Carbon/carbon (C/C) composites have attracted considerable attention for aerospace applications because of their superior characteristics, such as low density, high-temperature strength, low thermal expansion coefficient, high thermal conductivity, and excellent thermal shock resistance [1-3]. However, the oxidation and ablation resistances of C/C composites should be improved, because carbon can be easily oxidized at temperatures over 450 °C under aerobic conditions, which restricts the application of C/C composites in aerospace fields [4]. With the development of advanced space vehicles, the materials applied in hypersonic vehicles must be capable of prolonging operation in oxidizing atmospheres above 2000 °C [5]. The current thermal protection systems, such as the C/C-SiC composites, hardly meet this requirement, because the protecting silica layer becomes active under such temperatures [6]. Research has demonstrated that the introduction of ultrahigh-temperature ceramics (UHTCs), such as ZrC, ZrB2, HfC, and HfB2, into C/C composites is an effective approach for improving the oxidation and ablation resistances at high temperatures [7-10]. Among these UHTCs, ZrB2 ceramics have attracted considerable attention because of their high melting point (3250 °C), high hardness, good thermal shock and ablation resistance, and oxidation products (ZrO2) with high melting point [11,12]. However, research has proven that the addition of SiC can improve the mechanical properties and oxidation resistance of ZrB2 composites [13]. Molten SiO2 bonded with ZrO2 helps seal defects, such as cracks and pores, during ablation at an ultra-high temperature after oxidation in the high-temperature aerobic environment [14]. Therefore, the antioxidation ablation performance of C/C composites can be improved considerably by introducing ZrB2-SiC composite ceramics into the matrix.
The technique of polymer infiltration and pyrolysis (PIP) and alternately stacked layers of ZrB2 powders have been used to introduce ZrB2 into C/C or C/C-SiC composites [15,16]. Nevertheless, the former has long total cycle time and high cost, whereas the latter has a limited amount of ZrB2. Slurry infiltration (SI) has been favored by researchers due to its unique advantages of short preparation cycle and simple operation. Distilled water and ceramics were utilized by TANG et al [17] to prepare a slurry, and the properties of composites relied on the capability of the air pump and the gravity of the slurry to introduce ZrB2 particles into a preform. However, even with pressure assist technique, the UHTC particles were mainly concentrated in the preform from the surface to a 2 mm depth. ZrB2 powders, polycarbosilane (PCS), and divinyl benzene were made into the slurry by HU et al [18], and the slurry was pressed into a preform by high pressure. Nevertheless, powders accumulated on the preform surface. The conventional SI technique has the problems of poor fluidity and wettability of the slurry, which cause the powders to accumulate on the preform surface easily. No open literature was reported on the preparation of C/C-ZrB2-SiC composites by slurry impregnation with high solid loading and without high pressure.
Therefore, in this work, a new method of high-solid-loading slurry impregnation (HSLSI) was used in our research to distribute ZrB2 evenly in a preform and increase the ZrB2 content as much as possible. Ethanol was used to improve the wettability between ZrB2 slurry and C/C composites, and the slurry with high solid loading and low viscosity was prepared and impregnated into the preform with atmospheric pressure. The as-fabricated composites were densified by PIP. Finally, the microstructural, mechanical, and ablation properties of C/C-ZrB2-SiC composites were investigated.
2 Experimental
2.1 Raw materials and preparation
T300 PAN-based carbon fibers (Toray, Japan) were used to fabricate integrated needled felts. The felts with a density of 0.45 g/cm3 were prepared by alternate 0° nonwoven carbon fiber cloth, short-cut fiber webs, and 90° nonwoven carbon fiber cloth by a needle-punching technique. The felts were densified to 0.75 g/cm3 by chemical vapor infiltration. The C/C-ZrB2-SiC composites were fabricated via three steps. Firstly, the ZrB2 slurry was prepared. Commercially available ZrB2 powders (1 μm, 99.90% purity), ethanol, and deionized water were used as starting materials, and poly- ethyleneimine (PEI, Aladdin Co., Ltd., Shanghai, China) was used as a dispersant. The wettability between carbon-based materials with water is poor, but it can be effectively improved by hydrophilic groups (—OH) [19]. All raw materials were mixed based on a certain proportion and then milled in a planetary ball for 2 h to form a homogeneous slurry of 40 vol.% solid loading. Typical slurry compositions consisted of 60.85 g ZrB2 powders, 0.24 g PEI, 12.51 g deionized water, and 1.75 g ethanol. The pH values of the suspensions were adjusted by using HCl and ammonia water (NH3·H2O). Secondly, the ZrB2 slurry was directly impregnated into the preform by capillary force and gravity, and no powders accumulated on the preform surface. After the impregnation process, the preform was dried in an air dry oven at 120 °C for 10 h. The put-on mass was compared with that in the theory of density of ZrB2, and its volume content was determined. Thirdly, PCS with a ceramic yield of approximately 65 wt.% was dissolved in n-hexane with a mass ratio of 1:1 and used as the precursor for SiC during PIP. The C/C-ZrB2-SiC composites were obtained by eight cycles of PCS through the PIP process, and the pyrolysis of the PCS was conducted at 1400 °C for 1 h in an atmosphere of flowing argon.
