Trans. Nonferrous Met. Soc. China 27(2017) 1156-1164
Acidithiobacillus ferrooxidans enhanced heavy metals immobilization efficiency in acidic aqueous system through bio-mediated coprecipitation
Min GAN, Ming-ming LI, Jian ZENG, Xin-xing LIU, Jian-yu ZHU, Yue-hua HU, Guan-zhou QIU
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 15 August 2016; accepted 28 March 2017
Abstract:
This study investigated the promotion effect of A. ferrooxidans on complex heavy metals coprecipitation process. A. ferrooxidans significantly enhanced the ferrous oxidation, which also promoted the formation of iron-oxyhydroxysulphate. Cu(II) concentration reduced to 0.058 mmol/L in A. ferrooxidans inoculated system, and Cd also reduced to the lowest concentration (0.085 mmol/L). Pb was mainly immobilized as anglesite and iron-oxyhydroxysulphate promoted the removal of remanent Pb in solution. The precipitates are characterized by XRD, SEM, and FTIR analysis. The main component of the iron-oxyhydroxysulphate was well crystallized jarosite. A. ferrooxidans contributed to the formation of schwertmannite in later monovalent cation lack stage. Higher ferrous iron oxidation rate and Fe(III) supply rate in A. ferrooxidans inoculated system facilitated polyhedron crystal formation and the increase of particle diameter. Complex heavy metals could be incorporated into iron oxyhydroxysulphate crystal, and efficiently removed from acidic wastewater through A. ferrooxidans mediated coprecipitation.
Key words:
coprecipitation; Acidithiobacillus ferrooxidans; complex heavy metals; iron-oxyhydroxysulphate; acidic water;
1 Introduction
Heavy metal pollution has come into the forefront as a major world problem due to its potential damage to human health and ecology [1-3]. Copper (Cu) is a major micronutrient for all living organisms, which can activate many kinds of enzymes in protein metabolism. However, it has been proved that excessive intake of Cu causes damage in intestinal and stomach systems [4]. Lead (Pb) is readily absorbed by human body through the gastrointestinal tract. 70%-90% of the Pb assimilated into bones, then liver and kidneys, which leads to renal tumors [5]. Exposure to lower amounts of cadmium (Cd) may cause gastrointestinal irritation, such as vomiting, and diarrhea [6]. Cu, Cd and Pb are regarded as the most hazardous elements to human health. These metals are widely spread in the acidic environment because of the anthropogenic activities, and cause adverse health effects.
Currently, chemical precipitation, filtration, ion exchange, adsorption, electrodeposition, and membrane systems are widely employed in water purification area [7-9]. In recent years, coprecipitation technique has raised increasing concerns for the removal of trace metal ions from acidic water system [10]. Results of several studies suggest that metals can coprecipitate with iron under acidic condition, or incorporate into noncrystalline iron oxides [11]. Metal coprecipitation with iron oxides is a process that occurs in soil, sediments, and water, which plays an important role in limiting heavy metals solubility, toxicity, and bioavailability [12]. Acid mine drainage usually contains iron ion and heavy metals, while the low chemical oxidation rate of ferrous iron is not conducive to the formation of secondary iron minerals and the corresponding metal immobilization process. Acidithiobacillus ferrooxidans (A. ferrooxidans) commonly exists in acid mine effluents, which accelerates the sulfide mines and mineral weathering process, and shows a particular tolerance to heavy metals [13-15]. The simplicity in operation, low consumption in chemicals and in-situ treatment make the coprecipitation a promising technique.
Ferrous iron oxidation in acid mine water is predominantly mediated by iron-oxidizing bacteria, because abiotic oxidation using molecular oxygen as oxidant is very slow [16]. When A. ferrooxidans is introduced to the coprecipitation system, it can greatly promote the ferrous iron oxidation rate and plays as role as the template of the secondary iron-based mineral, which can enhance the heavy metal immobilization rate and efficiency correspondingly [17,18]. Along with biologically induced iron precipitation, Fe(III)- oxyhydroxide and Fe(III)-hydroxysulfate minerals such as schwertmannite (Fe8O8(OH)6SO4) and jarosite (KFe3(SO4)2(OH)6), can scavenge metals from acid mine drainage [19,20]. In light of these effects, the precipitation behavior of trace metals maybe differs with that in the abiotic system. In order to understand the fate of the complex trace metals in acid systems, this study investigate the behavior of Cd2+, Cu2+ and Pb2+ in biotic and abiotic systems, and the possibility of immobilizing ternary heavy metals by iron-oxyhydroxysulphate precipitation under the augmentation of A. ferroxidans. The varying parameters including pH, redox potential, ferrous iron oxidation and precipitation, heavy metals immobilization during experiment are taken into account for explaining the coprecipitation mechanism. A systematic research on coprecipitation can help to understand the metals immobilization behavior in acidic environment. Furthermore, it can be used in metals contaminated acidic environment remediation such as acid mine drainage, acid industrial waste water and fluorine-containing rare earth acid wastewater.
2 Experimental
2.1 Microorganism and medium
A. ferrooxidans ATCC 23270 was obtained from American Type Culture Collection (ATCC), and cultivated with FeSO4·7H2O (44.7 g/L) in the 9K medium (0.5 g/L MgSO4·7H2O, 3.0 g/L (NH4)2SO4, 0.5 g/L K2HPO4, 0.1 g/L KCl and 0.01g/L Ca(NO3)2), which was sterilized at 120 °C for 20 min. The initial pH value of these media was adjusted to 2.5 with 1.0 mol/L H2SO4. The cultures of A. ferrooxidans were incubated at 30 °C and 170 r/min. When the bacteria reached the logarithmic growth period (4 d), cells were harvested by centrifugation at 12000 r/min and washed twice with distilled water (pH 2.0). The cell concentration was determined by direct cell counting before the experiment.
