FeTi基储氢材料的研究及进展
冯佃臣 况家瑾 孙学贤 袁倩 袁泽明 张羊换
内蒙古科技大学材料与冶金学院
山东坤能环保科技有限公司
摘 要:
氢能具有资源丰富,能量高,清洁环保等优点,被视为21世纪最具发展潜力的清洁能源。很多国家和地区都对它进行了大量的研究,使其充分利用并发挥氢的性能。而对于氢能储运是氢能充分利用的关键。现今有很多的学者研究优异的储氢材料来提高氢能的储存效率。而其中FeTi系合金便是一种良好的储氢材料。FeTi系合金中的元素在自然中含量丰富价格便宜,并且FeTi系合金作为储氢材料具有吸放氢温度低等优点,因此它被认为是有很大应用前景的储氢材料。但FeTi基储氢材料也存在一些问题如:活化困难、容易与合金中的很多杂质元素及空气作用中毒而使活性失效等,这些问题都会制约FeTi基储氢材料的发展。本文就目前一些学者通过掺入合金元素(如Zr,V,Mn等)、机械变形和热处理等手段改善FeTi基合金的储氢性能的研究工作进行了总结,并针对FeTi基合金存在的这些问题,提出了防止其与一些杂质元素作用而活性失效以及改善其储氢材料性能措施的建议,为储氢材料领域的研究提供参考。
关键词:
储氢材料 ;FeTi基合金 ;研究进展 ;活化性能 ;
中图分类号: TG139.7
作者简介: 冯佃臣(1977-),男,内蒙古乌兰察布人,博士,副教授,研究方向:储氢材料、金属腐蚀与防护、金属热处理,电话:0472-5951572,E-mail:fdc21@imust.cn;
收稿日期: 2020-01-14
基金: 国家自然科学基金项目(5190115,51871125,51761032); 内蒙古自然科学基金项目(2020MS05073,219BS05005); 内蒙古科技大学校内基金项目(2019QDL-B111)资助;
Research and Progress in FeTi Based Hydrogen Storage Materials
Feng Dianchen Kuang Jiajin Sun Xuexian Yuan Qian Yuan Zeming Zhang Yanghuan
School of Materials and Metallurgy,Inner Mongolia University of Science and Technology
Shandong Kun Neng Environmental Protection Technology Co.
Abstract:
As a clean and high density energy,hydrogen energy was widely used in fuel cell vehicles,hydrogen power generation,hydrogen heating and many other fields. The key to make full use of hydrogen energy was to improve the storage and transportation of hydrogen energy. In order to make full use of the properties of hydrogen volatilization,many researchers had done a lot of researches on hydrogen storage alloys. Among them,FeTi based alloy was a good hydrogen storage material. The elements in the FeTi based alloy were rich in content and cheap in price in nature,and the FeTi based alloy had the advantage of low hydrogen absorption and desorption temperature in the preparation of hydrogen storage materials,so it was considered to be a hydrogen storage material with great application prospects. However,there were many problems in FeTi based hydrogen storage materials,such as the difficulty of activation,the possibility of poisoning due to interaction with impurities in the alloy and air,etc. These problems restrict the development of FeTi based hydrogen storage materials. The methods to improve the hydrogen storage properties of FeTi based alloys by adding alloying elements(such as Zr,V,Mn,etc.),mechanical deformation and heat treatment,and to inhibit the activity failure by controlling its own impurity elements and adding Zr were summarized. In order to improve the activation property of FeTi based alloy,several strategies were adopted.(1)The hydrogen storage properties of FeTi based alloy were improved by adding alloying elements(Zr,V,Ce,Mn,Ni).(2)The hydrogen storage properties of FeTi based alloy were improved by ball milling,cold rolling,mechanical milling,forging,equal channel angular pressing and high pressure torsion.(3)By controlling the cooling rate,the microstructure of FeTi based alloy was changed,and the hydrogen absorption kinetics of FeTi based alloy was improved.(4)The hydrogen storage properties of the oxidized FeTi based alloys were restored by heat treatment. The content of impurity elements(such as C,Si,Al,O,etc.)should be strictly controlled during the preparation of FeTi based alloy to prevent the active failure of FeTi based alloy. The introduction of high content of Zr into FeTi based alloy can effectively reduce the interaction between the alloy and air,thus weakening the adverse effects.The results were listed as follows:(1)The dissociation energy of hydrogen molecule could be degraded by adding alloy elements,such as easily oxidized element(Mn),because it would be preferentially oxidized and form segregation layer on the alloy surface. In addition,the oxide formed by these elements was embedded on the surface of the alloy,which reduced the compactness of the dense layer of TiO2 layer and improves its activation performance. In addition,if the elements(Zr,Si)forming the second phase were added,the second phase was easily formed in the alloy,and the distribution was uniform,which became the channel for hydrogen atoms to enter the main TiFe phase in the process of hydrogen absorption. In addition,these elements were also easy to form Ti rich phase,which would absorb hydrogen prior to TiFe phase,leading to the formation of microcracks and nucleation sites,promoting hydrogen absorption and improving activation properties.(2)Through ball milling,cold rolling,mechanical milling,forging,equal channel angular pressing,high pressure torsion and other mechanical deformation methods,the grain size could be quickly and effectively reduced,the material defects could be induced,and the activation time could be shortened,so as to improve the hydrogen storage performance of FeTi based alloy.(3)By controlling the cooling rate,the microstructure of FeTi based alloy was changed and the contact between the secondary phase and the main phase was increased. The interface between the secondary phase and the main phase became larger,which led to the decrease of the diffusion distance of hydrogen between the secondary phase and the main phase of TiFe and improved the hydrogen absorption kinetics of FeTi based alloy.(4)The hydrogen storage properties of the oxidized FeTi based alloy were restored by heat treatment.(5)The introduction of high content of Zr into FeTi based alloy could effectively reduce the interaction with air,thus improving the adverse effects. The content of impurity elements(such as C,Si,Al,O,etc.)in FeTi based alloy should be strictly controlled to prevent the active failure of FeTi based alloy. FeTi based alloys had great prospects in the development of hydrogen storage materials. However,its application was limited due to its harsh activation conditions and easy poisoning. The activation properties could be improved by alloying,mechanical deformation and heat treatment. In addition,alloy elements could be added and the content of impurity elements could be strictly controlled to prevent its activity failure.
