
Reaction mechanism of preparation of titanium by
electro-deoxidation in molten salt
WANG Bin, LIU Kui-ren, CHEN Jian-she
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 30 October 2010; accepted 27 May 2011
Abstract:
The electro-deoxidation of TiO2 was investigated in molten CaCl2. Back electromotive force measurements, constant voltage electrolytic experiments, contrast experiments of different cathodes, and cyclic voltammograms were carried out for solving the puzzle of reduction mechanism. The results showed that the reduction process proceeded step by step. TiO2 was first reduced to Ti3O5 or Ti2O3, and then further reduced to Ti3O, Ti2O, TiO and Ti. In addition, direct electrochemical reduction of titanium dioxide was the primary cathodic reaction; meanwhile, some calciothermic reduction reactions also happened at the cathode. Cyclic voltammograms of solid titanium dioxide and molybdenum wire in molten salts with different compositions were also studied.
Key words:
titanium; electro-deoxidation; reaction mechanism; molten salt;
1 Introduction
Titanium has excellent physical and chemical properties. It is widely used in aviation, aerospace and production industries for its comprehensive properties of low density, high strength and corrosion resistance and [1]. Nowadays, the most widespread preparation process of titanium is Kroll process (magnesium reduction), which is a complex procedure, so the production cost of titanium is very high [2]. Many researches have been conducted on shorter processes of titanium production, such as the electro-deoxidation process [3], the calciothermic reduction process [4-5], the preform reduction process (PRP) [6], and the solid oxide membrane process (SOM) [7]. Among these processes mentioned above, the electro-deoxidation process has the advantages of short flow and non-pollution [8-9]. In this electrolysis process, a TiO2 pellet is used as the cathode. Although the conductivity of TiO2 pellet is low, TiO2 can be reduced to Ti after electrolyzing for several hours. SCHWANDT et al [10-11] and LI et al [12] indicated that the TiO2 was reduced by means of an electrochemical process, and the reduction was conducted in several steps. However, SUZUKI et al [13], PARK et al [14] and LIU et al [15] considered that the reduction is a calciothermic reduction process. It is very obvious that arguments exist among different researchers, and the reduction mechanism is still ambiguous.
In this work, the electrolysis experiments were carried out in a resistance furnace for studying the reduction mechanism comprehensively. The cathodic reduction processes were discussed in depth, focusing on reduction steps and effect of Ca metal.
2 Experimental
Current interrupt technique was used for measuring back electromotive force. TiO2 pellets and graphite rods were used the cathodes and the anodes, respectively. The electrolytic current was interrupted after electrolyzing for a period of time at 3.0 V, and at the same time, an hp34401A digital multimeter was used to measure the voltage value of the electrolytic system. Then the steady voltage value was the back electromotive force. The influence of electrolysis time on back electromotive force was investigated.
Constant voltage electrolytic experiments were designed for understanding reduction processes. The cell voltage was lower than the theoretical decomposition voltage of CaO. The applied voltage was 0.7-1.5 V, and was held for 4.5 h. After partial reduction, the pellets were washed in dilute hydrochloric acid and water. The washed pellets were dried thoroughly and then were ground for XRD analysis, using a D/max-2500PC X-ray diffractometer.
Electrolytic contrast experiments of different cathodes were designed for investigating the behavior of CaO. The cell voltage was 3.0 V, and the electrolysis time was 2 h. TiO2 pellets and graphite pellets with the same surface area were used as cathodes and anode, respectively. The current change with electrolysis time was studied.
A three-electrode cell was used for cyclic voltammetry using an IM6e electrochemical workstation. Graphite rods (d 10 mm×3 mm) were used as counter electrode and reference electrode. TiO2 pellets (d 20 mm×2 mm) or molybdenum wires (d 1.5 mm) were used as the test electrode. The scanning speed was 10 mV/s.
All the electrochemical experiments were conducted at 950 °C under an atmosphere of dried argon. The electrolyzer was a corundum crucible. The electrolytic melt was calcium chloride (analytically pure), which was thermally pre-dried at 300 °C. TiO2 pellets (d 20 mm× 3 mm) prepared with die-pressing TiO2 powders were used as cathodes. High purity graphite rods (d20 mm×50 mm) were used as the anodes. Molybdenum wires (d1.5 mm) were used as the lead wires.
