Trans. Nonferrous Met. Soc. China 23(2013) 494-500
Bioleaching and electrochemical property of marmatite by Leptospirillum ferrooxidans
Jin-rong BAN, Guo-hua GU, Ke-ting HU
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 3 November 2011; accepted 3 May 2012
Abstract:
The effects of several variables on the bioleaching of marmatite with pure L. ferrooxidans were investigated. The results show that zinc extraction increases with the decrease of pulp density. Adjusting pH to1.6 during the bioleaching process has a positive effect to the dissolution of marmatite. External addition of Fe3+ ions accelerates the bioleaching, while the concentration of additional Fe3+ over 2.5 g/L weakens the acceleration effect due to the inhibition effect on bacteria growth and the promotion of jarosite production. The electrochemical measurements were used to make further understanding on the dissolution of marmatite with and without additional Fe3+ in the presence of L. ferrooxidans. The experimental data illustrate that additional Fe3+ ions could increase the corrosion current density, which is favorable to zinc extraction. The EIS spectra show that rate-limiting step does not change when Fe3+ is added.
Key words:
bioleaching; marmatite; electrochemical measurements; L. ferrooxidans;
1 Introduction
Nowadays, about 90% of the world’s total zinc is produced through the extraction of zinc from sphalerite by roast-leach-electrowinning (RLE) and pressure hydrometallurgy. Bioleaching may be applied as an alternative to increase zinc production without the consequent production of sulfur dioxide (SO2). The potential benefits of commercial bioleaching of zinc minerals are significant in the exploitation of low-grade ores that are difficult to process using conventional technologies. In addition, zinc concentrate bioleaching, when compared with pyrometallurgy, has the advantage that it does not require roasting, sulfuric acid plants and washing of the gaseous effluents [1].
As is known, most nature sphalerite contains traces of iron in solid solution with the temperature and chemistry of the environment. High-iron zinc sulphide is often called marmatite, which is one of the most important zinc bearing resources. Marmatite widely presents in south-west China. The presence of iron in marmatite can drastically reduce its market value due to the formation of ferrite (ZnO·Fe2O3). While, the activation energies for leaching decreased with increasing iron content of the sphalerite [2].
Bioleaching of marmatite flotation concentrate was studied by SHI et al [3-6] and the technological feasibility was demonstrated. The shortcomings of their studies were that the flotation concentrate contained other minerals which may have an adverse impact on the discovering of the catalytic oxidation effects of bacteria on marmatite leaching.
Most work with regard to the bioleaching of zinc has been done in the presence of Acidithiobacillus ferrooxidans, which is one of the most important iron and sulfur oxidizing bacteria [3-4,6-8]. In contrast, there are few reports on bioleaching of zinc from sulfide ores using pure Leptospirillum ferrooxidans and most of them have used consortia including sulfur-oxidizing bacteria. Consortia are usually more suitable for processing different minerals but the control and monitoring of the microbial consortia in bio-mining are minimal or non-existent [4,7,9]. Therefore, testing the performance of individual species is important before constructing a competitive consortium [9]. One main objective in our study is to describe the performance of a pure strain of Leptospirillum ferrooxidans during the natural marmatite bioleaching process under the temperature of 40 °C.
The microbial leaching of sulfide ores is a chemical and electrochemical process involved in the metabolism of microorganisms. The bacteria attack on the sulfide surface is based on the use of recyclable chemical species (H+, Fe2+, thiol-compound), which disrupt chemical bonds at the sulfide interface and thereby induce disintegration [10]. The interfacial chemical and electrochemical mechanisms which they have practical relevance for bioleaching of minerals were studied, based on the bacterial energy cycle [11]. So, the analysis of data obtained by electrochemical studies can provide useful information to further understand the process of marmatite dissolution.