Keyword:
hydrogen storage materials; FeTi based alloy; research progress; activation property;
Received: 2020-01-14
随着工业的发展和物质生活水平的提高,人类对能源的需求也与日俱增。近年来,能源的消耗主要来自于化石燃料,如煤碳、石油和天然气等,但化石燃料不仅储备有限,而且还会造成环境污染。因此,迫切地需要一种新型的绿色清洁能源来取代化石燃料。氢能作为一种绿色清洁能源,其不仅储量丰富、来源广泛、而且质量能量密度高(142 MJ·kg-1 )。故此,氢能被广泛应用于燃料电池汽车、氢能发电、氢能供暖等诸多领域。氢能的规模化应用包括氢的制取、储运、应用,但由于氢易燃,易爆,体积能量密度低等特点,氢的储运成为制约氢规模化应用的瓶颈。因此,迫切需要一种高能量密度,高安全性的储氢材料。所以,对储氢材料的研究,既要提高其氢存储的安全性,也要提高其储氢的能量密度。
在众多的储氢合金中,Fe Ti基合金不仅能够在室温下可逆吸收大量的氢气,而且具有优秀的吸放氢动力学性能。另外,其资源广泛,价格低廉,并适用于大规模的生产,是当今最接近实用化的储氢材料,具有很可观的发展前景。但是,Fe Ti基合金也存在着活化困难的缺点,需要在高温高压的条件下才能活化,而且活化后的合金,在氧气、一氧化碳、水等环境下容易中毒,再次失去活性
[1 ]
,这严重地制约了Fe Ti基储氢合金的广泛应用。因此,如何解决活化问题成为提高Fe Ti基储氢材料性能的关键。
1 Fe Ti基储氢材料存在的问题
1.1 活化困难
Fe Ti基储氢材料的活化需要在高温高压的条件下,经过多次的活化才能完成,比较困难。由于在反应过程中Fe与氧的结合力没有Ti与氧的结合力大,所以在表面优先生成了Ti O2 ,此Ti O2 致密层地堆积,导致氢原子难以与主相Ti Fe相发生氢化反应,合金活化困难
[2 ,3 ,4 ,5 ,6 ,7 ]
。Ti具有很强的吸附性,其将O2 ,CO2 等气体聚集在合金表面,降低了合金表面氢分子离解能,使氢分子的离解成为了制约合金吸氢的因素,因此导致活化困难
[8 ]
。此未改性的Fe Ti基合金通常需要在673 K以上高温和67 MPa的高压下才能完成活化
[9 ,10 ,11 ]
,这在实际操作过程中,是一个非常严苛的条件。
1.2 容易毒化
Fe Ti基合金对杂质元素有高的敏感度,像C,Al,Si等杂质元素,极容易使其中毒,并失去活性,降低储氢性能。另外,氧气、一氧化碳、水等均能使Fe Ti基合金在吸氢过程中中毒,从而失去活性
[1 ]
。所以,如何控制杂质元素和气体是Fe Ti基合金储氢材料能否实际应用的一个重要课题。
2 Fe Ti基储氢材料的研究进展
针对Fe Ti基储氢材料活化困难、容易中毒等缺点,研究者已经做了许多改进研究工作,并取得了很大进展。包括合金化法,机械球磨,冷却速度等,现总结如下:
2.1 单一元素对Fe Ti基储氢材料的活化性能的改善
目前,加入的单一元素有Zr,V,Ce,Mn,Ni等。有研究表明,通过掺杂过渡族元素Zr
[2 ,5 ,12 ,13 ,14 ,15 ]
和V
[16 ,17 ,18 ]
能够改善Fe Ti基合金的首次吸氢动力学。Gosselin和Huot
[19 ]
研究了掺杂4%(质量分数)的Zr对Fe Ti基合金的影响,发现添加Zr后形成的富Zr的二次相可以作为将氢从样品表面运输到内部的通道,这对Fe Ti基合金在室温和4.5 MPa氢气压力下进行首次吸氢非常有帮助。Kumar等
[16 ]
研究了V对Fe Ti合金的影响,发现3.1%(质量分数)的V掺杂后在327 K时表现出较快的吸氢动力学,这是因为V加入引起Fe Ti系中晶格的膨胀,降低了氢扩散的活化势能,从而加速吸氢速度。而且V与氢具强相互作用,也有助于增加氢分子解离,从而促进吸氢动力学。Jain等
[20 ]
研究发现,Fe Ti基合金中加入Zr后,Fe Ti基合金的首次吸氢不需要任何预先热处理。
最近,Leng等
[21 ]
发现,在初始氢气压力为4.0MPa的情况下,加入少量的Ce到Ti Fe0.9 Mn0.1 合金中,可以显著改善活化性能,使其能在353 K的温度下吸收氢。这是由于形成Ce氢化物时,引起的晶格膨胀造成合金表面的微裂纹,从而改善了FeTi基合金吸氢性能。