2.2 Coprecipitation experimental design
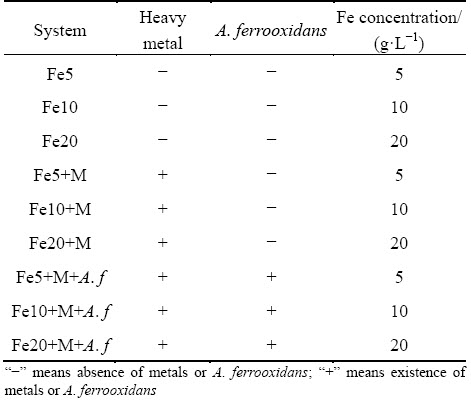
The complex heavy metals coprecipitation experiments were performed in 250 mL Erlenmeyer flask with 100 mL reaction volume. The experiments were divided into three groups including the pure FeSO4 system, heavy metals added chemical coprecipitation system, and A. ferrooxidans inoculated coprecipitation system. The Fe(II) (FeSO4·7H2O) concentration of each treatment was set at 5, 10 and 20 g/L consecutively. Table 1 showed the specific settings. The initial Cu (CuSO4·5H2O), Cd (CdSO4), and Pb (Pb(NO3)2) concentrations were set at 1.0, 0.13 and 0.3 mmol/L, respectively. The initial pH of the reaction system was adjusted to 2.5 with 1.0 mol/L H2SO4, and the cells were inoculated into the system to ensure that the initial concentration was approximately 1×108 cell/mL at the beginning of the experiment. The pH value, redox potential (ORP), Fe(II), total iron, and heavy metals concentrations were monitored respectively at a scheduled interval. The precipitates were collected with 0.45 μm filter paper through filtration, washed twice with the acidic-distilled water (pH 2.0), and naturally dried. SEM, XRD and FTIR were performed to characterize the precipitates. Each experimental treatment was conducted in duplicate.
Table 1 Specific coprecipitation experiment design
2.3 Analytical method
pH value of reaction systems was determined by using a pHS-3C model digital pH-meter every day. ORP was measured by platinum electrode and reference electrode. The concentrations of Fe(II) and total iron in solutions were determined by using 1, 10-phenanthroline method. The concentrations of Cd, Cu and Pb were measured by atomic absorption spectrometry (EDS).
2.4 Characterization method
The dried precipitates were identified by scanning electron microscope (SEM), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) analysis. SEM analysis was performed with a JSM-6360LV instrument operated at accelerating voltage of 10 kV. Samples were mounted on Al-stubs with double-sided carbon tabs and coated with a thin layer of gold in a Pelco Model 3 Sputter 91000 coater. For XRD analysis, it was conducted with Cu Kα radiation (40 kV, 250 mA) in a RINT2000 vertical goniometer equipped with a fixed monochrometer and a theta-compensating slit. Samples were scanned from 5° to 80° with a step increment of 0.02° and 4 s counting time. The FTIR spectra were taken on a Nicolet Nexus 670 Fourier transform spectrometer at a resolution of 4 cm-1 using a KBr beam splitter, a DTGS detector with KBr window, and a sample shuttle for the transmittance measurements. The background was taken on a disk made from 400 mg KBr.
3 Results and discussion
3.1 Changes of pH and ORP in coprecipitation process
Figure 1(a) shows the variation of pH in the coprecipitation process. pH change is related to the concentrations of ferrous/ferric iron, heavy metals and the activity of A. ferrooxidans. It can be identified from Fig. 1(a) that there are apparent differences between the biotic and abiotic systems. At the initial phase, pH of the abiotic system is slightly raised, which is caused by ferrous iron oxidation in air condition (Eq. (1)). From approximately the third day, a dropping trend is observed due to the Fe(III) hydrolysis. pH of the abiotic system is higher than 2.0 during the reaction period. However, dropping speed in A. ferrooxidans inoculated system is significantly higher than that without bacteria, which maintains below 1.82. This shows that ferrous iron oxidation and the subsequent Fe(III) hydrolysis and iron-oxyhydroxysulphate formation (Eq. (2)) are greatly promoted by A. ferrooxidans [21]:
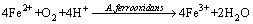 (1)
(1)
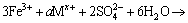
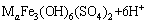 (2)
(2)
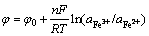 (3)
(3)
Oxidation reduction potential (ORP, Fig. 1(b)) variation reflects the ferrous oxidation and ferrous/ferric iron ratio (Eq. (3)). The ORP of the biotic system increases drastically in the initial stage, which also reveals that the ferrous oxidation is activated in A. ferrooxidans inoculated system. Subsequently, it maintains at higher than 560 mV. These phenomena illustrate that air oxidation of ferrous iron is insignificant in acidic aqueous environment. High ORP coupled with continuous decreasing pH can be considered as the indicators of the adaptation of A. ferrooxidans to coprecipitation system.