3 Results and discussion
It is well known that the back electromotive force is the same as the practical decomposition voltage of electrolytic system. Therefore, measuring the back electromotive force will help us to investigate the reactions occurring in the electrolytic cell. Figure 1 shows the back electromotive force of different electrolytic experiments. After electrolyzing for 3 min, the back electromotive force is about 0.3 V. Based on theoretical calculation [16], it is known that the voltage for reducing TiO2 to Ti3O5 or Ti2O3 is 0.17 V(CO is produced), 0.27 V(CO2 is produced) or 0.21 V(CO is produced), 0.32 V(CO2 is produced). The theoretical data are just similar to the equilibrium voltage (0.3 V). So it can be confirmed that reducing TiO2 to Ti3O5 and Ti2O3 are the main reactions at this stage. Similarly to the analysis above, when the electrolyzing time is 8 min, 15 min, 30 min and 2 h, the back electromotive force located at is 0.6-1.0 V. By comparing with theoretical data, it can be deduced that reducing TiO2, Ti3O5 and Ti2O3 to lower valence titanium oxides is primary reaction in these phases. Moreover, the main reactions are TiOx reduction to Ti in electrolyzer when the electrolytic time reaches 4 h or 6 h.
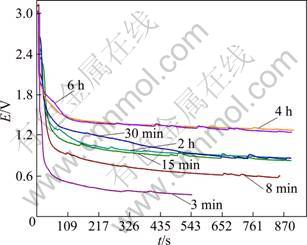
Fig. 1 Back electromotive force—time graph for samples electrolyzed for different time
Through the above analysis, it can be known that reduction process consists of several parts: tetravalent titanium in titanium dioxide is first reduced to low valent (Ti3O5, Ti2O3), and then low valent titanium is reduced to lower valent (Ti3O, Ti2O, TiO) or titanium.
3.2 Constant voltage electrolytic experiments
By calculation, CaO can be reduced at 950°C only when the cell voltage is higher than 1.52 V. However, the titanium oxides will be reduced if the cell voltages are higher than 0.17 V. Therefore, the cell voltages were selected at 1.5 V, 1.1 V and 0.7 V in this experiment. The current—time curves are shown in Fig. 2. When the cell voltage was 0.7 V, current changed slightly with time. However, the variation trends of curves are very different when the voltages were set at 1.1 V and 1.5 V. At the beginning, current decreased rapidly with time, and then a plateau appeared after 1 h. Curves analysis revealed that some electrode reactions occurred even when the voltage was as low as 1.5 V or 1.1 V. In addition, these reactions occurred easily at the beginning stage. With the time extending, reactions became difficult.
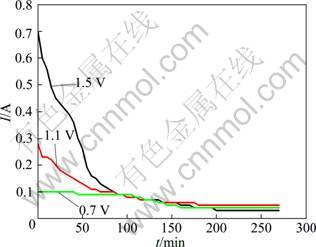
Fig. 2 Current—time curves of constant voltage electrolytic experiments
Figure 3 shows the XRD patterns of samples obtained after electrolyzing. Besides the main phase CaTiO3, some low valent titanium dioxides (Ti3O5, Ti4O7) also exist in the pellets. By theoretical calculation, CaO cannot be reduced under these conditions. Thus, it is confirmed that TiO2 can gain electrons and be reduced directly. The reduction processes are probably performed as follows: TiO2 grains near the lead wire were first reduced, and electrical conductivity of the whole pellet was improved significantly because conductivity of TiO2 is very sensitive to loss of oxygen. Then deoxidation process could be conducted easier, and the pellet was reduced smoothly.
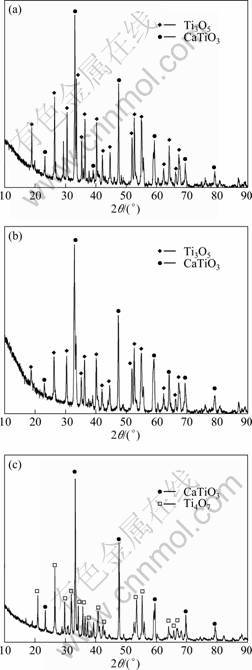
Fig. 3 XRD patterns of samples obtained by electrolyzing at different voltages for 4.5 h: (a) 1.5 V; (b) 1.1 V; (c) 0.7 V
Constant voltage electrolytic experiments revealed that TiO2 pellets can be reduced directly without any Ca metal.