据文献报道,少量的Mn,Al,Ni元素能够置换部分Fe,有利于活化过程
[22 ,23 ,24 ]
。一般来说,任何具有较高氧化稳定性的元素,都能促进Fe Ti的活化。特别是Mn,它可以化学吸附氢,使其产生明显的活化增强效应
[22 ,25 ,26 ]
。进一步的研究发现,Mn的加入,能够使Fe Ti基合金在室温下活化,但其不利于动力学性能。此外,Patel等
[27 ]
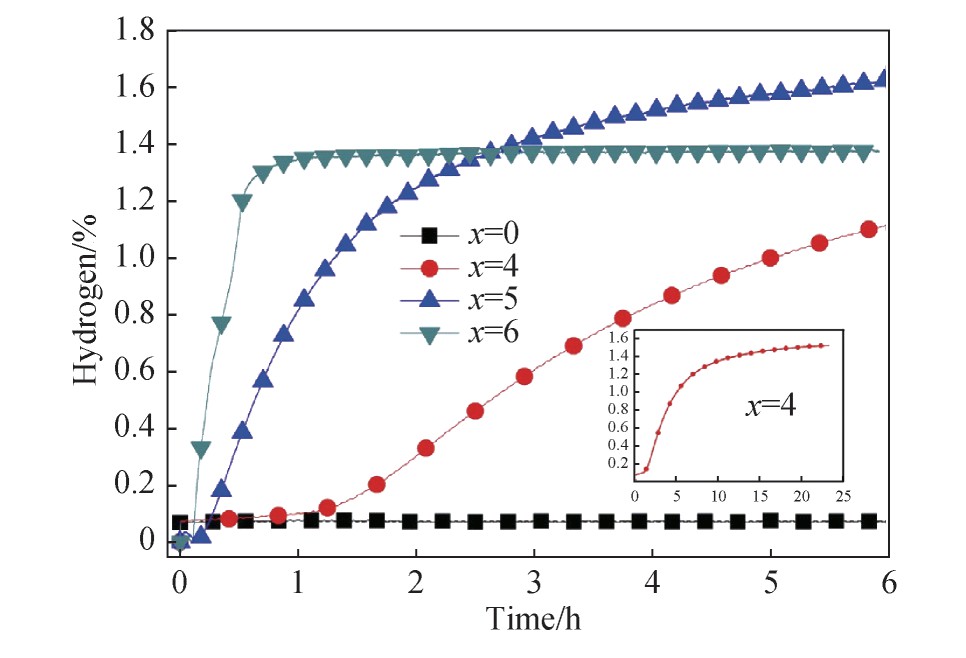
报道,含2%Mn和4%Zr的合金比仅含单一Zr的合金具有更好的吸氢动力学性能,如表1所示。这是因为第二相的形成促进了吸氢过程。这表明了混合两种元素对于Fe Ti基合金储氢性能的改善是更有效的。
2.2 多元合金化对Fe Ti基合金储氢性能的改善
Lv等
[28 ]
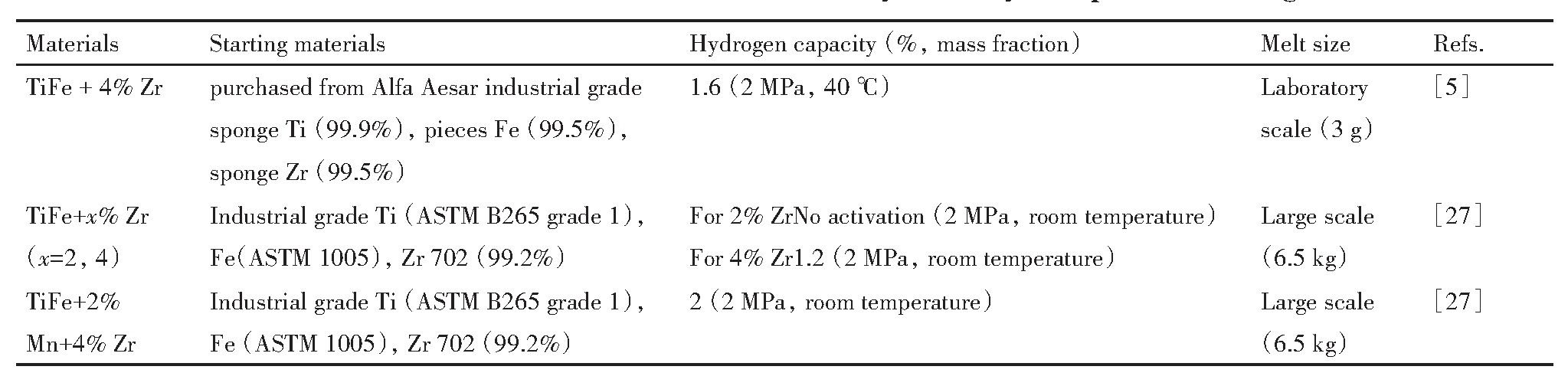
研究了Ti Fe+x%(Zr+2V)(x=0,4,5,6)合金组织和储氢性能,结果表明:掺杂后合金的晶体结构基本没有发生改变,均含有Ti Fe主相和类似于Mg Zn2 类的hcp二次相。如图1所示,其中掺杂(Zr+2V)可以明显改善Fe Ti基合金的首次吸氢动力学。因为合金在吸氢过程中,二次相较Ti Fe主相优先吸收氢,而且该二次相的膨胀可以使合金晶粒破碎,减小颗粒尺寸,并产生大量裂纹,不仅有利于氢的扩散,同时也使合金显露出更多新的表面,从而促进Fe Ti基储氢材料吸氢动力学。
Patel等
[27 ]
研究发现在2 MPa的氢压下,Ti Fe+2%Mn+4%Zr合金具有最快的动力学和最高的储氢容量(图2),在5 h后吸氢容量可达2%,这可能与形成相的微观结构和化学成分有关。与其他合金相比,在含2%Mn+4%Zr的合金中,二次相(Ti和Ti2 Fe)在Fe Ti合金中分布较细且均匀,其次第二相的化学组成(Ti和Ti2 Fe相)能起作用,促进首次吸氢动力学,并且使得吸氢容量变大。因此,多元合金化能有效提高Fe Ti基合金的储氢性能。
表1 Patel当前对Fe Ti基合金工作的研究与以往调查的区别 下载原图
Table1 Difference between Patel's current research onwork of Fe Ti system alloys and previous investigations
图1 Ti Fe+x%(Zr+2V)(x=0,4,5,6)合金在室温和2 MPa氢气压下的首次吸氢曲线
Fig.1 First hydrogenation curves of Ti Fe+x%(Zr+2V)(x=0,4,5 and 6)alloy at room temperature and under 2MPa hydrogen pressure
[28]
图2 室温和2 MPa氢气压下Fe Ti基合金的活化动力学
Fig.2 Activation kinetics of Fe Ti alloys at room temperature under 2 MPa hydrogen pressure
[27]
总而言之,元素的掺杂可以改善Fe Ti基合金的活化性能,主要原因有如下几个方面:(1)易氧化的元素(如Mn)的掺杂,这类元素由于其会被优先氧化,并在合金表面形成偏析层,能够降级氢分子的离解能。此外,这些元素形成的氧化物,镶嵌在合金的表面,降低了Ti O2 层致密层的紧实度,从而改善了其活化性能。(2)易形成第二相的元素(如Zr,Si)的掺杂,这类元素,容易在合金中原位形成第二相,且分布均匀,成为了吸氢过程中,氢原子进入主相Ti Fe相的通道。此外,这些元素也容易形成富Ti相,富Ti相会先于Ti Fe相吸氢,从而导致微裂纹和形核位点的形成,促进吸氢,改善活化性能。
2.3 机械变形对Fe Ti基合金储氢性能的研究
Chiang等
[7 ]
在氢气氛下研究Fe Ti基合金的吸氢性能。发现,球磨的钛铁粉在没有任何预先热处理的情况下可以吸氢,这表明了球磨促进了Fe Ti基合金的吸氢反应。另外Emami等
[30 ]