2.2 Sample characterization
Zeta potential measurements of ZrB2 in the absence and presence of PEI were conducted separately as a function of pH value (in the range of 2-11) via a zeta potential analyzer (Zetasizer Nano ZS, Malvern, UK) by using dilute suspensions (0.01 vol.%). The rheological behavior of the ZrB2 suspensions was determined using a rotational viscometer (AR2000EX, TA instrument, USA).
The bulk density and open porosity of the C/C-ZrB2-SiC composites were obtained through the Archimedes method. The phases were analyzed using an X-ray diffraction (XRD) analyzer (Rigaku Ltd., Japan; Cu Kα radiation). Flexural strength was evaluated by a three-point bending test (Instron-3369) with a span of 50 mm and a loading speed of 0.50 mm/min using bars with dimensions of 60 mm × 5 mm × 4 mm. Over five samples were measured for the test. The microstructure and morphology of the composites were characterized using a scanning electron microscope (SEM, FEI CO, NOVA Nano230) equipped with an energy-dispersive spectrometer (EDS).
Cylindrical samples (d30 mm × 10 mm) for ablation test were cut from the as-prepared C/C–ZrB2–SiC composites. The ablation test equipment consisted of an oxyacetylene gun, a manual control cabinet, two gas tanks, and several rubber pipes. The oxyacetylene gun tip with an inner diameter of 2 mm was perpendicular to the sample at 20 mm. During the test, the flow rates of oxygen and acetylene were 1.960 and 0.696 L/s, respectively; and the pressures of oxygen and acetylene were 0.4 and 0.095 MPa, respectively [20]. As detected by an optical pyrometer, the surface temperature of the sample center reached 2500 °C. The ablation duration was set to be 120 s. The linear and mass ablation rates of the tested samples were calculated using Eqs. (1) and (2), respectively:
Rl=(d0-d1)/t (1)
Rm=(m0-m1)/t (2)
where Rl is the linear ablation rate; d0 and d1 are the thickness values of samples in the center region before and after ablation, respectively; Rm is the mass ablation rate; m0 and m1 are the masses of the samples before and after ablation, respectively; t is the ablation time. A widely used C/C composite with a density of 1.82 g/cm3 was also investigated for comparison to evaluate the ablation property of the C/C-ZrB2-SiC composites. The final ablation rates of the composites are the average for five samples.
3 Results and discussion
3.1 Dispersion and rheological behavior of aqueous ZrB2 suspensions
Slurry with uniform dispersion, good stability, and low viscosity is an important factor for ensuring the uniform distribution of ceramics in the C/C preform. Otherwise, ceramics in the slurry can be easily separated from deionized water, which leads to the deposition of the ceramics on the C/C preform surface. In the slurry, the interaction between particles is mainly divided into two types, namely, the mutual attraction by van der Waals forces and the repulsive effect of electrostatic interactions, steric interactions, and other forces. Zeta potential is often used as a criterion for surface charge, because it is close to the stern potential and can be measured [21]. The zeta potential reflects the electrostatic dispersion effect. The dispersion of powders in the suspensions is good when the absolute value of the zeta potential (measured in mV) is high.
Figure 1(a) shows the zeta potential curves of ZrB2 powders in deionized water in the absence and presence of PEI. Under the condition that no PEI was added, the isoelectric point, which is the pH with zero net charge on particle surface, of ZrB2 powders was approximately 6. The absolute value of zeta potential for pristine ZrB2 reached the maximum at a pH value of approximately 10, and the absolute maximum was approximately 30.10 mV. However, the absolute maximum zeta potential for ZrB2 suspension was obtained when 0.40 wt.% PEI was used as dispersant at a pH value of 5, and the absolute maximum was 67.10 mV, which increased to 122.90% higher than that for pristine ZrB2 suspension. These results showed that the addition of a proper amount of PEI could significantly improve the dispersion effect of ZrB2 powders, and this phenomenon can be explained by two main reasons. On one hand, PEI, as a polymer dispersant with a branched chemical structure [22], may exhibit a remarkable steric stabilization behavior. On the other hand, PEI has a high
positive charge density that allows negatively charged substrates to be absorbed tightly, resulting in high electrostatic force in a suspension [18]. Excessively high or low concentrations of dispersants are not beneficial to the dispersion of particles in deionized water. When the concentration of PEI was lower than 0.40 wt.%, less surface of particles was covered with PEI, which decreased the electrostatic repulsion between particles and increased their aggregation. When the concentration of PEI was higher than 0.40 wt.%, the dispersants of particle surface adsorption were oversaturated, which not only increased the ionic strength of the solution and compressed the double-charge layer, resulting in the electrostatic repulsion decrease between particles, but also increased the bridging flocculation because of polyelectrolyte macromolecule [21]. In summary, selecting a suitable dispersant concentration is important to achieve good dispersibility of particles.