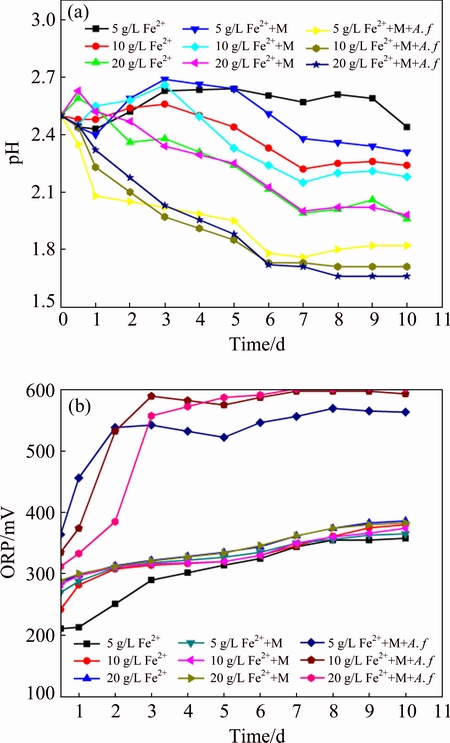
Fig. 1 Dynamic changes of pH (a) and ORP (b) during coprecipitation process
3.2 Changes of Fe(II) and total iron concentrations in coprecipitation process
The dynamic changes of ferrous iron and total iron directly reflect the oxidation ability of the bacteria and the iron-oxyhydroxysulphate formation. Figure 2(a) shows that ferrous iron concentration decreases rapidly in A. ferrooxidans existing system. However, the oxidation percentage is related to the initial FeSO4 dosage. Ferrous iron is completely oxidized within 24 h in system with 5 g/L FeSO4, while oxidation time extends to 72 h when the FeSO4 dosage is increased to 20 g/L. The efficient ferrous oxidation indicates that the activity of A. ferrooxidans is not inhibited by complex heavy metals. In metal-added abiotic systems, the ferrous iron concentrations decrease to 3.59, 6.31 and 12.16 g/L respectively within 10 d. However, according to previous research, the oxidation efficiency is just about 2% with pure chemical oxidation in acidic environment [22], implying that iron in abiotic system maybe precipitates as Fe(II) form. In coprecipitation process, ferrous iron oxidation rate is greatly promoted by A. ferrooxidans, and cells can gain energy from this process for proliferation. Due to the ferric iron hydrolysis and precipitation, the total iron concentration in solution also decreases [23]. The change of total iron concentration shows significant differences in two systems (Fig. 2(b)). In the biotic system, 73.68%, 56.32% and 63.5% iron transforms into precipitate within 3 d with the initial Fe(II) concentration increasing from 5 to 20 g/L, while the precipitation efficiency in heavy metals added abiotic treatment is only 28.14%, 37.00% and 39.22% correspondingly. The acidic environment (pH<2.0) is not conducive to the precipitation of ferric iron from the perspective of chemical thermodynamics. While ferric iron hydrolysis and precipitation in sulfate-rich acidic biotic solution illustrate that A. ferrooxidans activity facilitates this process. According to previous research, metals mainly bond to the ferric iron oxide while not the ferrous iron compounds. In this bio-mediated acidic coprecipitation system, the existence of A. ferrooxidans significantly promotes the ferrous iron oxidation and iron-oxyhydroxysulphate formation. It can be speculated that the promotion effect of A. ferrooxidans on iron-based secondary minerals can elevate metal immobilization efficiency correspondingly.
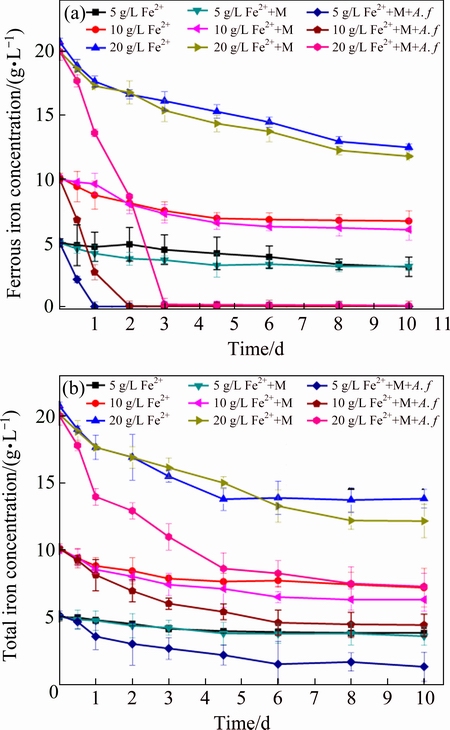
Fig. 2 Variations of Fe(II) (a) and total iron (b) concentrations during coprecipitation
3.3 Cu, Cd and Pb coprecipitation
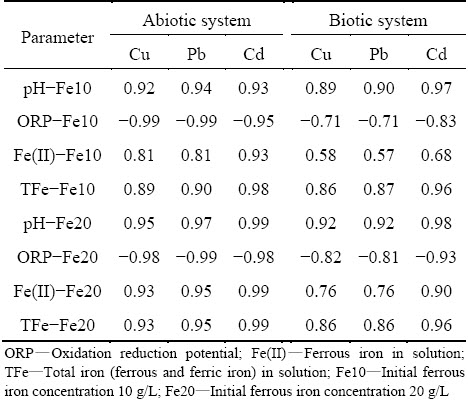
Figure 3 shows that Cu, Cd and Pb maintained a steady decreasing trend during the coprecipitation process. The initial Cu(II) concentration is 1.0 mmol/L, in most treatment, it sharply reduces to 0.12 mmol/L in the first day as well as the abiotic system, implying that chemical precipitation contributes substantially to Cu(II) immobilization in the initial stage. Cu(II) in A. ferrooxidans inoculated system with the highest Fe(II) dosage obtains the best coprecipitation effect, and it further reduces to 0.058 mmol/L on the 10th day. The coprecipitation efficiency in biotic system with 5 g/L Fe(II) ranks only second to the former. A. ferrooxidans is responsible for Fe(II) oxidation, as well as the iron-oxyhydroxysulphate formation. The result suggests that the formed iron-oxyhydroxysulphate accelerates the low concentration Cu(II) coprecipitation. As for the Cd (Fig. 3(b)), it exhibits a different characteristic change trend. The chemical precipitation is not significant in the first day except abiotic system with 5 g/L Fe(II) and the biotic system with 20 g/L Fe(II). The Cd concentration decreases continuously in both systems, indicating that Cd can be immobilized in either chemical or biotic coprecipitation process. However, in biotic system with the highest Fe dosage, Cd reduces to the lowest level (0.085 mmol/L), which shows the deep removal capacity in A. ferrooxidans existing system. Pb is added into the system as Pb(NO3)2 at initial concentration of 0.3 mmol/L. Lead nitrate is transformed into anglesite precipitate immediately, while there is still dissolved Pb in the solution. The precipitation behavior of remnant Pb is related to the A. ferrooxidans mediated biomineralization. Table 2 exhibits the Pearson correlation coefficient between heavy metals precipitation and environmental factors. The coefficient between pH/ORP and metal concentration in abiotic system (Fe(II) 10 g/L) exceeds 0.92 entirely, while that in biotic system is basically lower than 0.90. As to the ferrous iron (Fe(II)) and total iron concentration (TFe), it exhibits a similar variation trend. This phenomenon illustrates that A. ferrooxidans mediated coprecipitation process is more complex than the chemical one, and A. ferrooxidans plays a unique role in heavy metal coprecipitation. When Fe(II) concentration is increased to 20 g/L, the coprecipitation coefficients are remarkably increased in both systems especially for the biotic one. Heavy metals are removed through structural incorporation or substitution [17]. Higher iron concentration facilitates the iron-oxyhydroxysulphate formation, and promotes the coprecipitation efficient. The activity of A. ferrooxidans accelerates heavy metal coprecipitation kinetic and efficiency.