利用球磨技术合成了具有纳米晶结构的Fe Ti基合金。研究表明,球磨降低了合金粉末的颗粒尺寸,促进了首次吸氢反应。Manna等
[4 ]
研究发现,在空气中暴露后的Ti Fe+4%Zr合金,其吸氢性能可以通过球磨恢复。然而,吸氢速度仍然缓慢。Patel等
[29 ]
研究了球磨对Fe Ti基合金的吸氢性能的影响,研究表明球磨过程降低了颗粒尺寸并使合金出现了新鲜的表面层,促进了合金吸氢。这些研究都表明了通过球磨技术,能促进Fe Ti基储氢合金的吸氢反应。
Manna等
[4 ]
在对空气中暴露7 d的Ti Fe+4%Zr合金粉进行了研究,发现通过冷轧能够提高其储氢能力。但Ti Fe+4%Zr合金的氢容量会降低,这是因为在机械变形中形成了晶格缺陷和应力能够增加金属氢化物的平台压力。另外晶粒尺寸的减少,导致了晶界数量增加,为氢的扩散提供更多的通道,改善了合金的动力学性能。但是,晶界本身无法储存氢气,所以总的氢净容量有所下降。这是冷轧引起氢容量降低的原因。再者,Vega等
[3 ]
报道了在氩气氛下冷轧的Fe Ti基合金活化更容易,吸氢的最大容量约为1.4%。另外,通过高压扭转等严重的塑性变形来改变微观结构
[29 ]
,也能促进Fe Ti基合金的活化过程。Lv等
[28 ]
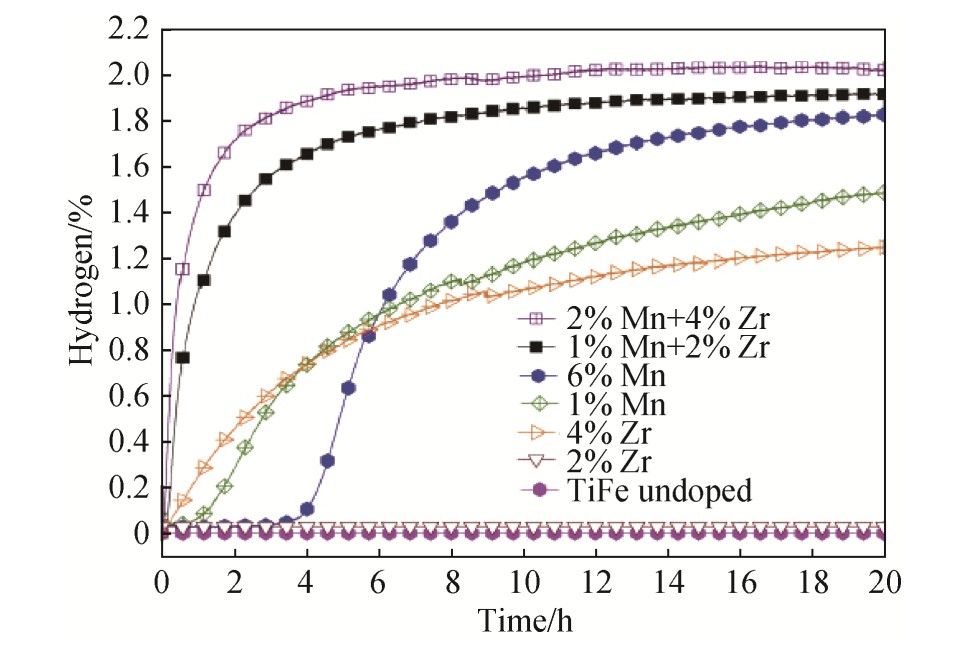
对在空气中暴露后的Ti Fe+x%(Zr+2V)(x=0,4,5,6)分别进行掺杂、冷轧和锻造工艺。研究了其对合金的微观结构和储氢性能的影响。得出结论为:(1)x=4的合金经锻造后,其首次吸氢动力学最好。如图3所示,在空气中不需要任何的热处理便可以吸氢,且吸氢容量最大为1.7%。吸氢动力学的加速是由于晶界、晶体缺陷的产生以及微观结构细化。吸氢容量较冷轧处理后的大的原因是经锻造后的样品氧化程度较低。(2)冷轧也可以改善吸氢动力学,但冷轧后合金的吸氢容量较锻造后合金低。这可能主要是由于冷轧会产生非常细小的颗粒,使得合金很容易在瞬间被氧化,从而降低合金的吸氢性能。
图3 室温和2 MPa氢气压下铸态、冷轧态和锻造态下Ti Fe+x%(Zr+2V),x=4合金的首次吸氢动力学
Fig.3 First hydrogenation kinetics of Ti Fe+x%(Zr+2V),x=4alloy in as-cast,cold rolling and forging states at room temperature and under 2 MPa hydrogen pressure
[28]
2.4 冷却速度对Fe Ti基合金储氢性能的改善研究
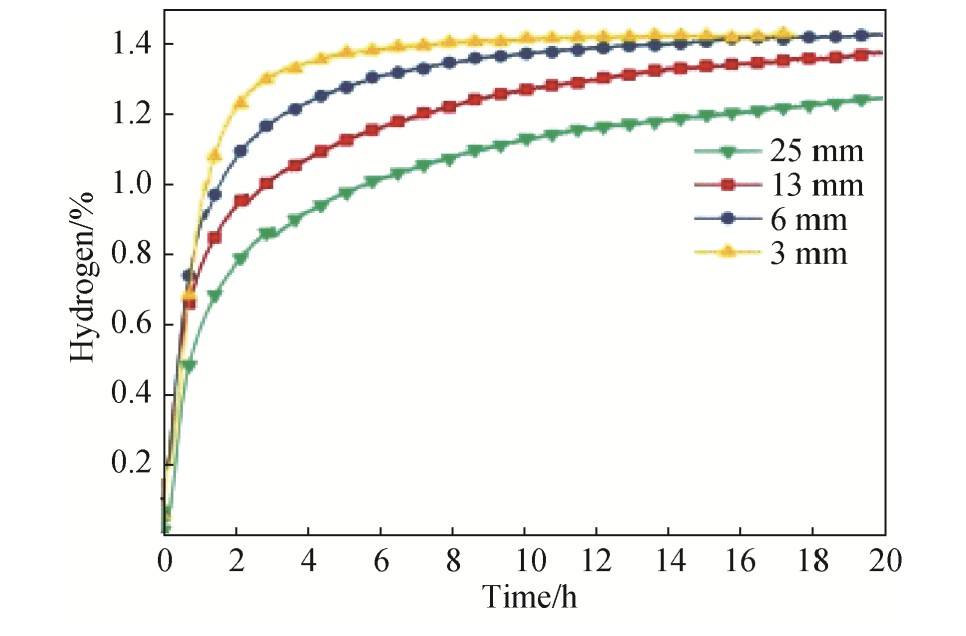
Patel等
[29 ]
采用阶梯模(图4)研究了不同冷却速率对加入4%Zr的Fe Ti基合金微观组织和吸氢行为的影响。采用感应熔炼法合成4%Zr的Fe Ti基合金钢锭,用厚度不同的阶梯模具,得到不同冷却速度下的合金。研究发现,快的冷却速度会使得二次相(Ti Fe2 ,Ti2 Fe,Ti)产生分布均匀的枝晶组织,并说明了,经过不同冷却速度获得的合金首次吸氢速率与Ti Fe主相和第二相的接触相界面总和成比例,另外还发现,更快的冷却速度会提高铸态合金的吸氢动力学性能,如图5所示
[29 ]
。因为随着冷却速率增加二次相与主相的接触相界面会变大,从而导致氢在第二相和Ti Fe主相间的扩散距离变小。所以能改善Fe Ti基合金吸氢动力学性能。
图4 25,13,6和3 mm厚的球墨铸铁阶梯模
Fig.4 A ductile cast iron step mold of thickness of 25,13,6and 3 mm
[29]
因此,通过改变铸锭的冷却速率,来改变合金微观结构和二次相的结晶程度,对促进Fe Ti基储氢材料的吸氢性能也很有意义。
3 针对Fe Ti基储氢材料存在的问题提出的解决措施和建议
3.1 提高其活化性能
3.1.1 通过合金化来改善Fe Ti基储氢合金的活化性能
根据以上研究可知,针对Fe Ti基储氢材料的活化困难,可以通过加入其他合金元素来改善其性能。
(1)加入单一过渡族元素。Zr,V,Mn,Ce,Ni等均能改善Fe Ti活化性能。这是因为这些元素在Fe Ti基合金系中会形成有益的二次相或者能与氢强作用。