3.3 Electrolytic contrast experiments of different cathodes
Current—time curves of electrolytic experiments with different cathodes are shown in Fig. 4. When the graphite pellet was used as cathode, the current was not faint. At the beginning, the current was even higher than 1.0 A, and then decreased slowly in the later stage. It can be confirmed that CaO reduction reactions caused the decrease of current because no particle can react except CaO at 3.0 V. In addition, Ca is lighter than molten salt, so some Ca metal produced at the cathode floated on the surface, and part may be oxidized, and then dissolved in molten salt again. This is also the reason why the current decreased slowly in the electrolytic process.
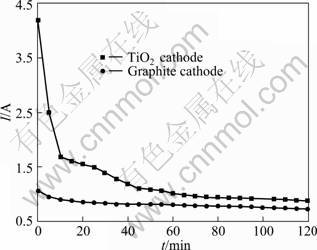
Fig. 4 Current—time curves of electrolytic experiments with different cathodes
When the TiO2 pellet was used as cathode, the current varied greatly. The initial current was 4.2 A, and then dropped rapidly with time, but it was higher than the current in experiment using the graphite cathode. This shows that TiO2 electrochemical reduction reactions induced much more current. In a word, the main reactions are TiO2 electrochemical reduction reactions in the normal electrolytic process; however, CaO reduction reactions exist indeed, and some calciothermic reduction reactions will also happen.
3.4 Cyclic voltammetry
Figure 5 presents two cyclic voltammograms (CVs). One was recorded on a molybdenum wire electrode and the other on a sintered TiO2 pellet. The CV recorded on the molybdenum wire exhibited no reaction until about -1.0 V when the current started to increase, forming a reduction peak, A1, at about -1.3 V. It apparently represents the reduction of CaO because the CaCl2 decomposition reaction is not limited by the diffusion process, and no other particles can take part in the electrode reactions. When the potential reached -1.5 V, the reduction current increased rapidly. And it is obviously due to the reduction of electrolyte CaCl2. Upon reversing the potential scan, the CV of the molybdenum wire showed a decreasing current that repeated the reduction current very well, suggesting that the electrode process depended only on electrode potential, and electron transfer was the controlled process. When the potential returned to -1.5 V, a re-oxidation peak, A0, occurred, which was due to the oxidation of Ca.
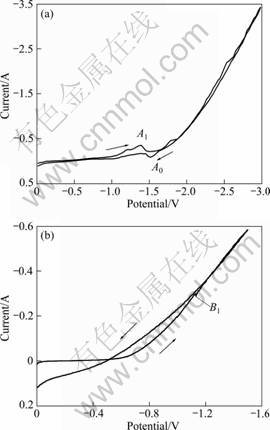
Fig. 5 Cyclic voltammograms of molybdenum wire and of TiO2 pellet in electrolyte CaCl2: (a) Molybdenum wire; (b) TiO2 pellet
The CV recorded on the TiO2 pellet showed a reduction current when the potential scanned to -0.6 V. Compared with Fig. 5(a), the potential of appearing reduction current shifts positively a lot. Therefore, the reduction current must have originated from TiO2 reduction. At the same time, the conclusion of existing direct electrochemical reduction reactions of TiO2 is also proved here. A small reduction current peak, B1, formed at about -1.2 V, representing the faint reaction of CaO reduction, for the reason that reduction of CaCl2 and TiO2 is not controlled by diffusion process. Later, the reduction current increased fast, suggesting that reduction reactions of CaCl2 and TiO2 took place simultaneously. To avoid the influence of much deposited Ca on the re-oxidation current, the potential scanning on TiO2 pellet electrode was reversed at -1.5 V. The current followed the same track as reduction current until about -1.1 V. Interestingly, there was no re-oxidation peak of Ca. The reasonable explanation is that Ca produced in this process was less because of the small scanning range. Meanwhile, Ca is easily dissolvable in CaCl2 melt. So, Ca produced at electrode must have dissolved in the electrolyte before the re-oxidation process. Besides, the curve did not show oxidation peak of TiOx as well. To reveal this uncertainty, TiO2 pellet after CV testing was analyzed by XRD. In Fig. 6, except a little Ti3O5 the primary phase is a stable composition, CaTiO3. The CaTiO3 phase must have hindered the oxidation process of low valent titanium.
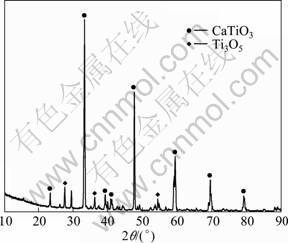
Fig. 6 XRD pattern of TiO2 pellet after CV testing
The measurement of CV in CaCl2 melt containing 0.5% (mass) CaO was executed as contrast experiment. And the results are shown in Fig. 7. The CV curves recorded on the molybdenum wire and TiO2 pellet formed reduction current peaks at about -1.3 V and -1.2 V, respectively. This result is similar to test results for pure CaCl2 except the peak intensity. Reduction current peaks, A2 and B2, were caused by reduction of CaO also. Results further prove that a certain amount of CaO exist in CaCl2, and the part of CaO can be reduced in electrolysis process.
