DOI:10.19476/j.ysxb.1004.0609.2017.04.015
LaFeO3复合氧化物的制备与红外辐射性能
刘庆生,游 拯,国 辉,曾少军
(江西理工大学 冶金与化学工程学院,赣州 341000)
摘 要:
采用溶胶-凝胶法制备钙钛矿型LaFe1-x-yMgxNiyO3的化合物,并利用TG-DTA、XRD、SEM和XPS等对其进行分析和表征。结果表明:掺杂样品在0.2~2 μm波段的红外发射率较未掺杂的LaFeO3的红外发射率大幅度提高,其原因在于,掺杂离子取代铁离子进入LaFeO3晶格中,为保持化合物电价平衡,部分Fe3+转变为Fe4+,引入杂质能级,氧空位浓度增加,促进杂质能级吸收和氧空位吸收;掺杂引起的晶格畸变使得晶格振动吸收增强;此外,电子在Fe3+和Fe4+之间的极化跃迁,亦极大地提高掺杂铁酸镧的红外辐射性能。
关键词:
文章编号:1004-0609(2017)-04-0781-08 中图分类号:O614.33 文献标志码:A
红外辐射材料是一种新型的功能材料,因其具有光热转化功能,所以在节能、环保、工业加热、医疗等领域得到广泛应用[1]。目前,工业上推广应用的红外辐射材料主要是非氧化物陶瓷,如SiC和SiB6[2-3],但是这类材料的抗氧化性能较差,无法在高温氧化环境下稳定存在。
近年来,越来越多的研究者致力于氧化物体系红外辐射节能材料的开发[4–6]。LIU等[4]制备了Nd3+掺杂的六铝酸盐陶瓷(LaMgAl11O19),其近红外波段(3~5 μm)的发射率为0.7~0.8左右;LU等[5]制备了铁酸盐基非晶涂层,其近红外波段(3~5 μm)的发射率为0.84左右。然而,总体而言,氧化物陶瓷在近红外波段的辐射性能尚不如非氧化物陶瓷。因此,研发兼具高发射率和抗氧化性的材料显得十分重要[7-8]。
LaFeO3是一种钙钛矿(ABO3)型复合氧化物,其熔点为1900 ℃,具有良好的高温热稳定性[9],由于其物理、化学性质优异,在固体电解液、固体氧化物燃料电池、电化学器件、传感器等多领域有着广泛的应用[9–12],但其红外辐射性能及相关应用却鲜见报道[13–16]。根据半导体光吸收理论,向基质中引入适当的杂质,形成对应于近红外波段的杂质能级,能够增强半导体在相应波段的吸收率。基尔霍夫热辐射定律指出,热平衡条件下物体在同一波段的吸收率等于其发射率。因此,通过对LaFeO3进行适当的化学掺杂,能够提高其近红外波段的辐射性能。本文作者研究发现,通过向LaFeO3基质中引入镁离子、镍离子杂质,大幅提高了其近红外波段的发射率。这表明掺杂的LaFeO3有望发展成为一种兼具高发射率、耐高温、抗氧化等综合性能的新一代红外辐射节能材料。
1 实验
1.1 样品的制备
采用溶胶–凝胶法制备4种组成不同的LaFe1-x-yMgxNiyO3粉体。以La(NO3)3·nH2O(分析纯)、Mg(NO3)2(分析纯)、Fe(NO3)3·9H2O(分析纯)、Ni(NO3)2·6H2O(分析纯)为原料,按化学计量比配料,溶解在去离子水中,加入一定的柠檬酸络合剂。将所得混合液置于80 ℃的磁力搅拌器中恒温搅拌使之成为溶胶,溶胶经陈化形成凝胶。将凝胶移入100 ℃恒温干燥箱中预处理10 h,再用研钵将胶状物研细,随后将其放入马弗炉中,1300 ℃煅烧2 h,最后制备出钙钛矿型LaFeO3、LaFe2/3Mg1/3O3、LaFe2/3Ni1/3O3、LaFe1/3Mg1/3Ni1/3O3粉末。