(2)加入两种或两种以上的元素。添加Ti Fe+x%(Zr+2V)可以明显改善Fe Ti基合金的首次吸氢动力学,因为形成hcp结构的Mg Zn2 二次相能够先于Ti Fe主相吸氢,且其吸氢膨胀会使合金颗粒断裂,并产生大量裂纹,暴露出更多新的表面,从而促进Fe Ti基合金的吸氢性能
[28 ]
。其中,添加含2%Mn+4%Zr的Fe Ti基合金具有更快的动力学和更高的容量。
图5 室温和2 MPa下的氢条件下随不同厚度的模具活化Ti Fe+4%Zr的时间
Fig.5 Activation time of Ti Fe+4%Zr with different thickness of die at room temperature and 2 MPa hydrogen pres-sure
[29]
3.1.2 通过热处理改善Fe Ti基合金的储氢性能
Modi等
[31 ]
对在空气中完全氧化2 h而丧失储氢能力的Ti Fe0.85 Mn0.15 合金进行研究,发现氧化后的合金在氢气压力下,温度为573 K时进行15 min的热处理,会使得该合金的储氢能力迅速恢复。其原因为,加热过程中氧化铁还原和Fe0.82 Mn0.18 fcc相的再活化结果,促进氢通过合金的富氧层表面,从而恢复合金的吸氢动力学。这说明通过一定的热处理可以恢复被氧化的Fe Ti基储氢合金的吸氢能力。
3.1.3 通过机械形变来改善Fe Ti基储氢材料的活化性能
机械变形法可以快速、有效地降低晶粒尺寸并诱发材料
[32 ,33 ]
缺陷,从而改善吸氢动力学性能,缩短活化时间。因此,它是改善储氢材料
[34 ,35 ,36 ,37 ,38 ,39 ]
首次吸氢动力学的有效途径。常用的机械变形方法有球磨
[4 ,40 ,41 ,42 ]
、冷轧
[4 ,32 ,43 ,44 ]
、机械铣削、锻造
[45 ]
、等角度挤压
[46 ,47 ,48 ,49 ]
、高压扭转
[50 ,51 ,52 ]
等。其中锻造是缩短Fe Ti基合金孕育时间,并改善Fe Ti基合金首次吸氢动力学的最有效的方法。此外,锻造加工简单,不仅明显加快吸氢反应,降低氧化程度,而且也不以牺牲吸氢容量为代价。所以,对于提高储氢材料活化性能,锻造是最理想的机械加工方法。
3.2 解决Fe Ti基储氢材料的中毒问题
Fe Ti基储氢材料容易与杂质元素和杂质气体发生反应,使合金毒化,从而降低其储氢性能和活化性能。
3.2.1 减弱外界杂质对Fe Ti基储氢材料消极效应的研究
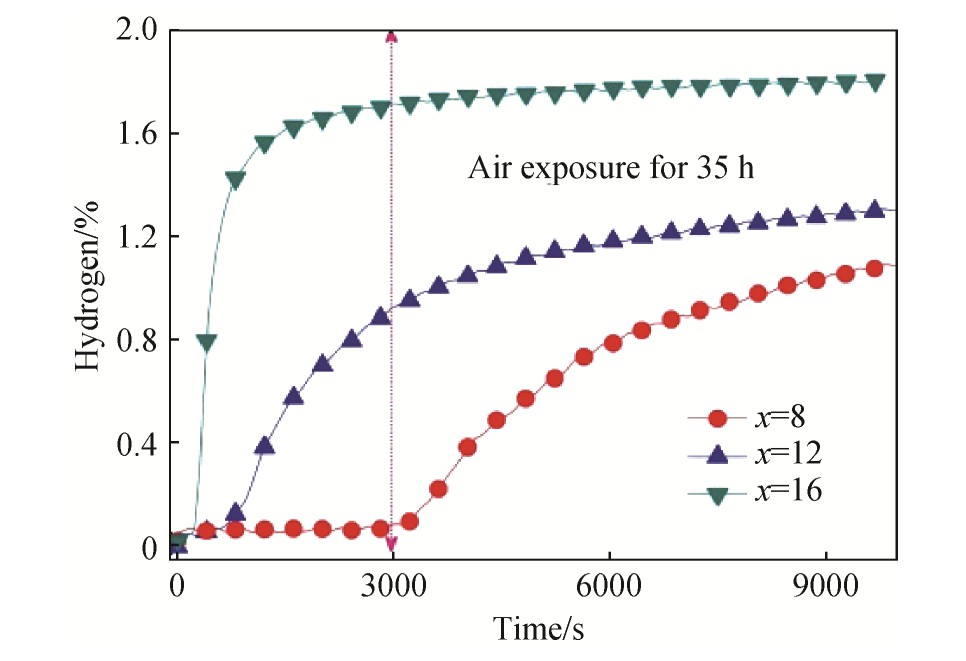
通过加入合金元素可以提高抗毒能力。例如加入高含量的Zr能有效提高Fe Ti基合金的抗毒能力。有研究表明:Fe Ti+x%Zr(x=8,12,16)的合金系中,x=16的Zr在Fe Ti基储氢材料中显示出了最高吸氢容量和最短活化时间,如图6所示。这是因为,添加高Zr可以有效抵御空气与Fe Ti基合金的接触,从而改善中毒程度
[53 ]
。
3.2.2 减弱自身杂质对Fe Ti基储氢材料的消极效应的研究
(1)C和Fe Ti生成Ti C,降低Fe Ti储氢能力。还有含Al的Fe Ti基合金储氢能力会降低
[51 ]
。Si的混入会降低Fe Ti基合金的吸氢能力,但却可以省去加热活化装置,但当Si含量小于0.12%时影响不大
[54 ]
。所以适当控制储氢材料中C,Al,Si含量,对其后期活化是有利的。
图6 在空气中暴露35 h的Ti Fe+x%Zr(x=8,12,16)合金在室温和2 MPa氢气压下的首次吸氢动力学
Fig.6 First hydrogenation kinetics of Ti Fe+x%Zr(x=8,12and 16)alloys exposed in air for 35 h at room tempera-ture under 2 MPa hydrogen pressure
[53]
(2)制备Fe Ti基合金时,要严格控制氧元素量,含氧量要低于0.1%
[55 ]
,最好控制低于0.01%
[56 ]
。为防止Fe Ti中毒,通常在纯氢气中多次活化,可以消除中毒现象
[57 ]
。
