
Preparation and sintering properties of zirconia-mullite-corundum composites using fly ash and zircon
MA Bei-yue(马北越), LI Ying(厉 英), CUI Shao-gang(崔绍刚), ZHAI Yu-chun(翟玉春)
School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China
Received 13 May 2010; accepted 8 September 2010
Abstract:
Zirconia-mullite-corundum composites were successfully prepared from fly ash, zircon and alumina powder by a reaction sintering process. The phase and microstructure evolutions of the composite synthesized at desired temperatures of 1 400, 1 500 and 1 600 °C for 4 h were characterized by X-ray diffractometry and scanning electronic microscopy, respectively. The influences of sintering temperature on shrinkage ratio, apparent porosity and bulk density of the synthesized composite were investigated. The formation process of the composites was discussed in detail. The results show that the zirconia-mullite-corundum composites with good sintering properties can be prepared at 1 600 °C for 4 h. Zirconia particles can be homogeneously distributed in mullite matrix, and the zirconia particles are around 5 μm. The formation process of zirconia-mullite-corundum composites consists of decomposition of zircon and mullitization process.
Key words:
zirconia; mullite; sintering properties; reaction sintering process; fly ash; zircon;
1 Introduction
Zirconia(ZrO2)-mullite(Al6Si2O13) composites have been found widespread applications due to their excellent properties such as high melting point, strength and fracture toughness as well as good wear and thermal shock resistance[1-2]. The various currently available methods for preparing the ZrO2-mullite composites mainly include reaction sintering process[3-5], sol-gel method[6], microwave sintering method[7], thermal plasma method[8], semi-colloidal technique[9], and liquid infiltration technology[10]. And the reaction sintering process is considered a promising technology for preparing ZrO2-mullite composites because it has some advantages such as using cheap raw materials, simple production technology and low manufacturing cost[10]. In recent years, some researchers have focused on using various raw materials to fabricate the ZrO2-mullite composites with low cost by reaction sintering process. The raw materials mainly involve ZrO2, alumina (Al2O3) and silica (SiO2), aluminum (Al) dross and zircon (ZrSiO4)[3], Al2O3 and ZrSiO4[2, 4-5] as well as metal Al and ZrSiO4. During the preparation process of ZrO2-mullite composites from above raw materials, the following chemical reactions maybe occur:
2SiO2(s)+3Al2O3(s)=Al6Si2O13(s) (1)
4ZrSiO4(s)+12Al(s,l)+9O2(g)=4ZrO2(s)+2Al6Si2O13(s) (2)
2ZrSiO4(s)+3Al2O3(s)=2ZrO2(s)+Al6Si2O13(s) (3)
Fly ash is an industrial by-product from the combustion of raw coal in thermal power plants, and its chemical composition mainly includes Al2O3 and SiO2[11]. Fly ash has been causing serious problems involving storing and environmental pollution. It has been reported that the total mass of fly ash in China will approximately reach 3.0×1010 t until 2020. Hence, comprehensive utilization of massive fly ash has been gaining much attention. So far, the application fields of fly ash mainly include building, agriculture, chemistry industry and ceramic[11-13]. Zircon consists of ZrO2 and SiO2. At present, the present reserve (calculated from ZrO2) of zircon in the world is approximately 3.2×107 t, and zircon has been mainly used to fabricate ceramic, glass and refractories[14]. However, there has been no report on preparation of ZrO2-mullite-corundum composites from waste fly ash and zircon.
In this study, ZrO2-mullite-corundum composites were prepared by reaction sintering process, using fly ash, zircon and alumina powder as the raw materials. The influences of sintering temperature on the phase composition, microstructure and sintering properties of the composites were investigated, and the formation process of the composites was discussed in detail. The present work can provide a new route to fabricate ZrO2-mullite-corundum composites with low cost from large availability industrial waste and natural raw materials, and is favorable for decreasing the manufacturing cost of ZrO2-mullite-corundum composites with high performance.
2 Experimental
2.1 Raw materials
Fly ash (mesh size≤44 μm) was chosen as Al2O3 and SiO2 sources, zircon (mesh size≤44 μm) as ZrO2 and SiO2 sources, and alumina powder (mesh size≤44 μm) as Al2O3 source for the preparation of ZrO2-mullite- corundum composites. The chemical compositions of the raw materials are listed in Table 1. As shown in Fig.1, the crystalline phases of fly ash mainly include mullite as well as small amounts of corundum (Al2O3) and quartz (SiO2).