1.2 表征
利用同步热分析仪(TG–DTA;Setsys Evolution 18型,法国生产)对LaFe1-x-yMgxNiyO3样品的反应合成过程进行差热分析。利用X 射线粉末衍射仪(XRD;Cu Kα,步长0.02°,扫描速度6 (°)/min,D8 Focus,德国生产)分析样品的物相组成。利用扫描电子显微镜(Hitachi S-4300型,日本生产)分析样品的显微结构和形貌。红外光谱分析仪(NEXUS-870型,美国生产)分析样品的红外光学特性。利用X射线光电子能谱仪(XPS;Al Kα,hν=1486.71 eV,英国生产)分析样品中各元素的价态变化。利用紫外-可见-近红外分光光度计(UV-VIS-NI;Cary5000型,加拿大生产)及其附带的积分球测试样品的光谱吸收率,以此为基础,利用图解积分法计算样品在0.2~2 μm波段的红外发射率[17]。
2 结果与讨论
2.1 热重与差热分析
图1所示为各样品的TG–DTA曲线。由图1可知,4条DTA曲线上60 ℃左右均有一个吸热峰,可能是样品中凝胶熔融吸热所致。在300~700 ℃之间,TG曲线有大量的质量损失现象出现,各样品的质量损失率分别为15.81 %、42.46 %、35.86 %和56.99 %,这
是样品在加热过程中,有机物发生分解燃烧,产生了水和CO2,并放出大量的热量所致,与此同时,各样品逐渐由无定型转变为钙钛矿结构。在700 ℃以后继续升温,TG曲线和DTA曲线均无明显变化,这表明已经形成完整的钙钛矿结构。
2.2 样品的物相分析
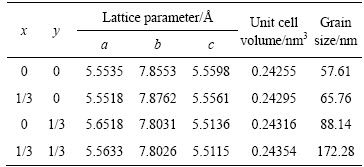
图2所示为各样品的XRD谱,各样品均可以通过标准衍射卡片JCPDS 37–1493进行指标化,标定为正方钙钛矿结构,空间群Pn˙a(62)。在LaFe2/3Mg1/3O3、LaFe2/3Ni1/3O3、LaFe1/3Ni1/3Mg1/3O3的XRD谱中未观察到NiO、MgO或其他杂相,表明大部分镍离子、镁离子通过掺杂进入了LaFeO3晶格内。较之LaFeO3,LaFe2/3Mg1/3O3的衍射峰并未发生偏移,但其峰展宽,峰强减弱,粒径增大;而LaFe2/3Ni1/3O3的衍射峰向高角度发生偏移,其半峰宽窄,说明其结晶良好,粒径亦增大;LaFe1/3Ni1/3Mg1/3O3的衍射峰有劈裂现象,并向高角度偏移,其粒径亦较大。利用Jade6.0分析其晶胞参数并结合Scherrer公式:
 (1)
(1)
式中: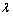 为X射线波长;K=0.89;
为X射线波长;K=0.89;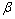 为衍射峰半高宽;
为衍射峰半高宽; 为衍射角。利用式(1)计算其晶粒尺寸,如表1所列。
为衍射角。利用式(1)计算其晶粒尺寸,如表1所列。
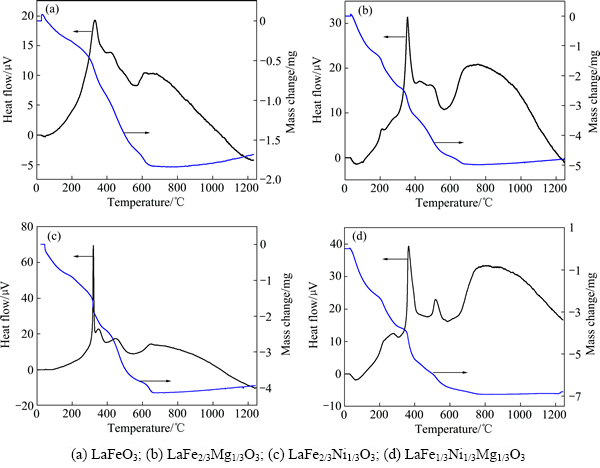
图1 样品的TG-DTA曲线