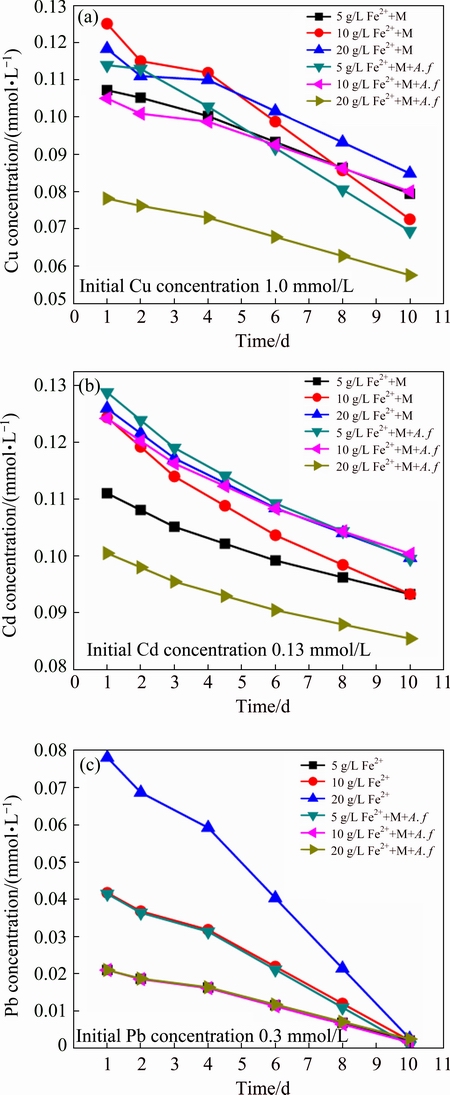
Fig. 3 Dynamic changes of Cu (a), Cd (b) and Pb (c) concentrations in solution
Table 2 Pearson correlation coefficients between heavy metals precipitation and environmental factors
3.4 Precipitate characterization
3.4.1 Morphology and element analysis
Morphology and element analysis directly reflect the influence of biological factors on secondary mineral formation. Figure 4(a1) shows that the precipitate formed in abiotic system with 5 g/L Fe(II) presents as irregular aggregates. With Fe concentration increasing to 10 g/L (Fig. 4(a2)) and 20 g/L (Fig. 4(a3)), the particles become larger and more regular. The precipitates formed in heavy metals added abiotic system (Figs. 4(b1)-(b3)) are similar to those in pure FeSO4-added system, which illustrates that the influence of metals on iron- oxyhydroxysulphate spatial structure is insignificant. In addition, the crystals produced by A. ferrooxidans (Figs. 4(c1)-(c3)) present as polyhedron-shaped tightly aggregate, and their surfaces are smoother than those in abiotic systems, which is related to the higher Fe(II) oxidation efficiency and supply rate of Fe(III) iron in A. ferrooxidans inoculated system [12]. This speculation is also confirmed by the energy dispersive spectroscopy analysis (Fig. 4). Table 3 shows that the iron contents of precipitate in biotic system reached 42.16%, 58.79% and 72.61%, respectively, increasing correspondingly with the increase of FeSO4 dosage. However, the iron content in precipitate formed in abiotic system is below 36.95%. Element sulfur exhibits a similar trend with iron, whose content in biotic system is also higher than others. This implies that A. ferrooxidans increases the 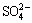 content in the formed iron-oxyhydroxysulphate. However, monovalent cation K shows a reverse trend with S and Fe. According to previous researches, monovalent cation can enhance the crystallinity; less monovalent cation facilitates the formation of amorphous iron based minerals, and the presence of wirelike structure schwertmannite (Sh) confirms it [21]. Iron-oxyhydroxysulphate incorporates metals into its crystal structure and leads to the immobilization of metals [24]. The promotion effect of A. ferrooxidans on iron-oxyhydroxysulphate formation facilitates metals coprecipitation.
content in the formed iron-oxyhydroxysulphate. However, monovalent cation K shows a reverse trend with S and Fe. According to previous researches, monovalent cation can enhance the crystallinity; less monovalent cation facilitates the formation of amorphous iron based minerals, and the presence of wirelike structure schwertmannite (Sh) confirms it [21]. Iron-oxyhydroxysulphate incorporates metals into its crystal structure and leads to the immobilization of metals [24]. The promotion effect of A. ferrooxidans on iron-oxyhydroxysulphate formation facilitates metals coprecipitation.
3.4.2 X-ray diffraction analysis
X-ray diffraction analysis is performed to identify the specific substance formed in different treatments. The precipitates are well crystallized according to the sharp peaks and the stable baseline of the XRD spectra (Fig. 5). Phase identification reveals that the main component of the precipitate in abiotic system only with FeSO4 (Fig. 5(a)) is jarosite (Ja, PDF card No. 22-0827), as well as a small amount of ammonium jarosite (Am, PDF card No. 26-1014) formed in the highest FeSO4 dosage condition.
In later coprecipitation stage, NH4+ may replace the K+ structure position with its concentration decreasing, resulting in the formation of ammonium jarosite. Figure 5(b) shows the XRD patterns of the precipitates formed in Cu, Cd and Pb added abiotic systems. The characteristic peaks of anglesite (An) indicate that Pb is predominantly bound to sulfate rather than phosphate. This may be attributed to much higher concentration of sulfate, relative to phosphate in the acid sulfate water. Compared with the spectra of heavy metals added abiotic system (Fig. 5(c)), more minor peaks can be searched in the biotic system, indicating that higher Fe(III) supply rate promoted the formation of iron-oxyhydroxysulphate, which may cover the signal of some minor constituents. Moreover, schwertmannite (Fe8O8(OH)6SO4) is identified in biotic system as minor constituent, which is formed in monovalent cation lack condition. The presence of schwertmannite in biotic system illustrates that A. ferrooxidans greatly promoted ferric iron precipitation. According to the previous literatures, metals are immobilized in jarosite by a series of precipitation, coprecipitation and adsorption reactions [25]. Bidentate inner-sphere complexes are formed between Cu and the singly coordinated surface sites, allowing for hydrolysis and dimer formation in Cu surface species [26,27].