Fig. 1 Zeta potential curves of ZrB2 in the absence and presence of PEI (a) and viscosity of ZrB2 suspension versus PEI concentration (b)
Figure 1(b) shows the curves for the rheological property of ZrB2 aqueous suspensions (at 40 vol.% solid loading) with different concentrations of PEI. The rheological property of the ZrB2 aqueous suspensions strongly depended on the added contents of PEI. At the dispersant concentration of 0.40 wt.%, the shear rate of 100 s-1 and pH 5, the viscosity of the slurry was 17.42 mPa·s. The viscosity of the dispersant concentration below or above 0.40 wt.% was higher than that at dispersant concentration of 0.40 wt.%. When the concentration of the dispersant was less than 0.40 wt.%, it could not be steadily adsorbed on the powder surface, and the zeta potential of ZrB2 particles was insufficient to establish a stable suspension, thereby increasing the viscosity of the suspension. When the dispersant concentration was higher than 0.40 wt.%, the over- saturated adsorption of PEI increased the ionic strength of the solution and compressed the double-charge layer, which decreased the electrostatic repulsion between particles [23]. The 0.40 wt.% PEI as dispersant appeared to be the optimum dispersant concentration to disperse ZrB2 powder, which induced the minimum viscosity value and maximum zeta potential. Thus, the influence of dispersant on the rheological property is significant. A suitable dispersant concentration would reduce the viscosity of suspensions.
3.2 Phase composition and microstructure
Figure 2(a) presents the cross-section morphology of the C/C preform. The preform was composed of four typical structural units: 90° non-woven layer, 0° non-woven layer, short-cut fiber web layer, and needle-punched fibers. The nonwoven layer had small pores, and some of them were in fiber bundles. The pores of the short-cut fiber web layer and needle-punched fibers were large, which provided the channels for slurry. After the HSLSI process, ZrB2 phases (with bright contrast due to containing heavy element Zr) were mainly distributed in the short-cut fiber web layer and needle-punched area (Fig. 2(b)), and only a small amount of ZrB2 was present in the intrabundle matrix (Fig. 2(c)). As shown in Fig. 2(d), the ZrB2 ceramics were loosely accumulated around the fiber, and the fiber was higher than the ceramic plane. Thus, the ceramics were partially detached during polishing due to the simple accumulation.
Figure 3 shows the density change of the composite during preparation. The bulk density and open porosity of the initial porous C/C preform were 0.75 g/cm3 and 59.60 vol.%, respectively. After SI, the density and open porosity of the C/C-ZrB2 preform were 2.15 g/cm3 and 36.30 vol.%, respectively. Approximately 23 vol.% of ZrB2 was introduced into the preform through the HSLSI process. The ZrB2 volume fraction of the C/C-ZrB2-SiC composite is higher than that reported in Refs. [24,25]. Therefore, a large amount of ZrB2 particles could be introduced into the composites through the HSLSI process. After the PIP process (eight cycles), the density of the C/C–ZrB2–SiC composite increased to 2.88 g/cm3, and the open porosity decreased to 7.10%.
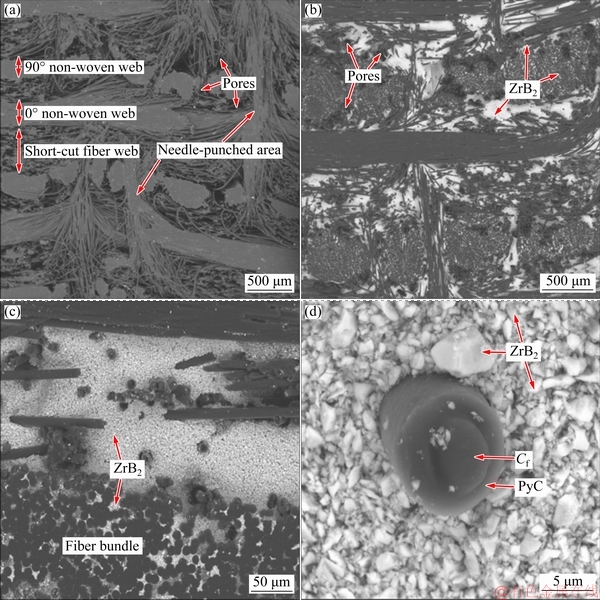
Fig. 2 Cross-section morphologies of C/C preform (a) and C/C-ZrB2 preform (b, c) and PyC and ZrB2 around carbon fiber (d)
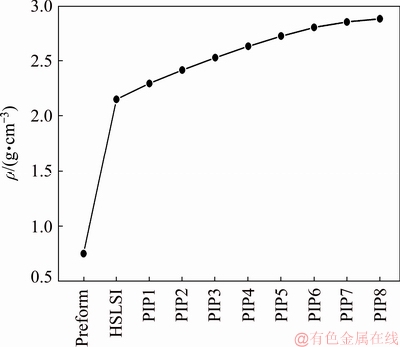
Fig. 3 Density change of composite during preparation
An XRD test was performed on the prepared C/C-ZrB2-SiC composites. The XRD analysis (Fig. 4) showed that the C/C-ZrB2-SiC composites were composed of carbon (C), ZrB2, and SiC, indicating that PCS was converted into SiC after heat treatment.