Fig. 1 TG-DTA curves of samples
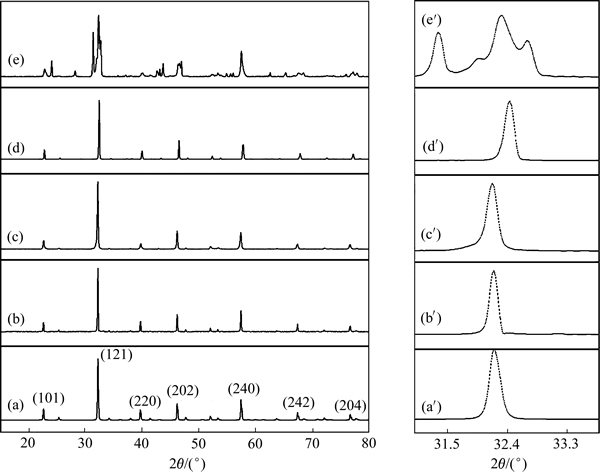
图2 JCPDS 37–1493,LaFeO3,LaFe2/3Mg1/3O3,LaFe2/3Ni1/3O3,LaFe1/3Ni1/3Mg1/3O3的XRD谱
Fig. 2 XRD patterns of JCPDS 37–1493((a), (a′)), LaFeO3((b), (b′)), LaFe2/3Mg1/3O3((c), (c′)), LaFe2/3Ni1/3O3((d). (d′)), LaFe1/3Ni1/3Mg1/3O3((e), (e′))
对于镍元素,理论上Ni2+(0.069 nm)、Ni3+(0.060 nm)都可以进入LaFeO3的晶胞内(晶胞参数见表1);LaFe2/3Ni1/3O3、LaFe1/3Ni1/3Mg1/3O3的粒径均大于未掺杂LaFeO3的,可推出镍元素是以+2价进入铁酸镧晶格的。
掺杂会导致LaFeO3的晶体结构发生畸变,晶格参数和晶胞体积随之改变(见表1)。Mg2+、Ni2+半径与Fe3+不同,分别部分取代Fe3+的晶格位置,在一定程度上会破坏LaFeO3晶格的对称性,产生晶格畸变,从而增强了晶格的振动活性,改变了分子振动与转动能级状态,从而促进了晶格的振动吸收,使中远红外辐射性能得以提升[18];由于不同价态掺杂使得部分Fe3+生成Fe4+,价态的改变有利于形成氧空穴,氧空穴浓度的增加使得近红外波段辐射率得以强化;Mg2+、Ni2+共掺杂亦是如此。
表1 LaFe1-x-yMgxNiyO3晶胞参数
Table 1 LaFe1-x-yMgxNiyO3 lattice parameters
2.3 样品的微观形貌分析
图3(a)、(b)、(c)、(d)所示分别为LaFeO3、LaFe2/3Mg1/3O3、LaFe2/3Ni1/3O3、LaFe1/3Ni1/3Mg1/3O3的SEM像。从图3可以看出,在未掺杂时,LaFeO3呈现无规则的球状,分散不均匀且团聚现象严重;引入掺杂元素之后,LaFeO3形貌明显改变,颗粒呈椭球状。
2.4 样品的光学性能分析
图4所示为样品的红外光谱。由于样品在1400~4000 cm-1的吸收峰主要为空气中水和CO2的吸收峰,故图中只给出了样品在400~1400 cm-1的红外光谱。从图4可以看出,红外光谱中波数在400和600 cm-1附近,分别为BO6八面体结构中Fe—O键的弯曲振动和伸缩振动[19]。掺杂之后,各样品的Fe—O键的伸缩振动频率均向高频移动,主要是由掺杂离子导致铁酸镧的Fe—O的键强增强所致。同时本实验中还观察到各样品的Fe—O键附近出现了一些振动,这些吸收带是晶格不对称振动引起的。
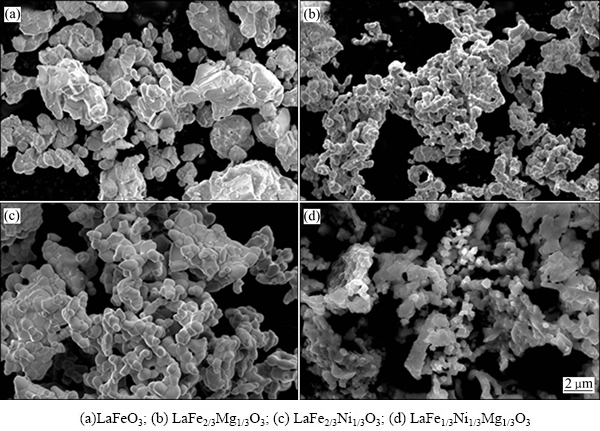
图3 样品的SEM像
Fig. 3 SEM images of samples

图4 样品的红外光谱
Fig. 4 IR spectra of samples
2.5 XPS分析
图5所示为样品中Fe 2p的XPS谱。LaFeO3样品中铁离子由+3和+2两种价态组成。结合图1中的TG-DTA分析可以推断,LaFeO3样品在高温煅烧过程中存在氧原子逸出现象,形成了氧空位,Fe3+捕获氧空位所释放的电子从而被还原成为Fe2+[20-21]。WANG等[22]认为,这种Fe2+和氧空位共存的现象在铁酸盐钙钛矿中是普遍存在的。