Table 1 Chemical compositions of raw materials (mass fraction, %)
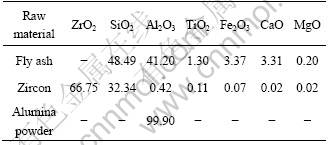
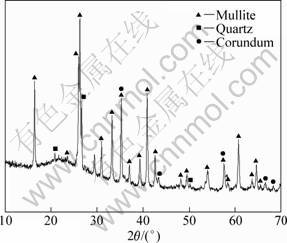
Fig.1 XRD pattern of fly ash
2.2 Preparation and sintering of samples
The overall reaction equation for synthesizing ZrO2-mullite-corundum composites from fly ash, zircon and alumina powder can be expressed as
ZrSiO4(s)+3SiO2(s)+6Al2O3(s)=ZrO2(s)+2Al6Si2O13(s) (4)
According to Eq. (4), the mass ratio of zircon to fly ash to alumina powder was 49:100:122. The raw materials were weighed in terms of the mass ratio, dry mixed for 18 h and formed to samples with 50 mm in diameter and 20 mm in height under a pressure of 200 MPa. Then the formed samples were dried fully at 120 °C, carried out in an electric furnace, and sintered at 1 400, 1 500 and 1 600 °C for 4 h, respectively. After the predetermined temperatures and times reached, the system was cooled to room temperature in air.
2.3 Characterization of samples
The sintered samples were characterized by carrying out shrinkage ratio, apparent porosity, bulk density, phase evolution and microstructure analyses. The diameter shrinkage ratio and volume shrinkage ratio were calculated according to Eqs.(5) and (6), respectively. The apparent porosity and bulk density were measured by immersion method in water under vacuum using Archimedes’ principle and calculated according to Eqs.(7) and (8)[15].
![]() (5)
(5)
![]() (6)
(6)
![]() (7)
(7)
![]() (8)
(8)
where ΔD and ΔV are the diameter shrinkage ratio and volume shrinkage ratio of the sintered samples (%); Pa is the apparent porosity of the sintered samples (%); Db is the bulk density of the sintered samples (g/cm3); D0 and H0 are the diameter and height of the samples before sintering (mm); D1 and H1 are the diameter and height of the samples after sintering (mm); m1 is the mass of a dried sample in air (g); m2 is the mass of the sample in water (g); m3 is the mass of the sample with free bubbles on the surface (g); and d is the density of water (g/cm3).
The phase compositions of the sintered samples were examined by X-ray diffractometer (XRD, Cu Kα radiation, 30 kV and 30 mA) and the microstructures were observed by scanning electronic microscope (SEM).
3 Results and discussion
3.1 Phase composition and microstructure
Fig.2 shows the XRD patterns of the samples sintered at different temperatures for 4 h. It can be observed that the sintering temperature has a great influence on the phase composition of samples. The sample synthesized at 1 400 °C mainly consists of zircon, mullite, monoclinic zirconia and corundum, and the zircon is a main crystalline phase, such as corresponding peaks at 20°, 27°, 35.5° and 53.5°, as shown in Fig.2(a). The presence of zirconia reveals that the decomposition of zircon starts at a lower temperature (1 400 °C). The other decomposition product (quartz) from zircon is not detected, which indicates that the formed quartz can further react with alumina in sample to produce mullite, so the formation of mullite also starts at 1 400 °C. In the sample sintered at 1 500 °C, zircon phase is not detected, and the crystalline phases mainly include zirconia, mullite and corundum. When the sample is sintered at 1 600 °C, the crystalline phases are the same as that of the sample sintered at 1500 °C, and the main crystalline phases involve mullite and zirconia, such as corresponding peaks at 16.5°, 26°, 26.5°, 33.5° and 35.5° as well as 28.5° and 31.5°, respectively, as shown in Fig.2(c). From 1 400 °C up to 1 600 °C, the peak intensities of mullite and zirconia strengthen obviously, whereas that of corundum weakens, and no new phase is formed in sample. Therefore, the ZrO2-mullite-corundum composites can be prepared at 1 500-1 600 °C from fly ash, zircon and alumina powder by reaction sintering process.