In order to discover the effect of iron-oxidizing bacteria of Leptospirillum ferrooxidans on the dissolution of natural marmatite and further understand the mechanisms of marmatite bioleaching, we have studied the effects of some facts, such as pulp density, pH and the external addition of different concentrations of Fe3+ ions, on the marmatite dissolution by bioleaching experiments with a pure iron-oxidizing bacterial strain of Leptospirillum ferrooxidans. Fe3+ ions used were applied in Fe2(SO4)3·xH2O(25%Fe). In addition, the electro- chemical measurements (Tafel and electrochemical impedance spectroscopy) were utilized to understand the leaching process of marmatite in different electrolytes with and without additional Fe3+ ions, in which the marmatite-carbon paste electrodes were used.
2 Materials and methods
2.1 Microorganisms and culture media
Leptospirillum ferrooxidans (LYZTS) was obtained from the Key Laboratory of Biometallurgy in Central South University. A sterilized iron-free 9K medium was used as a substrate for bacterial growth. The composition of iron-free 9K medium was 3.0 g/L (NH4)2SO4, 0.1 g/L KCl, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2. The culture was routinely sub-cultured using ferrous sulphate as an energy source in an incubator at the temperature of 40 °C. The pH of the culture was 1.6. Then the solution was filtered through a Whatman filter No.1 to remove the precipitate and centrifuged 9000×g for 20 min using a Centrifuge, Model TGL16M. The centrifuged cells were washed by sterilized sulphuric acid under pH 1.6. Washing and centrifugation were repeated three times before the cells were free from the precipitates. After that, the cells were re-suspended in the sterilized iron-free 9K medium with a concentration of 2×108 cell/mL. All the bacteria used in the experiments were original bacteria and were not adapted to marmatite before bioleaching.
2.2 Marmatite preparation
The natural marmatite samples used in experiments were obtained from a lead-zinc mine in Dachang, Guangxi Province, China. The samples were hand- selected before grounding which were ground to a particle size 100% minus 75 μm by a porcelain ball milling. The chemical analyses showed that the samples contained 51.00% Zn, 31.91% S and 13.20% Fe. Figure 1 shows marmatite as the major mineral component.
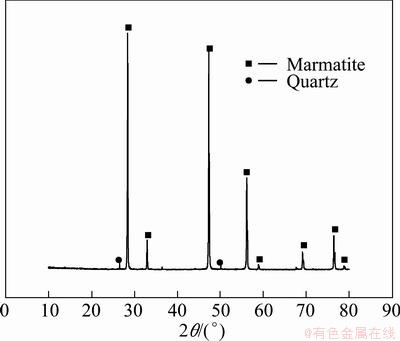
Fig. 1 XRD pattern of mineral sample
2.3 Bioleaching experiments
Bioleaching experiments were carried out in 250 mL Erlenmeyer flasks. Iron-free 9K medium (135 mL) adjusted to the required pH of 1.6 was transferred into each flask. The flasks were then autoclaved at 1×105 Pa and 121 °C for 20 min. Following autoclaving, each flask was inoculated with 15 mL culture producing a final volume of slurry approximately 150 mL, and then the marmatite sample was added. In all experiments, the initial bacterial population was 2×107 cell/mL. To facilitate the mixing and transfer of O2 and CO2, the flasks were shaken at a constant of 160 r/min. At predetermined time intervals, the water lost was compensated with sterilized distilled water, and if the pH changed, it was adjusted to pH 1.6 with 1:1 sulfuric acid or sodium hydroxide (2 mol/L). 4 mL liquid sample was removed periodically from the flasks and analyzed for the dissolved zinc content by atomic absorption spectrophotometry (AAS). The removed solutions were replaced with an equal volume of sterilized iron-free nutrient medium.
2.4 Electrochemical study
Electrochemical studies were conducted using a conventional three-electrode system electrochemical cell. The cell consists of an Ag, AgCl/KCl(sat’d)-reference electrode with a salt bridge (capillary), two graphite counter electrodes, and a particularly made working electrode by the method in Refs. [12-14]. 1 g base conductor material (powdered graphite) was mixed with 1 g powdered marmatite sample and 0.5 mL paraffin oil. They were compressed under pressure using a carbon paste electrode holder.