较之未掺杂的铁酸镧,掺杂之后(见图5(b)、(c)、(d)),其Fe 2p的结合能位置发生不同程度的偏移,这是由于掺杂离子以及不同的化学环境造成的。在引入杂质离子之后,样品中铁离子的化合价以+3和+4价为主。这是由于在掺杂过程中,杂质离子取代Fe3+进入LaFeO3晶格中,会在结构中形成正电荷缺陷,结合XRD分析可知,这是由于不同价态取代,使得部分Fe3+变为Fe4+,为保持化合物电中性,晶格中有氧空位产生。相应的缺陷反应如下:
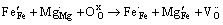 (2)
(2)
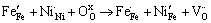 (3)
(3)
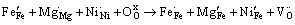 (4)
(4)
式中:缺陷均采用Krger-Vink符号表示;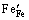 、
、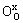 分别代表正常格位上的Fe3+和O2-离子;
分别代表正常格位上的Fe3+和O2-离子; 表示Fe4+离子;
表示Fe4+离子; 、
、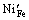 分别表示在Fe格位上的Mg2+、Ni2+离子;
分别表示在Fe格位上的Mg2+、Ni2+离子; 表示氧空位。
表示氧空位。
氧空位浓度增加有利于提高样品的红外辐射性能[23-24]。
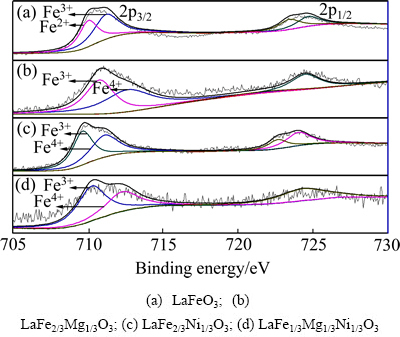
图5 样品的Fe 2p的XPS谱
Fig. 5 Fe 2p XPS spectra of samples
图6所示为样品中O 1s的XPS谱。4个样品中的氧均以晶格氧和吸附氧存在。晶格氧的结合能为528.90~529.89 eV,吸附氧的结合能为531.35~532.28 eV。样品吸附氧与晶格氧的比值分别为0.46、1.58、1.28和0.94,这说明掺杂之后样品的吸附氧与晶格氧的比值明显增大,吸附氧含量增加,有助于提高样品的吸收率[25]。
2.6 样品的光谱分析
图7给出了样品的光谱吸收率。LaFeO3的吸收率在200~500 nm波段大致稳定,在波长大于500 nm的波段大幅下降,吸收边约为600 nm。根据ARIMA等[26]的分析结果,LaFeO3的本征吸收边为590 nm,比较于该实验数据,本实验的结果与其较为一致。由图7可以看出,分别掺杂了Mg、Ni元素以及Mg、Ni共掺杂的铁酸镧样品在200~2000 nm波段范围内吸收率均稳定在0.95左右,远高于未掺杂LaFeO3的。这表明通过掺杂Mg2+、Ni2+显著提高了LaFeO3的吸收率,其中Mg2+–Ni2+共掺杂的铁酸镧吸收率最高;同时,掺杂后各试样的吸收边红移至波长为2000 nm的光谱区域。出现此现象的原因是掺杂使LaFeO3价带中氧空位浓度增加,带内吸收增强,在一定程度上提高了吸收率[27-28]。