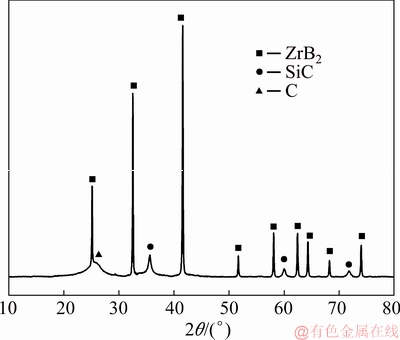
Fig. 4 XRD pattern of C/C-ZrB2-SiC composites
Figure 5(a) displays BSE image of a typical cross- section of the C/C-ZrB2-SiC composites. In comparison with Fig. 2(b), Fig. 5(a) demonstrates that the composites were further densified, and the pores were significantly reduced. A large amount of carbon fibers existed in each bundle, and the gaps between carbon fibers in the intrabundle matrix were only several micrometers, which was close to the size of ZrB2 particles. Therefore, in the HSLSI process, when the slurry flowed into the large pores in the preforms the particles on the outer surface of the fiber bundles would clog the intrabundle pores and prevent further particle ingress. In the subsequent process of PIP densification, most of the pores in the fiber bundles were filled with PCS-derived SiC (Fig. 5(b)), because the liquid formed by the mixing of PCS and n-hexane had good liquidity and could efficiently flow into the fiber bundles. The EDS analysis results confirmed the phases with gray contrast to be SiC. As shown in Fig. 5(c), the ceramics were distributed uniformly and compactly around the carbon fiber (Cf), and the ZrB2 powder had hardly fallen off in the polishing process under the fixed effect of SiC. The magnified graphs showed that PyC was surrounded by a thin layer of SiC (Fig. 5(c)). This phenomenon could be due to the irregular ZrB2 particles, which made covering the PyC surface completely difficult, whereas the PCS introduced by the PIP process could efficiently enter the gap between the PyC and ZrB2 particles due to good fluidity. The pyrolysis process could obtain a thin SiC layer, which could effectively protect PyC and carbon fibers.

Fig. 5 BSE images of cross-section of C/C-ZrB2-SiC composites
3.3 Mechanical properties
Table 1 summarizes the mechanical properties of C/C and C/C-ZrB2-SiC composites. The average flexural strength of the five specimens used in the tests of C/C-ZrB2-SiC composites was 309.30 MPa, which was 146% higher than that of C/C composites (125.60 MPa). Good mechanical properties of carbon fiber-reinforced composites are mainly dependent on the fiber–matrix or fiber–matrix–filler interfacial adhesion, because the load transfer from the matrix to the fibers would require a good bonding at the interfaces [26]. After C/C composites were infiltrated ZrB2 and SiC, the interfacial surface areas between the bundles and layers were increased, a strong bonding between the ceramics and PyC was observed, and the low porosity benefitted the load transfer to the fibers, which improved the mechanical properties.
Table 1 Mechanical properties of C/C and C/C–ZrB2–SiC composites

Figure 6 shows the load–displacement curve of the C/C-ZrB2-SiC composite. The sample exhibited a similar typical non-brittle fracture behavior. Before reaching the matrix-cracking stress, the C/C-ZrB2-SiC composite presented elastic deformation. However, with the increase in stress, the C/C-ZrB2-SiC composite displayed inelastic deformation behavior, indicating that multi-cracking occurred in the matrix [27]. When the loading reached the maximum that the material could bear, the load suddenly decreased to a certain extent, and the material failed. This mechanical behavior implied that the carbon fibers improved the toughness of ceramics and prevented catastrophic fracture.
Figure 7 presents the microscopic fracture profiles of the C/C-ZrB2-SiC composites. The fracture surface of the C/C-ZrB2-SiC composites was coarse, and the composites had some fibers pulled out (Fig. 7(a)). The fracture morphology demonstrated that ZrB2 ceramics could completely permeate the preform and distribute evenly in the matrix through the HSLSI process. The carbon fibers were not damaged in the HSLSI combined with the PIP process. Several holes were retained after the fibers were pulled out (Fig. 7(b)). When the composite was under load, the fibers were pulled out to avoid sudden fracture and improve the toughness of the composite (Fig. 7(c)). Figure 7(d) shows that the carbon fibers were pulled out, some of the fibers were separated from the PyC, whereas the PyC was closely bound to the ceramic matrix. Thus, the bonding between the ceramic matrix and PyC interphase was stronger than that of fibers and PyC interphase. The combination of the fracture morphology and the load–displacement curve of C/C-ZrB2-SiC composites confirmed the nonbrittle fracture behavior.