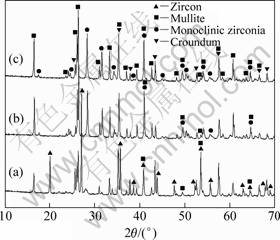
Fig.2 XRD patterns of samples sintered at different temperatures for 4 h: (a) 1 400 °C; (b) 1 500 °C; (c) 1 600 °C
Fig.3 shows the SEM photographs of the samples sintered at different temperatures for 4 h. It can be seen from Fig.3(a) that the sample sintered at 1 400 °C for 4 h presents amounts of gas porosities and zircon particles. When the sintering temperature reaches 1 500 °C, gas porosities coalescence can occur and white zirconia particles can be homogeneously distributed in gray mullite matrix (Fig.3(b)). The sample obtained at 1 600 °C for 4 h shows a more homogeneous microstructure with a continuous mullite matrix and dispersive zirconia particles (Fig.3(c)). However, some gas porosities and zirconia agglomerates can be observed. In Fig.3(d) at a higher magnification for the zone shown in Fig.3(c), the dispersive zirconia particles are around 5 μm.
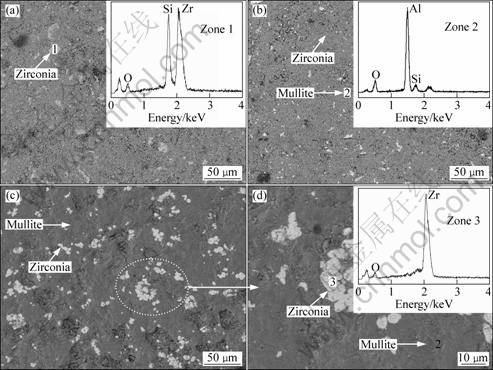
Fig.3 SEM photographs of samples sintered at different temperatures for 4 h: (a) 1 400 °C; (b) 1 500 °C; (c), (d) 1 600 °C
In Ref.[3], the ZrO2-mullite composites were prepared at 1 500 °C for 6 h by reaction sintering process, with aluminum dross and zircon as the raw materials. A homogeneous microstructure with a continuous mullite matrix and dispersive zirconia particles was obtained, and some gas porosities and zirconia agglomerates could be also observed. Additionally, the zirconia particles in the ZrO2-mullite composites were 5-10 μm.
3.2 Analysis of formation process
In this study, the preparation process of the ZrO2-mullite-corundum composites by reaction sintering the mixtures of fly ash, zircon and alumina powder at 1 400-1 600 °C is very complex and maybe involve many chemical reactions. During the synthesis process, the decomposition of zircon can promote the formation of mullite because the decomposition product (quartz) has a higher reaction activity, and can easily react with alumina to form mullite. It is concluded that whether zircon in sample can be decomposed is a key factor for the mullitization process. With the change of the sintering temperature (1 400-1 600 °C), the reaction products maybe include ZrO2, SiO2, Al2O3 and Al6Si2O13. According to the relational thermodynamic data in Refs.[5,16], the relationship between standard Gibbs free energy (![]() ) and temperature (T) can be obtained. The chemical reactions likely to occur during the synthesis process and the relational expressions of
) and temperature (T) can be obtained. The chemical reactions likely to occur during the synthesis process and the relational expressions of ![]() and T (
and T (![]() -T) are as follows:
-T) are as follows:
ZrSiO4(s)=ZrO2(s)+SiO2(s),
![]() (9)
(9)
2ZrSiO4(s)+3Al2O3(s)=2ZrO2(s)+Al6Si2O13(s),
![]() (10)
(10)
2SiO2(s)+3Al2O3(s)=Al6Si2O13(s),
![]() (11)
(11)
ZrSiO4(s)+3SiO2(s)+6Al2O3(s)=ZrO2(s)+2Al6Si2O13(s),
![]() (12)
(12)
Fig.4 shows the diagram of ![]() for ZrSiO4-SiO2-Al2O3 system obtained from Eqs.(9)-(12). It can be seen that
for ZrSiO4-SiO2-Al2O3 system obtained from Eqs.(9)-(12). It can be seen that ![]() ,
, ![]() and
and ![]() for reactions (10), (11) and (12), respectively, keep negative at the temperatures of 1 600-2 000 K. However,
for reactions (10), (11) and (12), respectively, keep negative at the temperatures of 1 600-2 000 K. However, ![]() for reaction (9) still keeps positive. This reveals that compared with reactions (10)-(12), the reaction (9) cannot generate easily at 1 600-2 000 K.
for reaction (9) still keeps positive. This reveals that compared with reactions (10)-(12), the reaction (9) cannot generate easily at 1 600-2 000 K.