KONISHI et al [15] found that the equilibrium distribution of bacterial cells between mineral and solution was attained within the first 30 min, so it was presumed that the electrode-solution interface might attain a relatively stable state within the first 30 min. All the measurements began after the marmatite electrode was immersed in the electrolyte for 30 min [12]. Before test, the exposed surface of the marmatite-carbon paste electrode was polished with 600 and 1000 grit silicon carbide papers in order to clean and refresh the work surface and then rinsed with distilled water. A Princeton Potentiostat/Galvanostat 283 A & Lock-in Amplifer produced by EG&G Princeton Applied Research Corp USA was used to perform the electrochemical measurements. The data were collected and analyzed by computer. All measurements were carried out neither agitating nor oxygen eliminating from the medium [13,14,16].
The Tafel curves were tested with a sweep rate of 1 mV/s and applied potentials of ±250 mV vs open circuit potential (OCP). The electrochemical impedance spectroscopy studies were carried out by applying a 5 mV amplitude sine-wave signal perturbation in the frequency range of 10-2-105 Hz under the open circuit potential and the impedance diagrams were shown in the form of Nyquist plots. Before all the measurements, the pH of the electrolyte was adjusted to 1.6. There are no further adjustments when Fe3+ ions were added into the electrolyte.
3 Results and discussion
3.1 Bioleaching of marmatite
3.1.1 Effect of pulp density
The effects of pulp density determined by bioleaching at a rotational speed of orbital shaker of 160 r/min are shown in Fig. 2. It can be seen a decrease in zinc dissolution rate when the mineral pulp density was increased, with the highest dissolution rate of 88.2% obtained at the pulp density of 1% (w/v). As is known, higher pulp density can lead to an increase in the friction between particles and attrition of bacterial cells, which might possibly affect interactions between particles and bacterial, and cell damage [17,18]. So the leaching rate of marmatite became lower when the pulp density increased higher with original L. ferrooxidans, even though the leaching time was 30 d.
3.1.2 Effect of controlling pH
There usually had an optimal pH for the bacteria to keep its best activity. But the pH changed during the leaching process due to the complex redox reactions in the system. In this work, the effect of controlling pH to the initial value of 1.6 at regular intervals on the dissolution of marmatite was examined. At the same time, the experiment which did not control pH during the process was taken as a comparison. The experiment results are shown in Fig. 3. It could notice that the zinc dissolution is higher when the pH is adjusted to the initial value of 1.6. The low zinc extraction can be credited to the higher pH values (data not shown here) under the condition of not controlling pH to 1.6 during the experiment process, which may make it difficult for the thriving of L. ferrooxidans. At the same time, iron (III) precipitation may probably take place in terms of the jarosite forming under higher pH values, thus reducing the oxidant (Fe(III)) concentration.
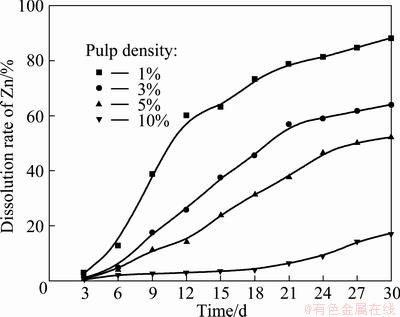
Fig. 2 Effects of pulp density on zinc dissolution by L. ferrooxidans (Particle size 75 μm, pH 1.6, rotational speed of 160 r/min, adjusting pH 1.6 everyday, and 10% inoculum addition in volume fraction
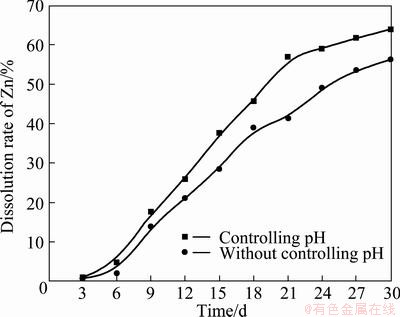
Fig. 3 Effects of pH on zinc dissolution by L. ferrooxidans (Particle size 75μm, rotational speed 160 r/min, 3%(w/v)pulp density, temperature 40 °C and 10% inoculum addition in volume fraction
3.1.3 Effect of external addition of Fe3+ ions
SHI and FANG [3] studied the chemical leaching of marmatite flotation concentrate by Fe3+ ion. The result indicated that the dissolution rate of marmatite rapidly decreased, and it even stopped, with the depletion of Fe3+ ions [3]. As L. ferrooxidans can regenerate Fe3+ ions by oxidizing Fe2+ ions, it may be a good way to add some Fe3+ ions into the system to further improve the dissolution rate of marmatite. So we investigated the effect of additional Fe3+ ions on the marmatite bioleaching with the purpose of finding an optimal amount of addition.