Fig. 6 Load–displacement curve of C/C-ZrB2-SiC composite
3.4 Ablation morphology and ablation behavior
Table 2 summarizes the results of the ablation properties of the C/C and C/C-ZrB2-SiC composites. After being exposed to oxyacetylene torch at 2500 °C for 120 s, the C/C composites with a density of 1.82 g/cm3 and the C/C-ZrB2-SiC composites had mass ablation rates of 5.76 and 0.40 mg/s, respectively. After adding ZrB2 and SiC ceramics into porous C/C composites, the order of magnitude was decreased by one. The linear ablation rates of C/C with a density of 1.82 g/cm3 and the C/C-ZrB2-SiC composites were 6.08 and 0.91 μm/s, respectively. Such results suggested that the co-addition of ZrB2 and SiC could play a positive role in improving the ablation resistance of C/C composites.
Figure 8 presents the surface morphologies of the C/C-ZrB2-SiC composites before and after ablation. Figure 8(a) shows the macromorphology of the C/C-ZrB2-SiC composites before ablation. This image proves that smooth and compact composites could be produced by the mixed process. Figure 8(b) exhibits the macrograph of the C/C-ZrB2-SiC composites after the ablation test at 2500 °C for 120 s. The specimens were efficiently protected after ablation, and the surface of the center ablation zone was formed with a relatively dense white layer and good adhesion to the substrate, without evident ablation pit and fiber denudation. The ablated sample surface was mainly divided into three regions, namely, center ablation (A), transition erosion (B), and brim oxidation (C). The brim of the ablated surface barely changed and exhibited better erosion than the central region, because the thermal gradient between the center and borders of the specimen rendered the ablation rate nonuniformly throughout the surface.

Fig. 7 Fracture morphologies of C/C-ZrB2-SiC composites
Table 2 Ablation properties of C/C and C/C-ZrB2-SiC composites

The reaction products of the sample center formed after the ablation test were characterized using XRD, as shown in Fig. 9. The results indicated that the white molten layer only consisted of ZrO2 for the oxidation of ZrB2 ceramics. ZrO2 was stable at 2500 °C because of its high melting temperature (2715 °C) considering the possible reaction products. However, at approximately 2500 °C, the SiO2 evaporated rapidly and was blown away by the high-speed oxyacetylene torch in the center ablation region [28]. An unusually low melting point of 450 °C and a high vapor pressure of B2O3, which vaporized rapidly at temperatures above 1100 °C, were considered [29]. The oxidation products of SiC and B2O3 were inadequately stable in the flame tip region; thus, no SiO2 and B2O3 peaks were observed in the XRD pattern.
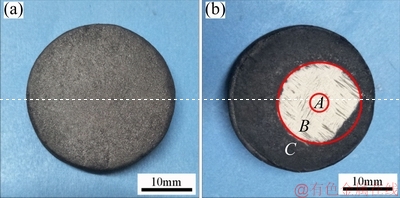
Fig. 8 Macrographs of C/C-ZrB2-SiC composite before (a) and after (b) ablation

Fig. 9 XRD pattern of C/C-ZrB2-SiC composites at ablation center
Figures 10(a-c) display the BSE images of micro- structures and EDS analysis of the center, transition, and brim ablation regions of C/C-ZrB2-SiC composites, respectively. At the ablation center of the specimen, the ablation surface covered a relatively dense oxide layer with minimal pores. The EDS analysis showed that the white particles were ZrO2 phase, which was consistent with the previous XRD results. The reason for this phenomenon was the oxidation of ZrB2 and SiC ceramic particles. Their oxides (SiO2 and B2O3) almost evaporated at such ultrahigh temperature in the initial stage of the ablation and were the main contributors to the increasing mass ablation rate and generated porosity. However, the ZrO2 particles adhered to the sample surface to form a continuous oxidation protective layer that could prevent further intrusion of oxygen and the oxidation of internal matrix. Ablation is a complex process that includes thermal, physical, chemical, and mechanical erosion under high temperature. In this work, the temperature of the center ablation area was approximately 2500 °C. Figure 10(b) presents the micro- topography of region B in Fig. 8(b), which shows the ablated surface covered with rough and porous white and gray glass phases. The EDS analysis showed that the mixture layer consisted of SiO2 and ZrO2, which could prevent the sample from being ablated further. The temperature in the transition oxidation region was lower than that at the ablation center. A relatively small amount of ZrO2 was formed, and most silicon oxides and the evaporation of B2O3 formed numerous holes. Figure 10(c) shows that the brim oxidation region was covered with a gray coating with irregular grains ranging from 10 to 50 μm. The EDS results proved that the ablation product was Si-Zr-O phase, and Si was the main element. Few ZrB2 was oxidized because the temperature of the ablation area was lower than that of the transition zone. The formation of this continuous layer effectively inhibited the inward transport of oxygen and prevented the direct exposure of the C/C-ZrB2-SiC composites to air in this temperature region.