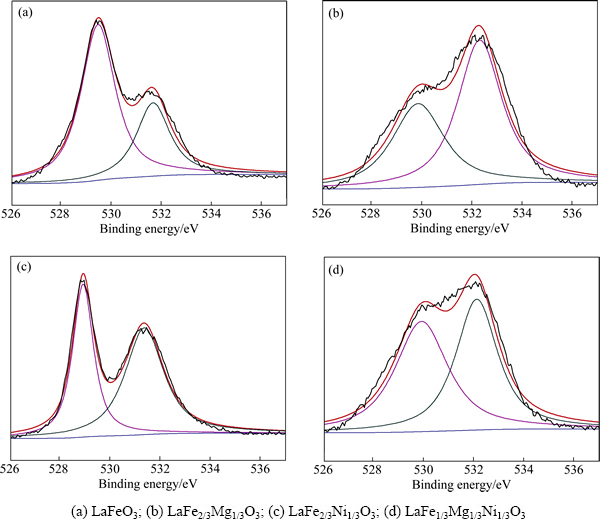
图6 样品的O 1s的XPS谱
Fig. 6 O 1s XPS spectra of samples

图7 样品的光谱吸收曲线
Fig. 7 Spectral absorption curves of samples
2.7 红外发射率测试
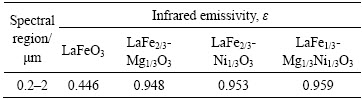
表2给出了LaFeO3、LaFe2/3Mg1/3O3、LaFe2/3- Ni1/3O3、LaFe1/3Mg1/3Ni1/3O3的红外发射率。较之LaFeO3,LaFe2/3Mg1/3O3、LaFe2/3Ni1/3O3、LaFe1/3Mg1/3- Ni1/3O3在0.2~2 μm近红外波段的发射率发生了显著提高。由基尔霍夫热辐射定律及综上所述可知,LaFe2/3Mg1/3O3、LaFe2/3Ni1/3O3、LaFe1/3Mg1/3Ni1/3O3近红外发射率的提高主要归因于掺杂引起的Fe3+ Fe4+小极化子吸收机制。这表明掺杂的LaFeO3具有优异的近红外辐射性能,能够强化高温过程的辐射传热能力,尤其以LaFe1/3Ni1/3Mg1/3O3在0.2~2 μm范围内吸收率与发射率最高,因此LaFe1/3Mg1/3Ni1/3O3有望成为一种新颖的红外辐射节能材料,在高温热工装备节能领域发挥作用。
Fe4+小极化子吸收机制。这表明掺杂的LaFeO3具有优异的近红外辐射性能,能够强化高温过程的辐射传热能力,尤其以LaFe1/3Ni1/3Mg1/3O3在0.2~2 μm范围内吸收率与发射率最高,因此LaFe1/3Mg1/3Ni1/3O3有望成为一种新颖的红外辐射节能材料,在高温热工装备节能领域发挥作用。
表2 样品的红外发射率
Table 2 Infrared emissivity of sample
3 结论
1) 采用溶胶-凝胶法(柠檬酸络合法)在1300 ℃制备出单一钙钛矿型复合氧化物LaFe1-x-yMgxNiyO3。引入掺杂元素之后,其粒径逐渐增大。
2) Mg2+、Ni2+通过掺杂进入了LaFeO3晶格内,在一定程度上会破坏LaFeO3晶格的对称性,产生晶格畸变,从而增强了晶格的振动活性,改变了分子振动与转动能级状态,从而促进了晶格的振动吸收;由于不同价态取代使得部分Fe3+生成Fe4+,有氧空位产生。晶格振动吸收与氧空位均有利于提升对应波段的红外辐射率。
3) 引入杂质元素之后,LaFeO3复合氧化物的吸收率与发射率均远高于未掺杂铁酸镧的,其中LaFe1/3Mg1/3Ni1/3O3在0.2~2 μm范围内具有最高吸收率与发射率。
REFERENCES
[1] 武晓燕, 于宏兵, 耿丽娟. 红外辐射陶瓷的研究进展[J]. 硅酸盐通报, 2013, 32(2): 280-282.
WU Xiao-yan, YU Hong-bing, GENG Li-juan. Study application and present development of infrared radiation ceramics[J]. Bulletin of the Chinese Ceramic Society, 2013, 32(2): 280-282.