Figure 4 depicts zinc dissolution in experiments carried out with different concentrations of additional Fe3+ ions. The zinc extractions confirm the results observed in other studies that external addition of Fe3+ ions created a chemical contribution and accelerated the dissolution under an appropriate concentration [3,19]. It can be seen that the presence of Fe3+ ions accelerate the marmatite dissolution in the beginning of the leaching experiments, since the marmatite oxidation occurs by a chemical mechanism according to the reaction (1). And then the produced Fe2+ ions can be oxidized into Fe3+ ions by L. ferrooxidans according to the reaction (2), thus generating the oxidizing agent necessary for the dissolution of marmatite. In the presence of 2.5 g/L Fe3+ ions, there was a substrate concentration suitable for the acceleration of marmatite bioleaching. Above 2.5 g/L Fe3+ ions, zinc dissolution became independent of the Fe3+ concentrations, while when the concentration of additional Fe3+ ions was 17.5 g/L, the promoting effect on zinc dissolution became weaker, but the leaching rate was still higher than that without additional Fe3+ ions. At the end of the leaching experiments, the zinc extraction rates were 79.80%, 81.18%, 80.78% and 75.29% for the different external additions of Fe3+ ions of 2.5, 7.5, 12.5 and 17.5 g/L, respectively. The zinc extraction rate was higher than that obtained without additional Fe3+ ions, which was only 61.27% at the pulp density of 3%. The results applied that the chemical oxidation of Fe3+ ions played an important role in the zinc extraction during the leaching process, especially in the initial stage when the cell density and bacterial oxidation ability of ferrous irons were lower. The results also suggested that too high concentration of Fe3+ ions in the leaching system might cause inhibition of bacterial activity and when the concentration of ferric ions was high enough, the precipitation of jarosite started to form. This reaction can be expressed as Eq.(3).
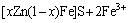 →xZn2++(3-x)Fe2++S0 (1)
→xZn2++(3-x)Fe2++S0 (1)
 (2)
(2)
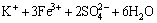 →KFe3(SO4)2(OH)6+6H+ (3)
→KFe3(SO4)2(OH)6+6H+ (3)
3.1.4 XRD analyses of leaching residues
From bioleaching results mentioned above, we can notice that the dissolution rate of marmatite is not complete. In Fig. 5, the X-ray diffraction patterns of residues leached by original L. ferrooxidans after 30 d show that jarosite and sulfur formed during the leaching process. The lower leaching rate may have a relation to the passivation layer formed on the particle surface, which can hinder the bioleaching of marmatite. It also can be seen that the intensity of jarosite formed in the leaching experiment with additional 2.5 g/L Fe3+ ions was stronger than that without additional Fe3+ ions. This suggested that there were more jarosite produced on the surface of particle and then inhibited the dissolution of marmatite.