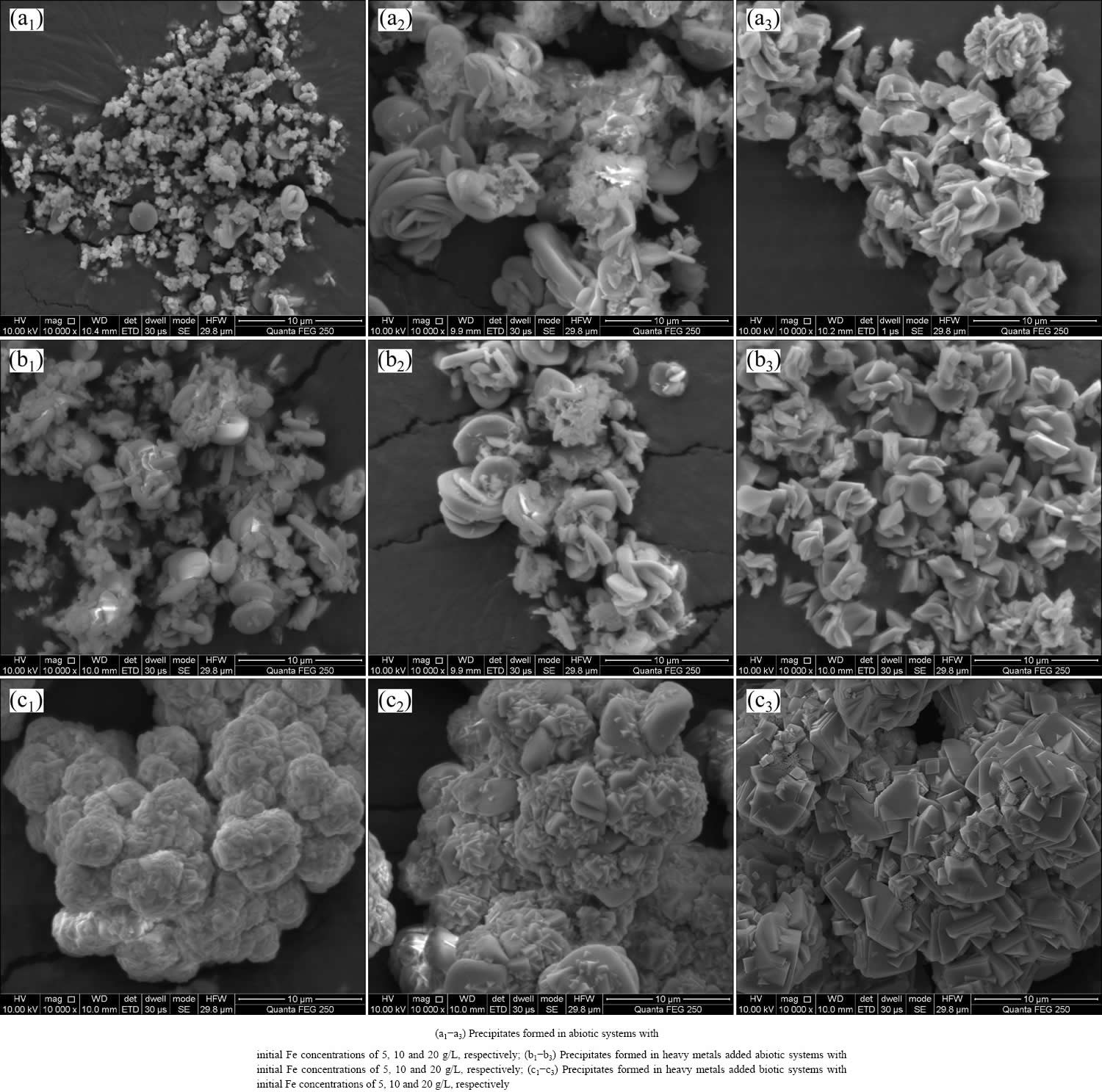
Fig. 4 Morphologies of precipitates produced by chemical and microbial methods
Table 3 Element content and grain size of precipitates in different treatments
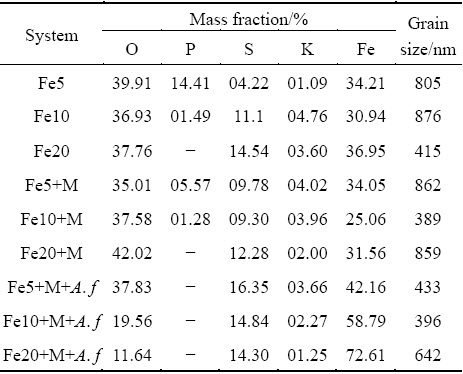
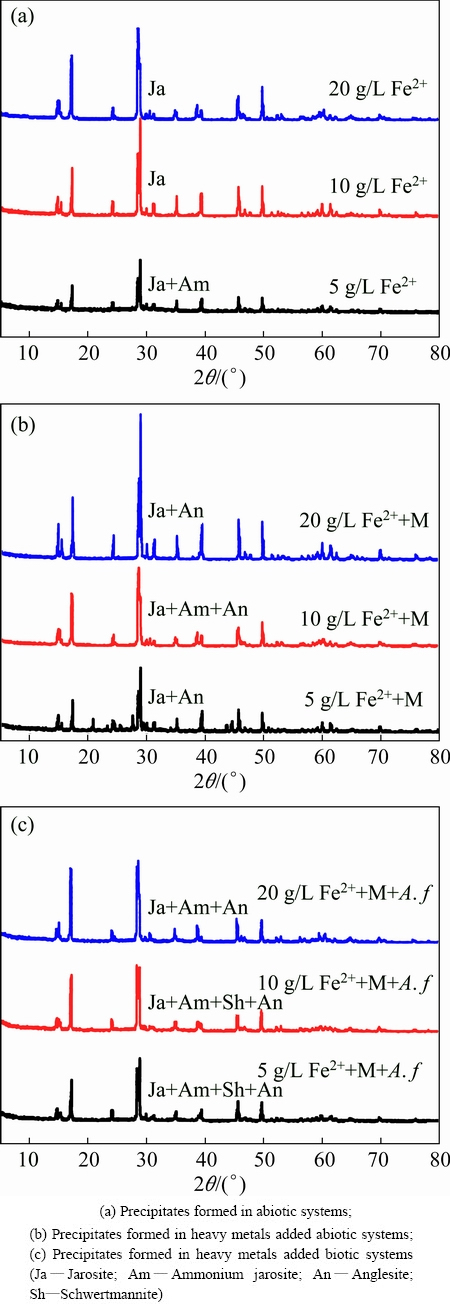
Fig. 5 XRD patterns of precipitates formed in different treatment conditions
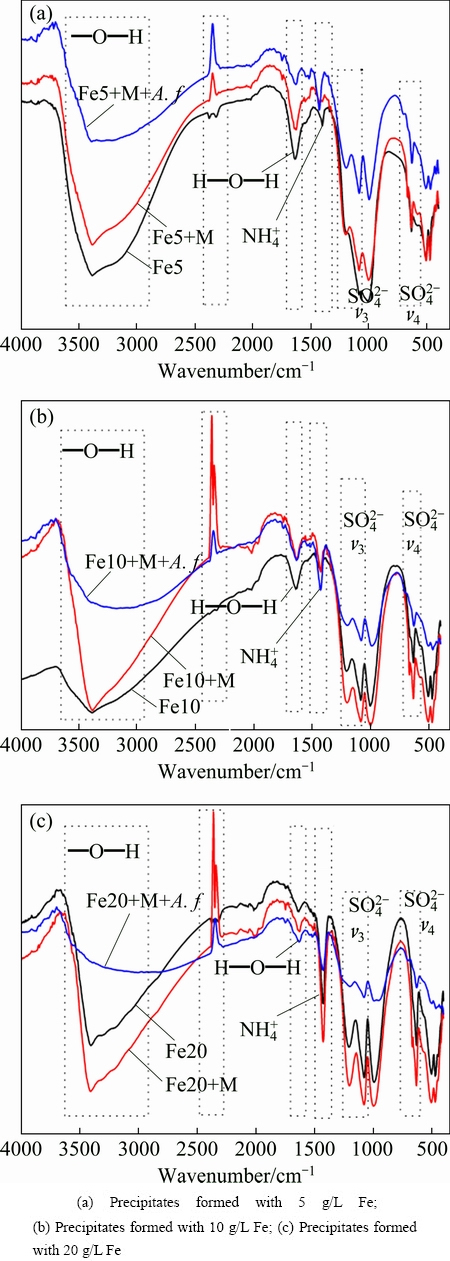
Fig. 6 FTIR spectra of precipitates formed in different treatments
3.4.3 FTIR analysis
The main absorption bands of the FTIR spectra (Fig. 6) show the similarity with the standard jarosite. While the spectrum of each sample has its own characteristics in peak shape and intensity, indicating the heterogeneity of precipitate formed in different systems. The intense absorption observed in region of 2900-3700 cm-1 can be attributed to O—H stretching (vOH) [21]. However, the transmittance of precipitate formed in biotic system is much higher than that in abiotic system, especially for metals added system. Hydroxyl group is a critical group in metals adsorption and precipitation process, and metals may incorporate into the crystal through cation exchange. Metals are adsorbed or substituted with hydroxyl group. The band around 1630 cm-1 is attributed to H—O—H deformation [18]. The consistency in peak position and transmissivity indicates that water molecule contents in the samples are basically identical. Three intense absorption bands are observed in range of 1000-1200 cm-1, the first two absorption bands are due to the υ3 (doublet) vibration of 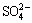 [12], while the band observed around 1000 cm-1 is the deformation of —O—H. The
[12], while the band observed around 1000 cm-1 is the deformation of —O—H. The 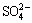 and —O—H transmittances of samples formed in biotic system are higher than 40%, and the corresponding transmittances in abiotic system are lower than 10%. The contents of pivotal group sulfate and hydroxyl in the A. ferrooxidans inoculated system are less uniformly than those in abiotic system. Heavy metals may be adsorbed, substituted with hydroxyl or sulfate groups in biotic system. The peak around 627.9 cm-1 and a weak shoulder observed near 676 cm-1 can be attributed to the v4 vibration mode of the sulfate. The band located near 1430 cm-1 is the characteristic peak of