(3)其他特例情况。将铁钛系的氧化物和Fe Ti基合金混合可以促进合金材料储氢性能。例如:Fe Tix (x=1.02~1.30)和0.5%~9.0%Ti10 Fe7 O3 的混合物,不经活化,在低氢压和室温条件下便能够吸氢
[54 ]
。
另外,Si加入Fe Ti基合金中,只要适当控制其含量,便能对改善合金的活化性能产生积极的影响,同时也不改变合金的储氢性能。C和Al混入会降低Fe Ti储氢能力,再者,氧混入时容易造成储氢材料中毒,但形成的铁钛系的氧化物反而能够促进其吸氢。所以对于杂质混入的研究仍需要进一步探索。
4 总结与展望
Fe Ti基合金在储氢材料的发展中具有很大前景。但其活化需要在高温高压才能完成,并且杂质也会影响Fe Ti基合金的活性,使其毒化,并降低其储氢性能。
通过合金化Zr,V,Mn等元素能够改善Fe Ti基储氢材料首次吸氢动力学。再者,混合Zr+2V和2Mn+4Zr多元金属也可以明显改善Fe Ti基合金的首次吸氢动力学;此外,球磨、冷轧、机械铣削、锻造、等径角挤压、高压扭转等机械变形方法也是改善Fe Ti基合金活化性能的有效手段;通过控制冷却速度改变Fe Ti基合金的微观组织,也能够改善其吸氢性能。热处理也能恢复被氧化Fe Ti基合金的储氢性能。
对于Fe Ti基合金的中毒,在Fe Ti基合金中引入高含量的Zr能有效降低与空气的相互作用,从而改善其不利影响。特别需要注意,制备Fe Ti基合金时要严格控制其中杂质元素(如C,Si,Al,O等)的含量,以防止Fe Ti基合金活性失效。
参考文献
[1] Sandrock G D,Goodell P D.Surface poisoning of La Ni5 ,Fe Ti and(Fe,Mn)Ti by O2 ,Co and H2 O[J].Journal of the Less Common Metals,1980,73(1):161.
[2] Lv P,Huot J.Hydrogenation improvement of Ti Fe by adding Zr Mn2 [J].Energy,2017,138:375.
[3] Vega L E R,Leiva D R,Leal Neto R M,Silva W B,Silva R A,Ishikawa T T,Kiminami C S,Botta W J.Mechanical activation of Ti Fe for hydrogen storage by cold rolling under inert atmosphere[J].International Journal of Hydrogen Energy,2018,43(5):2913.
[4] Manna J,Tougas B,Huot J.Mechanical activation of air exposed Ti Fe+4wt%Zr alloy for hydrogenation by cold rolling and ball milling[J].International Journal of Hydrogen Energy,2018,43(45):20795.
[5] Wang S,Du Z M,Zhang Z L,Han Z Y.Research progress on safety of lithium-ion batteries[J].Chinese Journal of Engineering,2018,40(8):901.(王爽,杜志明,张泽林,韩志跃.锂离子电池安全性研究进展[J].工程科学学报,2018,40(8):901.)
[6] Emami H,Edalati K,Matsuda J,Akiba E,Horita Z.Hydrogen storage performance of Ti Fe after processing by ball milling[J].Acta Materialia,2015,88:190.
[7] Chiang C H,Chin Z H,Perng T P.Hydrogenation of Ti Fe by high-energy ball milling[J].Journal of Alloys and Compounds,2000,307(1-2):259.