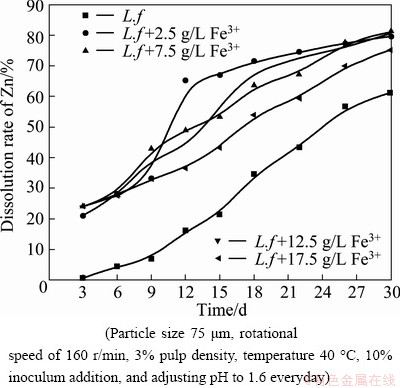
Fig. 4 Effects of different concentrations of Fe3+ on zinc dissolution by L. ferrooxidans
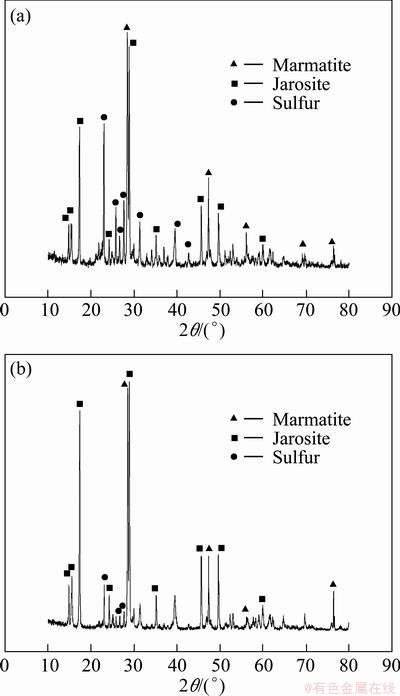
Fig. 5 X-ray diffraction patterns of residues leached by original L. ferrooxidans (a) and leached by original L. ferrooxidans with additional 2.5 g/L Fe3+ ions (b) after 30 d
3.2 Electrochemical study
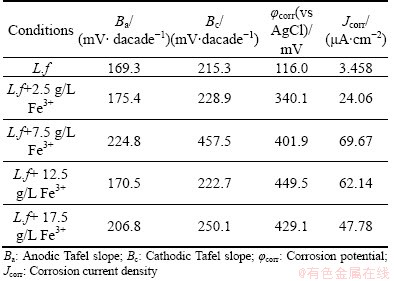
The Tafel polarization curves of the marmatite- carbon paste electrode with various concentrations of Fe3+ ions are described in Fig. 6. From the Tafel polarization curves, the electrochemical corrosion kinetic parameters can be obtained, which are listed in Table 1. As can be seen that the corrosion potential (φcorr) had a significant increase with the addition of Fe3+ ions, while there was a little decline when the concentration of Fe3+ ions was 17.5 g/L. The result is consistent with the tendency of open circuit potential (OCP) change. The open circuit potentials were 120, 368, 420, 475 and 460 mV for the Fe3+ concentrations of 0, 2.5, 7.5, 12.5, 17.5 g/L, respectively. Table 1 also shows that the corrosion current density (Jcorr) had a similar tendency with the corrosion potentials. The Jcorr in the electrolyte with Fe3+ ions was much higher than that without additional Fe3+ ions. The higher value of the corrosion current density suggests that the dissolution rate of the marmatite is higher during the experiments. In other words, the external addition of Fe3+ ions facilitates the extraction of marmatite [20]. This is little different from the results obtained by the bioleaching process. It may be attributed to the more complex leaching environment. At the beginning of the experiments, the original bacteria need time to acclimatize themselves to the solution with additional Fe3+ ions, and it needs much more time to thrive with higher concentrations of Fe3+ ions. As the bioleaching experiment processing, there were many ions dissolved into the solution. At the same time, the precipitation of jarosite and sulfur formed and accumulated on the particle surface, which made the further dissolution of marmatite more difficult. The more the concentration of Fe3+ ions in the solution is, the easier and the more the jarosite produced during the leaching process. Then it had some bad effects on the zinc dissolution in the bioleaching studies.