[2] 李 永, 李江涛, 杨 筠, 林志明. 燃烧合成Al掺杂SiC高发射率粉体材料及其在红外辐射涂料中应用[C]// 第十三届全国红外加热暨红外医学发展研讨会论文及论文摘要集. 锦州: 中国学术期刊(光盘版)电子杂质社, 2011: 4.
LI Yong, LI Jiang-tao, YANG Yun, LIN Zhi-ming. Combustion synthesis of Al-doped SiC powder of high emissivity materials and its application in infrared radiation coating[C]// The 13th National Seminar on Infrared Heating and Infrared Medical Development Set of Papers and Abstracts. Jinzhou: Chinese Journal of Academic Journals (CD-ROM), 2011: 4.
[3] 李 伶, 张文苑, 隋学叶, 杨 杰, 王开宇, 周长灵. 陶瓷隔热瓦耐高温高辐射率涂层的制备及表征[J]. 现代技术陶瓷, 2016, 37(2): 131-137.
LI Ling, ZHANG Wen-yuan, SUI Xue-ye, YANG Jie, WANG Kai-yu, ZHOU Chang-ling. Preparation and characterization of high temperature resistant and high emissivity multi-component coating for ceramic insulation tile[J]. Advanced Ceramics, 2016, 37(2): 131-137.
[4] LIU H, LIU Z, OUYANG J, WANG Y. Influences of lattice vibration and electron transition on thermal emissivity of Nd3+ doped LaMgAl11O19 hexaaluminates for metallic thermal protection system[J]. Applied Physics Letters, 2012, 101, 161903.
[5] LU L, FAN X, ZHANG J, HU X, LI G, ZHANG Z. Evolution of structure and infrared radiation properties for ferrite-based amorphous coating[J]. Applied Surface Science, 2014, 316: 82-87.
[6] WU X, YU H, DONG H. Enhanced infrared radiation properties of CoFe2O4 by doping with Y3+ via sol-gel auto-combustion[J]. Ceramics International, 2014, 40: 12883-12889.
[7] 谭伟民, 郁 飞, 倪维良, 潘云飞, 李文凯, 刘志远, 付 敏. 红外低发射率涂层的制备及表征[J]. 涂料技术与文摘, 2015, 36(9): 26-34.
TAN Wei-min, YU Fei, NI Wei-liang, PAN Yun-fei, LI Wen-kai, LIU Zhi-yuan, FU Min. Preparation and characterization of coating with low infrared emissivity[J]. Coatings Technology & Abstracts, 2015, 36(9): 26-34.
[8] 左凤娟, 成来飞, 张立同. ZrB2-SiC 多层陶瓷的抗氧化性[J]. 硅酸盐学报, 2012, 40(8): 1174-1178.
ZUO Feng-juan, CHENG Lai-fei, ZHANG Li-tong. Oxidation resistance of ZrB 2-SiC multilayer ceramics[J]. Journal of the Chinese Ceramic Society, 2012, 40(8): 1174-1178.
[9] JACOB K T, RANJANI R. Thermodynamic properties of LaFeO3-δ and LaFe12O19[J]. Materials Science and Engineering B, 2011, 176: 559-566.
[10] LIU L M, SUN K N, LI X K, ZHANG M, LIU Y B, ZHANG N Q, ZHOU X L. A novel doped CeO2-LaFeO3 composite oxide as both anode and cathode for solid oxide fuel cells[J]. International Journal of Hydrogen Energy, 2012, 37: 12574-12579.
[11] MUKGOPADHYAY K, MAHAPATRA A S, CHAKRABARTI P K. Multiferroic behavior, enhanced magnetization and exchange bias effect of Zn substituted nanocrystalline LaFeO3 (La(1-x)ZnxFeO3, x=0.10, and 0.30)[J]. Journal of Magnetism and Magnetic Materials, 2013, 329: 133-141.
[12] SONG P, WANG Q, ZHANG Z, YANG Z X. Synthesis and gas sensing properties of biomorphic LaFeO3 hollow fibers templated from cotton[J]. Sensors and Actuators B, 2010, 147: 248-254.
[13] REN Y Y,  R, GORTE R J, DENG C S. The effect of A-site cation (Ln=La, Pr, Sm) on the crystal structure, conductivity and oxygen reduction properties of Sr-doped ferrite perovskites[J]. Solid State Ionics, 2012, 212: 47-54.