Fig. 10 BSE images and EDS analysis of C/C-ZrB2-SiC composites in center (a), transition (b), and brim ablation (c) regions
During ablation, the surface of the center region suffered the highest thermal and pressure attacks. Reactions in the center region may include as follows:
2C(s)+O2(g)=2CO(g) (3)
C(s)+O2(g)=CO2(g) (4)
2SiC(s)+3O2(g)=2SiO2(l)+2CO(g) (5)
SiC(s)+O2(g)=SiO(g)+CO(g) (6)
SiO2(l)+CO(g)=SiO(g)+CO2(g) (7)
SiO2(l)=SiO2(g) (8)
2ZrB2(s)+5O2(g)=2ZrO2(s)+2B2O3(g) (9)
Figure 11 shows the center ablation schematic diagram for C/C-ZrB2-SiC composites. When the ablation began, the surface temperature of the sample increased rapidly, and the oxidative gases reacted with carbon fibers, carbon matrix, SiC, and ZrB2 (Eqs. (3)-(7) and (9)). Carbon was oxidized into CO (Eq. (3)) and CO2 (Eq. (4)), whereas the solid SiC was oxidized into liquid SiO2 (Eq. (5)). However, the ultrahigh temperature of the ablation center and the intense erosion of the flame resulted in the evaporation of low-viscosity SiO2 (Eq. (8)). As the temperature increased, the oxidation of SiC became highly active (Eq. (7)) and the gases of SiO and CO formed [30,31]. During ablation, the evaporation of gas produced by the oxidation of SiC and ZrB2 absorbed heat from the flame, and the continuous layer of ZrO2 acted as a barrier to prevent oxygen from further oxidation. Thus, the ablation resistance of C/C composites could be improved by the co-addition of ZrB2 and SiC.
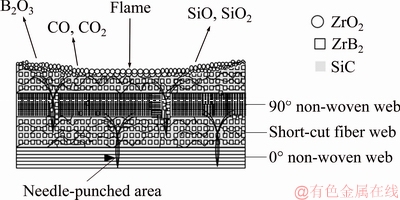
Fig. 11 Center ablation schematic diagram for C/C-ZrB2-SiC composites
4 Conclusions
(1) A ZrB2 slurry with a high solid loading up to 40 vol.% and low viscosity (17.42 MPa·s at 100 s-1) was prepared when the pH was 5 and the dispersant concentration was 0.40 wt.%.
(2) C/C-ZrB2-SiC composites were successfully prepared by combining HSLSI with PIP. After ZrB2 and SiC infiltration, the composites had a bulk density of 2.88 g/cm3 and an open porosity of 7.10%. The particles were evenly distributed in the regions of short-cut fiber web layer and needle-punched area.
(3) The flexural strength and ablation properties of the C/C-ZrB2-SiC composites were considerably higher than those of C/C composites. After ZrB2 and SiC infiltration, the flexural strength of the C/C composites increased from 125.60 to 309.30 MPa, and the linear and mass ablation rates decreased from 6.08 to 0.91 μm/s and from 5.76 to 0.40 mg/s, respectively.
(4) The surface of the C/C-ZrB2-SiC composites was covered by a continuous integrated ZrO2 layer, which acted as effective oxygen species and heat flux barrier, thereby protecting the composites against further ablation.
References
[1] FU Qian-gang, ZHANG Jia-ping, ZHANG Zheng-zhong, LI He-jun, SUN Can. SiC-MoSi2/ZrO2-MoSi2 coating to protect C/C composites against oxidation [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 2113-2117.
[2] LI Ke-zhi, SHEN Xue-tao, LI He-jun, ZHANG Shou-yang, FENG Tao, ZHANG Lei-lei. Ablation of the carbon/carbon composite nozzle-throats in a small solid rocket motor [J]. Carbon, 2011, 49: 1208-1215.
[3] LI Jun, YANG Xin, SU Zhe-an, XUE Liang, ZHONG Ping, LI Shuai-peng, HUANG Qi-zhong, LIU Hong-wei. Effect of ZrC-SiC content on microstructure ablation properties of C/C composites [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 2653-2664.
[4] YANG Xin, SU Zhe-an, HUANG Qi-zhong, CHAI Li-yuan. Preparation and oxidation resistance of mullite/SiC coating for carbon materials at 1150 °C [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2997-3002.
[5] LI Lu-lu, WANG Yi-guang, CHENG Lai-fei, ZHANG Li-tong. Preparation and properties of 2D C/SiC-ZrB2-TaC composites [J]. Ceramic International, 2011, 37: 891-896.
[6] LI Hou-bu, ZHANG Li-tong, CHENG Lai-fei, WANG Yi-guang. Ablation resistance of different coating structures for C/ZrB2-SiC composites under oxyacetylene torch flame [J]. International Journal of Applied Ceramic Technology, 2009, 6: 145-150.