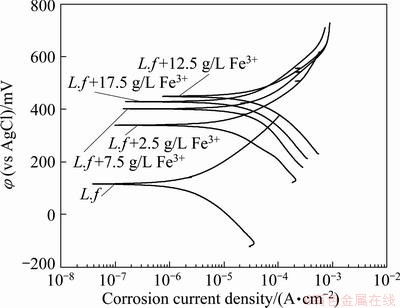
Fig. 6 Effects of Fe3+ ions on Tafel curves of marmatite-carbon paste electrode in the presence of pure original strain of L. ferrooxidans at 40 °C and scan rate of 1 mV/s with sweep range of ±250 mV vs OCP
Table 1 Tafel parameters of marmatite-carbon paste electrode with different concentrations of Fe3+ ions
Figures 7 and 8 illustrate the electrochemical impedance spectroscopy (EIS) of the marmatite-carbon paste electrode without and with different concentrations of Fe3+ ions in the presence of L. ferrooxidans under the open circuit potential.
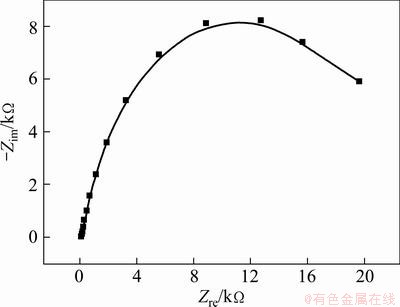
Fig. 7 Electrochemical impedance spectroscopy (EIS) of marmatite-carbon paste electrode in the presence of L. ferrooxidans at 40 °C without additional Fe3+ ions under open circuit potential
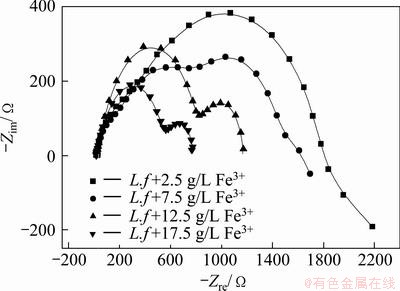
Fig. 8 Electrochemical impedance spectroscopy (EIS) of marmatite-carbon paste electrode with different concentrations of Fe3+ ions in the presence of L. ferrooxidans at 40 °C under open circuit potential
From Fig. 7, it can be seen a large capacitance loop at the tested frequency scope when the EIS measurement was carried out in the electrolyte without additional Fe3+ ions in the presence of L. ferrooxidans, which suggests that the oxidation reaction of the marmatite is controlled by the charge transfer [21].
Compared with Fig. 7, Fig. 8 shows an obvious change in the EIS spectra in the system with additional Fe3+ ions. The diagrams were dramatically depressed with the increase of Fe3+ ions, which implied the improving function of Fe3+ ions on the dissolution of marmatite. At the same time, the impedance spectra showed new features. There were two semicircles appeared in the system, while the first one was an incomplete capacitance arc at a higher frequency in the solution with 2.5 and 7.5 g/L Fe3+ ions. The results illustrate that the rate-limiting step was not changed [12,21,22]. The semicircle in the high frequency may be caused by the attachment of bacterial cells or/and the precipitation of reaction products. While the semicircle in the low frequency may have relation to the capacitance existed at the electrode-solution interface and the electrode transfer resistance of electrochemical reaction during the dissolution process of marmatite [12,21]. In Fig. 6, we can see that additional Fe3+ ions increased the current density, the reactions may occur easily and the reaction products may accumulate on the electrode surface, and then it may lead to the results that semicircle in the high frequency became larger when more addition Fe3+ ions presented in the electrolyte. The semicircle in the low frequency is affected by the redox potential by changing the double layer capacitance and ion transfer, when increasing additional Fe3+ ions were added into the electrolyte, the double layer capacitance was progressively depressed smaller. At the same time, the difference of the density of Fe3+ between the electrolyte solution and electrode became bigger, and then ion transfer of Fe3+ became easier from the electrolyte to the electrode. So the semicircle changes and becomes progressively smaller with the higher OCP in the system with increasing concentration of additional Fe3+. The result suggests that Fe3+ ions play a positive role in the extraction of zinc from marmatite.