R, GORTE R J, DENG C S. The effect of A-site cation (Ln=La, Pr, Sm) on the crystal structure, conductivity and oxygen reduction properties of Sr-doped ferrite perovskites[J]. Solid State Ionics, 2012, 212: 47-54.
[14] CHEN C, XU K, CUI Y, WANG C. Polaronic relaxation in LaFeO3[J]. Materials Letters, 2012, 89: 153-155.
[15] 陈永红, 魏亦军, 刘杏芹, 孟广耀. La1-xCaxFeO3-δ系阴极材料的GNP法合成及电性能研究[J]. 无机化学学报, 2005, 21(5): 673-678.
CHEN Yong-hong, WEI Yi-jun, LIU Xing-qin, MENG Guang-yao. Synthesis and characterization of La1-xCaxFeO3 -δ for cathodes by GNP method[J]. Chinese Journal of Inorganic Chemistry, 2005, 21(5): 673-678.
[16] CHANDRASEKHAR K D, MALLESH S, MURTHY J K, DAS A K, VENIMADHAV A. Role of defects and oxygen vacancies on dielectric and magnetic properties of Pb2+ ion doped LaFeO3 polycrystalline ceramics[J]. Physica B, 2014, 448: 304-311.
[17] YE J K, BU C H, HAN Z, WANG F, LI X W, CHEN Y X, LI J T. Flame-spraying synthesis and infrared emission property of Ca2+/ Cr3+ doped LaAlO3 microspheres[J]. Journal of the European Ceramic Society, 2015, 35: 3111-3118.
[18] WANG S. Effects of Fe on crystallization and properties of a new high infrared radiance glass-ceramics[J]. Environmental Science & Technology, 2010, 44: 4816-4820.
[19] ROMERO M,  R W, MARQUINA V,
R W, MARQUINA V,  -MAZARIEGO J L, ESCAMILLA R. Synthesis by molten salt method of the AFeO3 system (A=La, Gd) and its structural, vibrational and internal hyperfine magnetic field characterization[J]. Physica B, 2014, 443: 90-94.
-MAZARIEGO J L, ESCAMILLA R. Synthesis by molten salt method of the AFeO3 system (A=La, Gd) and its structural, vibrational and internal hyperfine magnetic field characterization[J]. Physica B, 2014, 443: 90-94.
[20] SAHA R, SHIREEN A, SHIRODKAR S N, WAGHMARE U V, SUNDARESAN A, RAO C N R. Multiferroic and magnetoelectric nature of GaFeO3, AlFeO3 and related oxides[J]. Solid State Communications, 2012, 152: 1964-1968.
[21] KE Q, LOU X, WANG Y, WANG J. Oxygen-vacancy-related relaxation and scaling behaviors of Bi0.9La0.1Fe0.98Mg0.02O3 ferroelectric thin films[J]. Physical Review B, 2010, 82: 024102.
[22] WANG J, NEATON J B, ZHENG H, NAGARAJAN V, OGALE S B, LIU B, VIEHLAND D, VAITHYANATHAN V, SCHLOM D G, WAGHMARB U V, SPALDIN N A, RABE K M, WUTTIG M, RAMESH R. Epitaxial BiFeO3 multiferroic thin film heterostructures[J]. Science, 2003, 299(5613): 1719-1722.
[23] YOON K J, ZINK P A, GOPALAN S, PAL U B, PEDERSONB L R. Defect chemistry and electrical properties of (La0.8Ca0.2)0.95FeO3-δ[J]. Journal of the Electrochemical Society, 2009, 156: B795-B800.
[24] ZINK P A, YOON K J, PAL U B, GOPALAN S. Analysis of the electronic and ionic conductivity of calcium-doped lanthanum ferrite[J]. Electrochemical & Solid State Letters, 2009, 12: B141-B143.
[25] HUANG J P, FAN C L, SONG G P, LI Y B, HE X D, ZHANG X J, SUN Y, DU S Y, ZHAO Y J. Enhanced infrared emissivity of CeO2 coating by La doping[J]. Applied Surface Science, 2013, 280: 605-609.
[26] ARIMA T, TOKURA Y, TORRANCE J B. Variation of optical gaps in perovskite-type 3d transition-metal oxides[J]. Physical Review B, 1993, 48, 17006.