[8] Kinaci A,Aydinol M K.Ab initio investigation of Fe TiH system[J].Hydrogen Energy,2007,32(13):2466.
[9] Miller H I,Murray J,Laury E,Reinhardt J,Goudy AJ.The hydriding and dehydriding kinetics of Fe Ti and Fe0.9 Ti Mn0.1 [J].Journal of Alloys and Compounds,1995,231(1-2):670.
[10] Khatamian D,Weatherly G C,Manchester F D.Some effects of activation for hydrogen absorption in Fe Ti powder[J].Acta Metallurgica,1983,31(10):1771.
[11] Schober T,Westlake D G.The activation of Fe Ti for hydrogen storage:a different view[J].Scripta Metallurgica,1981,15(8):913.
[12] Luo L S,Zhou J,Wen X Q,Liu W W,Guan J H.Effect of Ti/Cr on hydrogen absorption/desorption properties of(VFe)50 Ti26-x Cr24+x (0≤x≤2.0)hydrogen storage alloys[J].Nonferrous Metals Science and Engineering,2016,7(1):20.(罗林山,周健,文小强,刘雯雯,管建红.Ti/Cr比对(VFe)50 Ti26-x Cr24+x (0≤x≤2.0)储氢合金吸放氢性能的影响[J].有色金属科学与工程,2016,7(1):20.)
[13] Patel A K,Sharma P,Huot J.Effect of annealing on microstructure and hydrogenation properties of Ti Fe+Xwt%Zr(X=4,8)[J].International Journal of Hydrogen Energy,2018,43(12):6238.
[14] Lv P,Guzik M N,Sartori S,Huot J.Effect of ball milling and cryomilling on the microstructure and first hydrogenation properties of Ti Fe+4 wt%Zr alloy[J].Journal of Materials Research and Technology,2019,8(2):1828.
[15] Jain P,Gosselin C,Skryabina N,Fruchart D,Huot J.Hydrogenation properties of Ti Fe with Zr7 Ni10 alloy as additive[J].Journal of Alloys and Compounds,2015,636:375.
[16] Kumar S,Tiwari G P,Sonak S,Jain U,Krishnamurthy N.High performance Fe Ti-3.1 wt%V alloy for on board hydrogen storage solution[J].Energy,2014,75:520.
[17] Guéguen A,Latroche M.Influence of the addition of vanadium on the hydrogenation properties of the compounds Ti Fe0.9 Vx and Ti Fe0.8 Mn0.1 Vx (x=0,0.05 and 0.1)[J].Journal of Alloys and Compounds,2011,509(18):5562.
[18] Mitrokhin S V,Verbetsky V N,Kajumov R R,Cunmao H,Zhang Y F.Hydrogen sorption peculiarities in Fe Titype Ti-Fe-V-Mn alloys[J].Journal of Alloys and Compounds,1993,199(1-2):155.
[19] Gosselin C,Huot J.Hydrogenation properties of Ti Fe doped with zirconium[J].Materials,2015,8(11):7864.
[20] Jain P,Gosselin C,Huot J.Effect of Zr,Ni and Zr7 Ni10 alloy on hydrogen storage characteristics of Ti Fe alloy[J].International Journal of Hydrogen Energy,2015,40(47):16921.
[21] Leng H,Yu Z,Yin J,Wu Z,Chou K C.Effects of Ce on the hydrogen storage properties of Ti Fe0.9 Mn0.1 alloy[J].International Journal of Hydrogen Energy,2017,43(37):23731.
[22] Yang S Z,Ye R,Huang T S,Zhao S L,Chen B Z.Astudy of the activation of Fe Ti and Fe0.9 Ti Mn0.1 [J].International Journal of Hydrogen Energy,1988,13(7):433.
[23] Bruzzone G,Costa G,Ferretti M,Olcese G L.Hydrogen storage in aluminium-substituted Ti Fe compounds[J].International Journal of Hydrogen Energy,1981,6(2):181.
[24] Chung H S,Lee J Y.Effect of partial substitution of Mn and Ni for Fe in Fe Ti on hydriding kinetics[J].International Journal of Hydrogen Energy,1986,11(5):335.
[25] Lee S M,Perng T P.Effect of the second phase on the initiation of hydrogenation of Ti Fe1-x Mx (M=Cr,Mn)alloys[J].International Journal of Hydrogen Energy,1994,19(3):259.
[26] Zeaiter A,Nardin P,Yazdi M A P,Billard A.Outstanding shortening of the activation process stage for a Ti Febased hydrogen storage alloy[J].Materials Research Bulletin,2019,112:132.
[27] Patel A K,Duguay A,Tougas B,Schade C,Sharma P,Huot J.Microstructure and first hydrogenation properties of Ti Fe alloy with Zr and Mn as additives[J].International Journal of Hydrogen Energy,2020,45(1):787.
[28] Lv P,Liu Z C,Dixit V.Improved hydrogen storage properties of Ti Fe alloy by doping(Zr+2V)additive and using mechanical deformation[J]International Journal of Hydrogen Energy,2019,44(51):27843.
[29] Patel A K,Tougas B,Sharma P,Huot J.Effect of cooling rate on the microstructure and hydrogen storage properties of Ti Fe with 4 wt%Zr as an additive[J].Journal of Materials Research and Technology,2019,8(6):5623.
[30] Emami H,Edalati K,Matsuda J,Akiba E,Horita Z.Hydrogen storage performance of Ti Fe after processing by ball milling[J].Acta Materialia,2015,88:190.
[31] Modi P,Aguey-Zinsou K F.Titanium-iron-manganese(Ti Fe0.85 Mn0.15 )alloy for hydrogen storage:reactivation upon oxidation[J].International Journal of Hydrogen Energy,2019,44(31):16757.
[32] Khajavi S,Rajabi M,Huot J.Effect of cold rolling and ball milling on first hydrogenation of Ti0.5 Zr0.5 (Mn1-x Fex )Cr1 ,x=0,0.2,0.4[J].Journal of Alloys and Compounds,2019,775:912.