[7] LIU Chun-xuan, CHEN Jian-xun, SU Zhe-an, YANG Xin, CAO Liu-xu, HUANG Qing-zhong. Pyrolysis mechanism of ZrC precursor and fabrication of C/C-ZrC composites by precursor infiltration and pyrolysis [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 1779-1784.
[8] JAYASEELAN D D, de GUIMARAES S R, BROWN P, LEE W E. Reactive infiltration processing (RIP) of ultra high temperature ceramics (UHTC) into porous C/C composite tubes [J]. Journal of the European Ceramic Society, 2011, 31: 361-368.
[9] XUE Liang, SU Zhe-an, YANG Xin, HUANG Dong, YIN Teng, LIU Chun-xuan, HUANG Qi-zhong. Microstructure and ablation behavior of C/C-HfC composites prepared by precursor infiltration and pyrolysis [J]. Corrosion Science, 2015, 94: 165-170.
[10] ZHANG Jia-ping, FU Qian-gang, WANG Le. Preparation, ablation behavior and thermal retardant ability of C/C-HfB2-SiC composites [J]. Materials & Design, 2017, 132: 552-558.
[11] ZHU Su-min, FAHRENHOLTZ W G, HILMAS G E. Enhanced densification and mechanical properties of ZrB2-SiC processed by a preceramic polymer coating route [J]. Scripta Materialia, 2008, 59: 123-126.
[12] TONG Yong-gang, BAI Shu-xin, YE Yi-cong, ZHANG Hong, YANG Zhen-huan. Reactive melt infiltration of a ZrB2 modified C/ZrC composite by a eutectic Zr-B alloy [J]. Materials Letters, 2015, 138: 208-211.
[13] HU Ping, GUI Kai-xuan, HONG Wen-hu, ZHANG Xing-hong. Preparation of ZrB2-SiC ceramics by single-step and optimized two-step hot pressing using nanosized ZrB2 powders [J]. Materials Letters, 2017, 200: 14-17.
[14] GAO Dong, ZHANG Yue, XU Chun-lai, SONG Yang, SHI Xiao-bin. Formation mechanism of zircon phase in ZrB2-SiC ceramic composites during oxidation [J]. Journal of Inorganic Materials, 2011, 26: 433-437.
[15] LI He-jun, YAO Xi-yuan, ZHANG Yu-lei, LI Ke-zhi, GUO Ling-jun, LIU Lei. Effect of heat flux on ablation behaviour and mechanism of C/C-ZrB2-SiC composite under oxyacetylene torch flame [J]. Corrosion Science, 2013, 74: 265-270.
[16] TANG Su-fang, DENG Jing-yi, WANG Shi-jun, LIU Wen-chuan. Comparison of thermal and ablation behaviors of C/SiC composites and C/ZrB2-SiC composites [J]. Corrosion Science, 2009, 51: 54-61.
[17] TANG Su-fang, DENG Jing-yi, LIU Wen-chuan, YANG Ke. Mechanical and ablation properties of 2D-carbon/carbon composites pre-infiltrated with a SiC filler [J]. Carbon, 2006, 44: 2877-2882.
[18] HU Hai-feng, WANG Qi-kun, CHEN Zhao-hui, ZHANG Chang-rui, ZHANG Yu-di, WANG Jun. Preparation and characterization of C/SiC-ZrB2 composites by precursor infiltration and pyrolysis process [J]. Ceramic International, 2010, 36: 1011-1016.
[19] HE Zhang-xin, JIANG Ying-qiao, LI Yue-hua, ZHU Jing, ZHOU Hui-zhu, MENG Wei, WANG Ling, DAI Lei. Carbon layer-exfoliated, wettability-enhanced, SO3H-functionalized carbon paper: A superior positive electrode for vanadium redox flow battery [J]. Carbon, 2017, 127: 297-304.
[20] WANG Di-ni, ZENG Yi, XIONG Xiang, LI Guo-dong, CHEN Zhao-ke, SUN Wei, WANG Ya-lei. Preparation and ablation properties of ZrB2-SiC protective laminae for carbon/carbon composites [J]. Ceramic International, 2014, 40: 14215-14222.
[21] HE Ru-jie, HU Ping, ZHANG Xing-hong, HAN Wen-bo, WEI Chun-cheng, HOU Yang. Preparation of high solid loading, low viscosity ZrB2-SiC aqueous suspensions using PEI as dispersant [J]. Ceramic International, 2013, 39: 2267-2274.
[22] ONG B C, LEONG Y K, CHEN S B. Interparticle forces in spherical monodispersed silica dispersions: Effects of branched poly- ethylenimine and molecular weight [J]. Journal ofColloid and InterfaceScience, 2009, 337: 24-31.
[23] HE Ru-jie, HU Ping, ZHANG Xing-hong, LIU Chen. Gelcasting of complex-shaped ZrB2-SiC ultra high temperature [J]. Materials Science and Engineering A, 2012, 556: 494-499.