[27] VIDAL K,  L M, ORTEGA-SAN-
L M, ORTEGA-SAN- L, ROJO T, LARESGOITI A, ARRIORTUAET M I. Isolating the effect of doping in the structure and conductivity of (Ln1-xMx)FeO3-δ perovskites[J]. Solid State Ionics, 2007, 178: 1310-1316.
L, ROJO T, LARESGOITI A, ARRIORTUAET M I. Isolating the effect of doping in the structure and conductivity of (Ln1-xMx)FeO3-δ perovskites[J]. Solid State Ionics, 2007, 178: 1310-1316.
[28] CHEN X, LIU L, PETER Y Y, MAO S S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals[J]. Science, 2011, 331: 746-750.
Preparation and infrared radiation properties of LaFeO3 composite oxides
LIU Qing-sheng, YOU Zheng, GUO Hui, ZENG Shao-jun
(College of Metallurgical and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China)
Abstract: A perovskite LaFe1-x-yMgxNiyO3 compound was prepared by the sol-gel method and characterized by TG-DTA, XRD, SEM, and XPS. The result shows that the infrared emissivity of the doped samples in the range of 0.2–2 μm is greatly improved than that of the undoped LaFeO3 sample. The increase of infrared emissivity of samples can be mainly attributed to the substitution of the doped ion with Fe3+ in the LaFeO3 lattice. In order to keep the balance of electrovalent compound, the substitution of the ion doping with Fe3+ introduces the energy level of Fe4+ impurity and generates oxygen vacancies, thus increasing the impurity and oxygen vacancy absorption. The lattice distortion caused by doping strengthens the lattice vibration absorption. Furthermore, the polaron hopping of electrons between Fe3+ and Fe4+ also significantly strengthens the absorption properties within the infrared radiative properties of doped lanthanum ferrite.
Key words: perovskite; lanthanum ferrite; doping; absorptivity; infrared emissivity
Foundation item: Projects(51264011, 51564019) supported by the National Natural Science Foundation of China
Received date: 2016-04-15; Accepted date: 2016-08-28
Corresponding author: LIU Qing-sheng; Tel: +86-15970132969; E-mail: lqs_01259@126.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51264011,51564019)
收稿日期:2016-04-15;修订日期:2016-08-28
通信作者:刘庆生,副教授,博士;电话:15970132969;E-mail: lqs_01259@126.com
摘 要:采用溶胶-凝胶法制备钙钛矿型LaFe1-x-yMgxNiyO3的化合物,并利用TG-DTA、XRD、SEM和XPS等对其进行分析和表征。结果表明:掺杂样品在0.2~2 μm波段的红外发射率较未掺杂的LaFeO3的红外发射率大幅度提高,其原因在于,掺杂离子取代铁离子进入LaFeO3晶格中,为保持化合物电价平衡,部分Fe3+转变为Fe4+,引入杂质能级,氧空位浓度增加,促进杂质能级吸收和氧空位吸收;掺杂引起的晶格畸变使得晶格振动吸收增强;此外,电子在Fe3+和Fe4+之间的极化跃迁,亦极大地提高掺杂铁酸镧的红外辐射性能。
[1] 武晓燕, 于宏兵, 耿丽娟. 红外辐射陶瓷的研究进展[J]. 硅酸盐通报, 2013, 32(2): 280-282.
[3] 李 伶, 张文苑, 隋学叶, 杨 杰, 王开宇, 周长灵. 陶瓷隔热瓦耐高温高辐射率涂层的制备及表征[J]. 现代技术陶瓷, 2016, 37(2): 131-137.
[7] 谭伟民, 郁 飞, 倪维良, 潘云飞, 李文凯, 刘志远, 付 敏. 红外低发射率涂层的制备及表征[J]. 涂料技术与文摘, 2015, 36(9): 26-34.
[8] 左凤娟, 成来飞, 张立同. ZrB2-SiC 多层陶瓷的抗氧化性[J]. 硅酸盐学报, 2012, 40(8): 1174-1178.
[15] 陈永红, 魏亦军, 刘杏芹, 孟广耀. La1-xCaxFeO3-δ系阴极材料的GNP法合成及电性能研究[J]. 无机化学学报, 2005, 21(5): 673-678.