[33] Han S,Wang C X,Li Y,Lv Z Q,Wan Q Z,Yang C.Effect of forging ratio on microstructure and properties of18Ni(250) maraging steel[J].Forging&Stamping Technology,2020,45(10):192.(韩顺,王春旭,厉勇,吕知清,万奇志,杨超.锻比对18Ni(250)马氏体时效钢组织及性能的影响[J].锻压技术,2020,45(10):192.)
[34] Figueroa C G,Schouwenaars R,Cortés-Pérez J,Petrov R,Kestens L.Ultrafine gradient microstructure induced by severe plastic deformation under sliding contact conditions in copper[J].Materials Characterization,2018,138:263.
[35] Huot J.Nanocrystalline metal hydrides obtained by severe plastic deformations[J].Metals-Open Access Metallurgy Journal,2012,2(4):22.
[36] Kim Y S,Choi E,Kim W J.Characterization of the microstructures and the shape memory properties of the FeMn-Si-Cr-Ni-C shape memory alloy after severe plastic deformation by differential speed rolling and subsequent annealing[J].Materials Characterization,2018,136:12.
[37] Němec M,G?rtnerováV,J?ger A.Influence of severe plastic deformation on intermetallic particles in Mg-12wt%Zn alloy investigated using transmission electron microscopy[J].Materials Characterization,2016,119:129.
[38] Němec M,J?ger A,Tesa?K,G?rtnerováV.Influence of alloying element Zn on the microstructural,mechanical and corrosion properties of binary Mg-Zn alloys after severe plastic deformation[J].Materials Characterization,2017,134:69.
[39] Toth L S,Gu C.Ultrafine-grain metals by severe plastic deformation[J].Materials Characterization,2014,92(6):1.
[40] Zadorozhnyy V Y,Milovzorov G S,Klyamkin S N,Zadorozhnyy M Y,Gorshenkov M V,Kaloshkin S D.Preparation and hydrogen storage properties of nanocrystalline Ti Fe synthesized by mechanical alloying[J].Progress in Natural Science:Materials International,2017,27(1):149.
[41] Hong S H,Song M Y.Preparation of Mg-Mg H2 flakes by planetary ball milling with stearic acid and their hydrogen storage properties[J].Metals and Materials International,2016,22(3):544.
[42] Hong S H,Song M Y.Preparation of a sample with a single Mg H2 phase by horizontal ball milling and the first hydriding reaction of 90 wt%Mg-10 wt%Mg H2 [J].Metals and Materials International,2015,21(2):422.
[43] Lv P,Huot J.Hydrogenation improvement of Ti Fe by adding Zr Mn2 [J].Energy,2017,138:375.
[44] Vega L E R,Leiva D R,Neto Leal R M,Silva W B,Silva R A,Ishikawa T T,Kiminami C S,Botta W J.Mechanical activation of Ti Fe for hydrogen storage by cold rolling under inert atmosphere[J].International Journal of Hydrogen Energy,2018,43(5):2913.
[45] Gao P F,Yu C,Lei Z N,Zhan M.Research progress in macro and micro forming rules and control of titanium alloy complex components by isothermal forging[J].Journal of Plasticity Engineering,2020,27(7):21.(高鹏飞,于超,雷珍妮,詹梅.钛合金复杂构件等温锻宏微观成形规律与调控研究进展[J].塑性工程学报,2020,27(7):21.)
[46] Aal M I A E,Mahallawy N E,Shehata F A,Hameed MA E,Yoon E Y,Kim H S.Wear properties of ECAP-processed ultrafine grained Al-Cu alloys[J].Materials Science&Engineering:A(Structural Materials:Properties,Microstructure and Processing),2010,527(16-17):3726.
[47] Jorge A M,Prokofiev E,Lima G F D,Rauch E,Veron M,Botta W J,Kawasaki M,Langdon T G.An investigation of hydrogen storage in a magnesium-based alloy processed by equal-channel angular pressing[J].International Journal of Hydrogen Energy,2013,38(20):8306.
[48] Révész A,Gajdics M,Varga L K,Krállics G,Péter L,Spassov T.Hydrogen storage of nanocrystalline Mg-Ni alloy processed by equal-channel angular pressing and cold rolling[J].International Journal of Hydrogen Energy,2014,39(18):9911.
[49] Wang L S,Jiang J H,Ma A,Li Y H.A critical review of Mg-based hydrogen storage materials processed by equal channel angular pressing[J].Metals-Open Access Metallurgy Journal,2017,7(9):324.
[50] Endo N,Suzuki S,Goshome K,Maeda T.Operation of a bench-scale Ti Fe-based alloy tank under mild conditions for low-cost stationary hydrogen storage[J].International Journal of Hydrogen Energy,2017,42(8):5246.
[51] Edalati K,Matsuda J,Yanagida A,Akiba E,Horita Z.Activation of Ti Fe for hydrogen storage by plastic deformation using groove rolling and high-pressure torsion:similarities and differences[J].International Journal of Hydrogen Energy,2014,39(28):15589.
[52] Edalati K,Matsuda J,Iwaoka H,Toh S,Akiba E,Horita Z.High-pressure torsion of Ti Fe intermetallics for activation of hydrogen storage at room temperature with heterogeneous nanostructure[J].International Journal of Hydrogen Energy,2013,38(11):4622.
[53] Lv P,Liu Z C.Effect of high zirconium content on hydrogenation properties and anti-poisoning ability of air-exposed Ti Fe alloy[J].Journal of Materials Research and Technology,2019,8(6):5972.
[54] Zhao D L,Han Z G,Zhai T T,Yuan Z M,Qi Y,Zhang Y H.Advances in activation property of hydrogen storage for Ti Fe-based alloy[J].Chinese Journal of Rare Metals,2020,44(4):337.(赵栋梁,韩忠刚,翟亭亭,袁泽明,祁焱,张羊换.Ti Fe基合金储氢活化性能研究进展[J].稀有金属,2020,44(4):337.)
[55] Busch G,Schlapbach L,Stucki F,Fischer P,Andresen A F.Hydrogen storage in Fe Ti:surface segregation and its catalytic effect on hydrogenation and structural studies by means of neutron diffraction[J].International Journal of Hydrogen Energy,1979,4(1):29.
[56] Sandrock G D.The mtallurgy and production of rechargeable hydrides[J].Hydrides for Energy Storage,1978:353.
[57] Schlapbach L,Riesterer T.The activation of Fe Ti for hydrogen absorption[J].Applied Physics A,1983,32(4):169.