In this study, when the sintering temperature rises to 1 673 K (1 400 °C), ZrSiO4 can easily react with Al2O3 to produce Al6Si2O13 and ZrO2 (reaction (10)), which is confirmed by the XRD patterns (Fig. 2). ![]() -
-![]() can be decreased gradually with the increase of temperature, and compared with
can be decreased gradually with the increase of temperature, and compared with ![]() and
and ![]() , the decrease trend of
, the decrease trend of ![]() and
and ![]() is lower. Moreover, at the experimental temperatures of 1 673-1 873 K (1 400-1 600 °C),
is lower. Moreover, at the experimental temperatures of 1 673-1 873 K (1 400-1 600 °C), ![]() for reactions (9)-(12) is as follows:
for reactions (9)-(12) is as follows: ![]() <
<![]() <
<![]() <
<![]() , indicating that the mullite can be easily formed from zircon, quartz and alumina. It can be concluded that the formation process of ZrO2-mullite-curundum composites consists of the decomposition of zircon and the mullitization process.
, indicating that the mullite can be easily formed from zircon, quartz and alumina. It can be concluded that the formation process of ZrO2-mullite-curundum composites consists of the decomposition of zircon and the mullitization process.
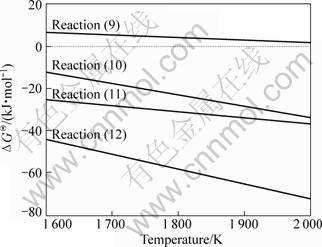
Fig.4 Diagram of![]() for ZrSiO4-SiO2-Al2O3 system
for ZrSiO4-SiO2-Al2O3 system
3.3 Sintering properties
Fig.5 shows the influences of sintering temperature on the diameter shrinkage ratio, volume shrinkage ratio, apparent porosity and bulk density of samples. It can be seen from Fig.5(a) that with increasing the temperature, the diameter shrinkage ratio and volume shrinkage ratio are increased gradually. For the sample sintered at 1 400 °C for 4 h, the diameter shrinkage ratio and volume shrinkage ratio are 5.87% and 16.72%, respectively. At 1 500 °C, they are increased to 7.61% and 21.66%, respectively. When the temperature reaches 1 600 °C, they are further increased to 10.62% and 29.49%, respectively. It can be seen from Fig.5(b) that sintering temperature has an effective influence on the apparent porosity and bulk density of samples. With the increase of sintering temperature, the apparent porosity of samples is decreased obviously, while the bulk density is increased gradually. When the samples are sintered at 1 400 °C up to 1 600 °C, the apparent porosity is decreased from 23.60% to 0.71%, and the bulk density is increased from 2.55 to 3.03 g/cm3, respectively. Therefore, increasing temperature can greatly promote the sintering of samples, and favor the fabrication of the ZrO2-mullite-corundum composites with high density.
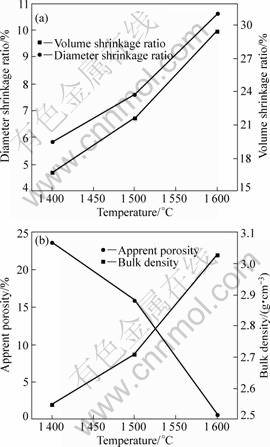
Fig.5 Sintering properties of samples sintered at different temperatures for 4 h: (a) Diameter shrinkage ratio and volume shrinkage ratio; (b) Apparent porosity and bulk density
4 Conclusions
1) ZrO2-mullite-corundum composites can be successfully prepared at 1 500-1 600 °C by reaction sintering process, with fly ash, zircon and alumina powder as the raw materials. Zirconia particles can be homogeneously distributed in mullite matrix, and the dispersive zirconia particles are around 5 μm.