and —O—H transmittances of samples formed in biotic system are higher than 40%, and the corresponding transmittances in abiotic system are lower than 10%. The contents of pivotal group sulfate and hydroxyl in the A. ferrooxidans inoculated system are less uniformly than those in abiotic system. Heavy metals may be adsorbed, substituted with hydroxyl or sulfate groups in biotic system. The peak around 627.9 cm-1 and a weak shoulder observed near 676 cm-1 can be attributed to the v4 vibration mode of the sulfate. The band located near 1430 cm-1 is the characteristic peak of 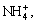 which confirms the existence of ammonium jarosite in the precipitate mixture. It can be identified from the spectra that the content of
which confirms the existence of ammonium jarosite in the precipitate mixture. It can be identified from the spectra that the content of 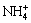 is positively related to the initial FeSO4 dosage. This phenomenon illustrates that the formation of ammonium jarosite occurs in the later stage of the reaction. The bands observed near 504.0 and 466 cm-1 are vibrations of FeO6 coordination octahedral [28]. Note that the intense peak around 2359 cm-1 just appeared in heavy metals added system, which has not been reported in previous researches. This band can be regarded as the characteristic peak between heavy metals and iron-oxyhydroxysulphate. Transmittances of critical group hydroxyl and sulfate in metals-existing biotic system are significantly lower than those in other two systems, which indicates that these sites are occupied by heavy metals. The above results have proved the composition of the iron-oxyhydroxysulphate and the critical groups for metals immobilization. Metals are immobilized through precipitation, structural incorporation or substitution. Heavy metals immobilization mechanism through bio-mediated coprecipitation in acidic aqueous system is summarized as Fig. 7.
is positively related to the initial FeSO4 dosage. This phenomenon illustrates that the formation of ammonium jarosite occurs in the later stage of the reaction. The bands observed near 504.0 and 466 cm-1 are vibrations of FeO6 coordination octahedral [28]. Note that the intense peak around 2359 cm-1 just appeared in heavy metals added system, which has not been reported in previous researches. This band can be regarded as the characteristic peak between heavy metals and iron-oxyhydroxysulphate. Transmittances of critical group hydroxyl and sulfate in metals-existing biotic system are significantly lower than those in other two systems, which indicates that these sites are occupied by heavy metals. The above results have proved the composition of the iron-oxyhydroxysulphate and the critical groups for metals immobilization. Metals are immobilized through precipitation, structural incorporation or substitution. Heavy metals immobilization mechanism through bio-mediated coprecipitation in acidic aqueous system is summarized as Fig. 7.