4 Conclusions
1) The pulp density has an obvious effect on the dissolution of marmatite. Controlling pH during the leaching process can facilitate the dissolution of zinc. External addition of Fe3+ can promote zinc extraction, while the positive effects may decline when the concentration of additional Fe3+ ions was too high. Fe3+ ions play an important role in the dissolution of marmatite, especially in the initial stage when the cell density and bacterial oxidation ability of ferrous irons were lower. The best zinc dissolution, about 81.18%, was achieved with additional Fe3+ ions of 7.5 g/L, which is about 20% higher than that without additional Fe3+ ions. The XRD analyses show that there are more jarosite produced when Fe3+ ions are added. The jarosite and sulfur accumulate on the particle surface and then inhibit the zinc dissolution.
2) The electrochemical behaviors of the marmatite-carbon paste electrode show that external addition of Fe3+ ions increases the corrosion current density, which means that the dissolution of marmatite becomes rapid, accordingly promotes the dissolution of marmatite. The EIS spectra indicate that the rate limiting step does not change when Fe3+ ions are added. The dissolution of marmatite is controlled by the charge transfer process.
References
[1] RODRIGUEZ Y, BALLESTER M L, GONZALEZ F, MUNOZ J A. New information on the sphalerite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy 2003, 71(1): 57-66.
[2] WEISENER C G, SMART R S, GERSON A R. A comparison of the kinetics and mechanism of acid leaching of sphalerite containing low and high concentrations of iron [J]. Int J Miner Process, 2004, 74(1): 239-249.
[3] SHI Shao-yuan, FANG Zhao-heng. Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans [J]. Hydrometallurgy, 2004, 75(1): 1-10.
[4] SHI Shao-yuan, FANG Zhao-heng. Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(3): 569-575.
[5] SHI Shao-yuan, FANG Zhao-heng, NI Jin-ren. Bioleaching of marmatite flotation concentrate with a moderately thermoacidophilic iron-oxidizing bacterial strain [J]. Mineral Engineering, 2005, 18(11): 1127-1129.
[6] SHI Shao-yuan, FANG Zhao-heng. Bioleaching of marmatite flotation concentrate by adapted mixed mesoacidophilic cultures in an air-lift reactor [J]. Int J Miner Process, 2005, 76(1): 3-12.
[7] WANG Jun, QIU Guan-zhou, QIN Wen-qing, ZHANG Yan-sheng. Microbial leaching of marmatite by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 937-942.
[8] CHEN Song, QIN Wen-qing, QIU Guan-zhou. Effect of Cu2+ ions on bioleaching of marmatite [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1518-1522.
[9] GIAVENO A, LAVALLE L, CHIACCHIARINI P, DONATI E. Bioleaching of zinc from low-grade complex sulfide ores in an airlift by isolated Leptospirillum ferrooxidans [J]. Hydrometallurgy, 2007, 89(1): 117-126.
[10] TRIBUTSCH H, ROJAS-CHAPANA J A. Metal sulfide semiconductor electrochemical mechanisms induced by bacterial activity [J]. Electrochimica Acta, 2000, 45(28): 4705-4716.
[11] HANSFORD G S, VARGAS T. Chemical and electrochemical basis of bioleaching processes [J]. Hydrometallurgy, 2001, 59(2): 135-145.
[12] SHI Shao-yuan, FANG Zhao-heng, NI Jin-ren. Electrochemical impedance spectroscopy of marmatite-carbon paste electrode in the presence and absence of Acidithiobacillus ferroooxidans [J]. Electrochemistry Communications, 2005, 7(11): 1177-1182.
[13] OLUBAMBI P A, POTGIETER J H, NDLOVU S, BORODE J O. Electrochemical studies on interplay of mineralogical variation and particle size on bioleaching low grade complex sulphide ores [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(5): 1312-1325.
[14] CHOI W K, TORMA A E, OHLINE R W, GHALI E. Electrochemical aspects of zinc sulphide leaching by Thiobacillus ferrooxidans [J]. Hydrometallurgy, 1993, 33(1): 137-152.
[15] YASUHIRO K, HIROTUGU N, SATORU A. Bioleaching of sphalerite by the acidophilic thermophile Acidianus brierleyi [J]. Hydrometallurgy, 1998, 47(2): 339-352.