2) The ZrO2-mullite-corundum composites with high density can be prepared at 1 600 °C for 4 h, the diameter shrinkage ratio, volume shrinkage ratio and bulk density of the composites are 10.62%, 29.49% and 3.03 g/cm3, respectively.
3) The formation process of the ZrO2-mullite- corundum composites includes the decomposition of zircon and the mullitization process.
References
[1] RENDTORFF N, GARRIDO L, AGLIETTI E. Mullite-zirconia- zircon composites: Properties and thermal shock resistance [J]. Ceram Int, 2009, 35(2): 779-786.
[2] GARRIDO L B, AGLIETTI E F. Reaction-sintered mullite-zirconia composites by colloidal processing of alumina-zircon-CeO2 mixtures [J]. Mater Sci Eng A, 2004, 369(1/2): 250-257.
[3] IBARRA CASTRO M N, ALMANZA ROBLES J M, CORT?S HERN?NDEZ D A, ESCOBEDO BOCARDO J C, TORRES TORRES J. Development of mullite-zirconia composites from a mixture of aluminum dross and zircon [J]. Ceram Int, 2009, 35(2): 921-924.
[4] EBADZADEH T. Porous mullite-ZrO2 composites from reaction sintering of zircon and aluminum [J]. Ceram Int, 2005, 31(8): 1091-1095.
[5] ZHAO S K, HUANG Y, WANG C A, HUANG X Y, GUO J K. Mullite formation from reaction sintering of ZrSiO4/α-Al2O3 mixtures [J]. Mater Lett, 2003, 57(11): 1716-1722.
[6] YUAN Q M, TAN J Q, SHEN J Y, ZHU X H, YANG Z F. Processing and microstructure of mullite–zirconia composites prepared from sol-gel powder [J]. J Am Ceram Soc, 1986, 69(3): 268-269.
[7] RAABE J, BOBRYK E, PETROVSKY V. Fabrication of mullite-zirconia composites by microwave sintering of corundum/amorphous silica particles and sol-gel substrates [J]. Ceram Int, 2001, 27(1): 81-84.
[8] BHATTACHARJEE S, SINGH S K, GALGALI R K. Preparation of zirconia toughened mullite by thermal plasma [J]. Mater Lett, 2000, 43(1/2): 77-80.
[9] MAITRA S, RAHAMAN A, SARKAR A, TARAFDAR A. Zirconia-mullite materials prepared from semi-colloidal route derived precursors [J]. Ceram Int, 2006, 32(2): 201-206.
[10] PARK H C, YANG T Y, YOON S Y, STEVENS R. Preparation of zirconia-mullite composites by an infiltration route [J]. Mater Sci Eng A, 2005, 405(1/2): 233-238.
[11] AHMARUZZAMAN M. A review on the utilization of fly ash [J]. Prog Energ Combust, 2010, 36(3): 327-363.
[12] LI J H, MA H W, HUANG W H. Effect of V2O5 on the properties of mullite ceramics synthesized from high-aluminum fly ash and bauxite [J]. J Hazard Mater, 2009, 166(2/3): 1535-1539.
[13] JI H M, LU H X, HAO X G, WU P. High purity alumina powders extracted from fly ash by the calcining-leaching process [J]. J Chin Ceram Soc, 2007, 35(12): 1657-1660.
[14] MA B Y, YU J K. Influence of processing parameters on the phase composition of ZrN-Si3N4 synthesized from zircon [J]. Rare Metals, 2009, 28(4): 367-371.
[15] CHEN Min, YU Jing-kun, WANG Nan. Refractories and fuel combustion [M]. Shenyang: Northeastern University Press, 2005. (in Chinese)
[16] LIANG Ying-jiao, CHE Yin-chang. Handbook of thermodynamic data in inorganic [M]. Shenyang: Northeastern University Press, 1993. (in Chinese)
Foundation item: Project(N100302002)supported by the Fundamental Research Funds for the Central Universities, China; Project (20100471467) supported by the China Postdoctoral Science Foundation
Corresponding author: MA Bei-yue; Tel: +86-24-83673860; E-mail: beiyue_ma@yahoo.com.cn
DOI: 10.1016/S1003-6326(10)60650-4