4 Conclusions
1) This research investigates complex heavy metals immobilization behavior in chemical and bio-mediated coprecipitation process in acidic water system.
2) Ferrous oxidation and iron-oxyhydroxysulphate formation are promoted by A. ferrooxidans activity. Heavy metals coprecipitation kinetic and removal efficiency are enhanced with the increasing iron concentration and the inoculation of A. ferrooxidans. The main component of the precipitate is well crystallized jarosite.
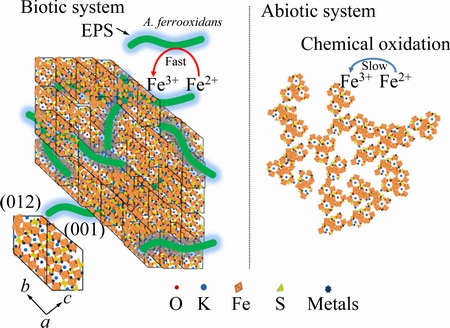
Fig. 7 Schematic of heavy metals coprecipitation in biotic and abiotic systems
3) Higher Fe(II) oxidation efficiency and Fe(III) supply rate in A. ferrooxidans inoculated system facilitate larger grain diameter and polyhedron shaped crystal formation.
4) Complex heavy metals can be incorporated into iron oxyhydroxysulphate crystal and efficiently removed from acidic wastewater through A. ferrooxidans mediated coprecipitation. Coprecipitation is a promising technique for heavy metals in-situ treatment in acidic environment.
References
[1] CHEN J Q, WANG Z X, XIE W, ZHU J J, ZHOU W B. Source and hazard identification of heavy metals in soils of Changsha based on TIN model and direct exposure method [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 642-651.
[2] ZHANG C, LAI C, ZENG G M, HUANG D L, YANG C P, WANG Y, ZHOU Y Y, GHENG M. Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution [J]. Water Research, 2016, 95: 103-112.
[3] DHANARANI S, VISWANATHAN E, PIRUTHIVIRAJ P, ARIVALAGAN P, KALIANNAN T. Comparative study on the biosorption of aluminum by free and immobilized cells of Bacillus safensis KTSMBNL 26 isolated from explosive contaminated soil [J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 69: 61-67.
[4] GAETKE L M, CHOW C K. Copper toxicity, oxidative stress, and antioxidant nutrients [J]. Toxicology, 2003, 189: 147-163.
[5] VERMA S, DUBEY R. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants [J]. Plant Science, 2003, 164: 645-655.
[6] MULLER L. Consequences of cadmium toxicity in rat hepatocytes: Mitochondrial dysfunction and lipid peroxidation [J]. Toxicology, 1986, 40: 285-295.
[7] ZENG X F, TWARDOWSKA I, WEI S H, SUN L N, WANG J, ZHU J Y, CAI J C. Removal of trace metals and improvement of dredged sediment dewaterability by bioleaching combined with Fenton-like reaction [J]. Journal of Hazardous Materials, 2015, 288: 51-59.
[8] ARIVALAGAN P, SINGARAJ D, HARIDASS V, KALIANNAN T. Removal of cadmium from aqueous solution by batch studies using Bacillus cereus [J]. Ecological Engineering, 2014, 71: 728-735.
[9] KARTHIK C, RAMKUMAR V S, PUGAZHENDHI A, GOPALAKRISHNAN K, ARULSELVI P I. Biosorption and biotransformation of Cr(VI) by novel Cellulosimicrobium funkei strain AR6 [J]. Journal of the Taiwan Institute of Chemical Engineers, 2016.
[10] KURNIAWAN T A, CHAN G Y S, LO W H. Physico-chemical treatment techniques for wastewater laden with heavy metals [J]. Chemical Engineering Journal, 2006, 118: 83-98.
[11] LEE G, BIGHAM J M, FAURE G. Removal of trace metals by coprecipitation with Fe, Al and Mn from natural waters contaminated with acid mine drainage in the Ducktown Mining District, Tennessee [J]. Applied Geochemistry, 2002, 17: 569-581.
[12] LI M M, ZHU J Y, GAN M, WANG Q F, JIE S Q, CHAI L Y. Characteristics of chromium coprecipitation mediated by Acidithiobacillus ferrooxidans DC [J]. Water Air and Soil Pollution, 2014, 225: 2071-2084.
[13] GAN M, ZHOU S, LI M M, ZHU J Y, LIU X X, CHAI L Y. Bioleaching of multiple heavy metals from contaminated sediment by mesophile consortium [J]. Environmental Science and Pollution Research, 2015, 22: 5807-5816.
[14] NIE H Y, YANG C, ZHU N W, WU P X, ZHANG T, ZHANG Y Q, XIANG Y J. Isolation of Acidithiobacillus ferrooxidans strain Z1 and its mechanism of bioleaching copper from waste printed circuit boards [J]. Journal of Chemical Technology and Biotechnology, 2015, 90: 714-721.
[15] NATARAJAN K. Microbial aspects of acid mine drainage and its bioremediation [J]. Transactions of Nonferrous Metals Society of China, 2008, 18: 1352-1360.
[16] SINGER P C, STUMM W. Acidic mine drainage: The rate-determining step [J]. Science, 1970, 167: 1121-1123.
[17] MA Y Q, LIN C X. Arsenate immobilization associated with microbial oxidation of ferrous ion in complex acid sulfate water [J]. Journal of Hazardous Materials, 2012, 217: 238-245.