[24] FAN Qian-guo, CUI Hong, YAN Lian-sheng, ZHANG Qiang, MENG Xiang-li, YANG Xing. Ablation resistance properties of Ultra–high temperature composites C/C-SiC-ZrB2 by slurry impregnation method [J]. Journal of Inorganic Materials, 2013, 28: 1014-1018.
[25] TONG Chang-qing, CHENG Lai-fei, LIU Yong-sheng, ZHANG Li-tong. Ablation performance of 2D C/SiC-ZrB2 composites [J]. Chinese Journal of Aeronautics, 2012, 32: 69-74.
[26] PARK S J, CHO M S, LEE J R, PAK P K. Influence of molybdenum disilicide filler on carbon–carbon composites [J]. Carbon, 1999, 37: 1685-1689.
[27] MEI Hui, CHEN Ting, ZHANG Ding, CHENG Lai-fei. Oxidation degradation and mechanical reduction on C/SiC composites with artificial notch defects [J]. Ceramic International, 2018, 44: 13873-13878.
[28] LIU Yue, FU Qian-gang, WANG Bei-bei, LIU Tian-yu, SUN Jia. The ablation behavior and mechanical property of C/C-SiC-ZrB2 composites fabricated by reactive melt infiltration [J]. Ceramic International, 2017, 43: 6138-6147.
[29] TRIPP W C, GRAHAM H C. Thermogravimetric study of the oxidation of ZrB2 in the temperature range of 800 °C to 1500 °C [J]. Journal of the Electrochemical Society,1971, 118: 1195-1199.
[30] HAN Jie-cai, HU Ping, ZHANG Xing-hong, MENG Song-he, HAN Wen-bo. Oxidation–resistant ZrB2-SiC composites at 2200 °C [J]. Composites Science and Technology, 2008, 68: 799-806.
[31] ZHOU Shan-bao, LI Wei-jie, HU Ping, HONG Chang-qing, WENG Ling. Ablation behavior of ZrB2-SiC-ZrO2 ceramic composites by means of the oxyacetylene torch [J]. Corrosion Science, 2009, 51: 2071-2079.
高固相含量浆料浸渍结合PIP法制备C/C–ZrB2–SiC复合材料及其性能
冉丽萍,饶 菲,彭 可,尹 欢,易茂中
中南大学 粉末冶金国家重点实验室,长沙 410083
摘 要:往C/C复合材料中加入超高温陶瓷,以满足其在高温有氧环境中的应用。采用高固相含量浆料浸渍结合前驱体浸渍裂解工艺制备C/C-ZrB2-SiC复合材料,研究ZrB2浆料的分散性和流变行为,以及C/C-ZrB2-SiC复合材料的显微组织、力学性能和烧蚀性能。结果表明:硼化锆浆料在分散剂聚乙烯胺含量为0.40 wt.%、pH值为5的条件下具有良好的分散性和较低的黏度。陶瓷相均匀地分布在C/C复合材料的网胎层和针刺区; C/C-ZrB2- SiC复合材料的抗弯强度为309.30 MPa,其在2500 °C氧乙炔火焰烧蚀条件下显示出优异的烧蚀性能,质量烧蚀率和线烧蚀率分别为0.40 mg/s和0.91 μm/s。此外,在烧蚀过程中,复合材料表面形成连续、致密的二氧化锆层,能有效地降低氧气向材料内部的扩散速率,对复合材料形成良好的保护。
关键词:C/C-ZrB2-SiC复合材料;显微组织;力学性能;烧蚀性能
(Edited by Wei-ping CHEN)
Foundation item: Project (GFZX0101040101-2012C20X) supported by the National Basic Research Program of China; Project (2017JJ2320) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: Ke PENG; Tel: +86-731-88879422; E-mail: pengkecsu@csu.edu.cn
DOI: 10.1016/S1003-6326(19)65120-4
Abstract: Ultrahigh-temperature ceramics were added to C/C composites to meet their application requirement in a high-temperature oxidizing environment. C/C-ZrB2-SiC composites were fabricated by high-solid-loading slurry impregnation with polymer infiltration and pyrolysis. The dispersion and rheological behavior of ZrB2 slurry and the microstructural, mechanical, and ablation properties of the C/C-ZrB2-SiC composites were investigated. Results indicated that a well-dispersed and low-viscosity ZrB2 slurry was obtained using 0.40 wt.% polyethyleneimine as a dispersant at pH 5. Ceramics were uniformly distributed in the short-cut fiber layer and needle-punched area. The flexural strength of the C/C-ZrB2-SiC composites was 309.30 MPa. The composites exhibited satisfactory ablation resistance under the oxyacetylene flame of 2500 °C, and the mass and linear ablation rates were 0.40 mg/s and 0.91 μm/s, respectively. A continuous and compact ZrO2 layer, which could effectively reduce the diffusion rate of oxygen and protect the composites from being ablated, was formed.