[16] LIU Yun, DANG Zhi, WU Ping-xiao, LU Jing, SHU Xiao-hua, ZHENG Liu-chun. Influence of ferric iron on the electrochemical behavior of pyrite [J]. Ionics, 2011, 17(2): 169-176.
[17] OLUBAMBI P A, NDLOVU S, POTGIETER J H, BORODE J O. Role of ore mineralogy in optimizing conditions for bioleaching low-grade complex sulphide ores [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(5): 1234-1246.
[18] ACEVEDO F, GENTINA J C, VALENCIA P. Optimization of pulp density and particle size in the biooxidation of a pyritic gold concentrate by Sulfolobus metallicus [J]. World Journal of Microbiology and Biotechnology, 2004, 20(8): 865-869.
[19] PINA P S, LEAO V A, SILVA C A, DAMAN D, FRENAY J. The effect of ferrous and ferric iron on sphalerite bioleaching with Acidithiobacillus sp. [J]. Mineral Engineering, 2005, 18(5): 549-551.
[20] SHI Shao-yuan, FANG Zhao-heng, NI Jin-ren. Electrochemistry of marmatite - carbon paste electrode in the presence of bacterial strains [J]. Bioelectrochemistry, 2006, 68(1): 113-118.
[21] ZENG C L, WANG W, WU W T. Electrochemical impedance models for molten salt corrosion [J]. Corrosion Science, 2001, 43(4): 787-801.
[22] BEVILAQUA D, DIEZ-PEREZ I, FUGIVARA C S, SANZ F, BENEDETTI A V, GARCIA J R O. Oxidative dissolution of chalcopyrite by Acidithiobacillus ferrooxidans analyzed by electrochemical impedance spectroscopy and atomic force microscopy [J]. Bioelectrochemistry, 2004, 64(1): 79-84.
铁闪锌矿的Leptospirillum ferrooxidans菌浸出及电化学性能
班进荣,顾帼华,胡可婷
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:采用纯种L.ferrooxidans菌研究矿浆浓度、pH及外加Fe3+离子对铁闪锌矿生物浸出的影响。结果表明,锌的浸出率随着矿浆浓度的降低而增加。在生物浸出过程中调节pH值到1.6对铁闪锌矿的溶解有促进作用。外加Fe3+离子加速了铁闪锌矿的生物浸出,但当外加Fe3+离子浓度超过2.5 g/L时,促进作用变弱。这是因为高浓度的Fe3+离子会对细菌生长产生抑制作用且促进黄钾铁矾的生成。在L. ferrooxidans菌存在条件下,利用电化学测试方法进一步了解有、无外加Fe3+离子时铁闪锌矿的溶解过程。实验数据表明,外加Fe3+离子可以增加腐蚀电流密度,有利于锌的提取。交流阻抗谱表明,添加Fe3+离子后没有改变反应过程的控制步骤。
关键词:生物浸出;铁闪锌矿;电化学测试;L. ferrooxidans菌
(Edited by Xiang-qun LI)
Foundation item: Project (2010CB630903) supported by the National Basic Research Program of China
Corresponding author: Guo-hua GU; Tel: +86-731-88830545; E-mail: guguohua@126.com
DOI: 10.1016/S1003-6326(13)62490-5
Abstract: The effects of several variables on the bioleaching of marmatite with pure L. ferrooxidans were investigated. The results show that zinc extraction increases with the decrease of pulp density. Adjusting pH to1.6 during the bioleaching process has a positive effect to the dissolution of marmatite. External addition of Fe3+ ions accelerates the bioleaching, while the concentration of additional Fe3+ over 2.5 g/L weakens the acceleration effect due to the inhibition effect on bacteria growth and the promotion of jarosite production. The electrochemical measurements were used to make further understanding on the dissolution of marmatite with and without additional Fe3+ in the presence of L. ferrooxidans. The experimental data illustrate that additional Fe3+ ions could increase the corrosion current density, which is favorable to zinc extraction. The EIS spectra show that rate-limiting step does not change when Fe3+ is added.