[18] GAN M, SUN S J, ZHENG Z H, TANG H J, SHENG J R, ZHU J Y, LIU X X. Adsorption of Cr(VI) and Cu(II) by AlPO4 modified biosynthetic schwertmannite [J]. Applied Surface Science, 2015, 356: 986-997.
[19] DING J N, JIAN G, WU X L, ZHANG C G, WANG D Z, QIU G Z. Jarosite-type precipitates mediated by YN22, Sulfobacillus thermosulfidooxidans, and their influences on strain [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 1038-1044.
[20] LI H J, YANG H Y, CHEN G B. Catalytic performance of biological method seeds on jarosite process [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 557-564.
[21] ZHU J Y, GAN M, ZHANG D, HU Y H, CHAI L Y. The nature of Schwertmannite and Jarosite mediated by two strains of Acidithiobacillus ferrooxidans with different ferrous oxidation ability [J]. Materials Science and Engineering C, 2013, 33: 2679-2685.
[22] WANG H M, GONG L F, CRAVOTTA C A, YANG X F, TUOVINEN O H, DONG H L, FU X. Inhibition of bacterial oxidation of ferrous iron by lead nitrate in sulfate-rich systems [J]. Journal of Hazardous Materials, 2013, 244: 718-725.
[23] NAZARI B, JORJANI E, HANI H, MANAFI Z, RIAHI A. Formation of jarosite and its effect on important ions for Acidithiobacillus ferrooxidans bacteria [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 1152-1160.
[24] EGAL M, CASIOT C, MORIN G, PARMENTIER M, BRUNEEL O, LEBRUN S, ELBAZ P F. Kinetic control on the formation of tooeleite, schwertmannite and jarosite by Acidithiobacillus ferrooxidans strains in an As(III)-rich acid mine water [J]. Chemical Geology, 2009, 265: 432-441.
[25] MCGREGOR R, BLOWES D, JAMBOR J, ROBERTSON W. The solid-phase controls on the mobility of heavy metals at the copper cliff tailings area, Sudbury, Ontario, Canada [J]. Journal of Contaminant Hydrology, 1998, 33: 247-271.
[26] OTERO-FARI A A, GAGO R, ANTELO J, FIOL S, ARCE F. Surface complexation modelling of arsenic and copper immobilization by iron oxide precipitates derived from acid mine drainage [J]. Journal of Mexican Geological Society, 2015, 67: 493-508.
[27] CUI M, JANG M, CHO S H, KHIM J, CANNON F S. A continuous pilot-scale system using coal-mine drainage sludge to treat acid mine drainage contaminated with high concentrations of Pb, Zn, and other heavy metals [J]. Journal of Hazardous Materials, 2012, 215: 122-128.
[28] GAN M, ZHENG Z H, SUN S J, ZHU J Y, LIU X X. The influence of aluminum chloride on biosynthetic schwertmannite and Cu(II)/Cr(VI) adsorption [J]. RSC Advances, 2015, 5: 94500-94512.
基于Acidithiobacillus ferrooxidans介导的共沉淀对酸性体系中重金属的固定
甘 敏,李明明,曾 健,刘新星,朱建裕,胡岳华,邱冠周
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:研究Acidithiobacillus ferrooxidans对酸性水体中复合重金属共沉淀过程的促进作用。A. ferrooxidans显著地提高了共沉淀体系中Fe(II)离子的氧化速率及硫酸羟基铁氧体的形成。在A. ferrooxidans存在的条件下Cu(II)浓度降低至0.058 mmol/L,而Cd在生物体系中浓度降至最低0.085 mmol/L。Pb主要以铅矾形式沉淀,硫酸羟基铁氧体促进了残余铅离子的沉淀。通过XRD、SEM和FTIR等技术对形成产物进行系统表征。共沉淀体系中主要的组分为高结晶度的黄钾铁矾,而A. ferrooxidans促进施式矿物在一价阳离子缺乏的反应后期形成。A. ferrooxidans存在体系中较高的Fe(II)离子氧化速率和Fe(III)离子供应速度有利于多面体晶体形成及其晶粒尺径增加。复合重金属离子进入硫酸羟基铁氧体的晶体结构,通过A. ferrooxidans介导的共沉淀能够高效地将重金属离子从酸性废水中去除。
关键词:共沉淀;嗜酸氧化亚铁硫杆菌;复合重金属;硫酸羟基铁氧体;酸性水体
(Edited by Wei-ping CHEN)
Foundation item: Project (51174239) supported by the National Natural Science Foundation of China; Project supported by the Shanghai Tongji Gao Tingyao Environment Protection Science & Technology Development Foundation, China; Project supported by the Hunan Provincial Co-Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources, China; Project (2017M610506) supported by Postdoctoral Foundation for MG from Chinese PD Science Foundation, China; Project (185690) supported by PD Research Funding Plan in Hunan and Central South University, China
Corresponding author: Jian-yu ZHU; Tel: +86-731-88836944; E-mail: zhujy@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(17)60135-3
Abstract: This study investigated the promotion effect of A. ferrooxidans on complex heavy metals coprecipitation process. A. ferrooxidans significantly enhanced the ferrous oxidation, which also promoted the formation of iron-oxyhydroxysulphate. Cu(II) concentration reduced to 0.058 mmol/L in A. ferrooxidans inoculated system, and Cd also reduced to the lowest concentration (0.085 mmol/L). Pb was mainly immobilized as anglesite and iron-oxyhydroxysulphate promoted the removal of remanent Pb in solution. The precipitates are characterized by XRD, SEM, and FTIR analysis. The main component of the iron-oxyhydroxysulphate was well crystallized jarosite. A. ferrooxidans contributed to the formation of schwertmannite in later monovalent cation lack stage. Higher ferrous iron oxidation rate and Fe(III) supply rate in A. ferrooxidans inoculated system facilitated polyhedron crystal formation and the increase of particle diameter. Complex heavy metals could be incorporated into iron oxyhydroxysulphate crystal, and efficiently removed from acidic wastewater through A. ferrooxidans mediated coprecipitation.