4 Conclusions
1) TiO2 reduction proceed step by step: tetravalent titanium in titanium dioxide was first reduced to low valent (Ti3O5, Ti2O3), and then low valent titanium was reduced to lower valent (Ti3O, Ti2O, TiO) or titanium.
2) TiO2 pellets can be electrochemical reduced directly without any Ca metal.
3) In the normal electrolytic process, direct electrochemical reduction of titanium dioxide was the primary cathodic reaction, meanwhile, CaO reduction reactions existed indeed, and some calciothermic reduction reactions must have happened.
4) Electron transfer is the main controlled process in the experiment of electro-deoxidation of TiO2.
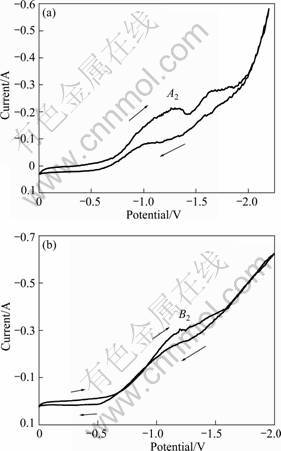
Fig. 7 Cyclic voltammograms of molybdenum wire and TiO2 pellet in CaCl2 melt containing 0.5% (mass fraction) CaO: (a) Molybdenum wire; (b) TiO2 pellet powder
References
[1] JAHEDI M, ZAHIRI S, GULIZIA S, TIGANIS B, TANG C, FRASER D. Direct manufacturing of titanium parts by cold spray [J]. Materials Science Forum, 2009, 618-619: 505-508.
[2] MO Wei, DENG Guo-zhu, LUO Fang-cheng. Titanium metallurgy [M]. Beijing: Metallurgical Industry Press, 1998: 8-9. (in Chinese)
[3] CHEN G Z, FRAY D J, FARTHING T W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride [J]. Nature, 2000, 407(9): 361-363.
[4] ONO K, SUZUKI R O. A new concept for producing Ti sponge: Calciothermic reduction [J]. JOM, 2002, 54(2): 59-61.
[5] SUZUKI R O. Calciothermic reduction of TiO2 and in situ electrolysis of CaO in the molten CaCl2 [J]. Journal of Physics and Chemistry of Solids, 2005, 66: 461-465.
[6] OKABE T H, ODA T, MITSUDA Y. Titanium powder production by preform reduction process (PRP) [J]. Journal of Alloys and Compounds, 2004, 364: 156-163.
[7] PAL U B, WOOLLEY D E, KENNEY G B. Emerging SOM technology for the green synthesis of metals from oxides [J]. JOM, 2001, 53(10): 32-35.
[8] FRAY D J, FARTHING T W, CHEN Z. Removal of oxygen from metal oxides and solid solutions by electrolysis in a fused salt: US, US 2004/0159559A1 [P]. 2004-02-12.
[9] MA M, WANG D H, WANG W G, HU X H, JIN X B, CHEN G Z. Extraction of titanium from different titania precursors by the FFC Cambridge process [J]. Journal of Physics and Chemistry of Solids, 2006, 420: 37-45.
[10] SCHWANDT C, FRAY D J. Determination of the kinetic pathway in the electrochemical reduction of titanium dioxide in molten calcium chloride [J]. Electrochemical Acta, 2005, 51: 66-76.
[11] ALEXANDER D T L, SCHWANDT C, FRAY D J. Microstructural kinetics of phase transformations during electrochemical reduction of titanium dioxide in molten calcium chloride [J]. Acta Materialia, 2006, 54: 2933-2944.
[12] LI Ying-jun, WANG Shu-lan, ZHONG He-xiang, SUN Hua. Mechanism and electro-potential of TiO2 electrochemical reduction [J]. Nonferrous Metals, 2003, 55(4): 68-70. (in Chinese)
[13] SUZUKI R O, AIZAWA M, ONO K. Calcium-deoxidation of niobium and titanium in Ca-saturated CaCl2 molten salt [J]. Journal of Alloys and Compounds, 1999, 288: 173-182.
[14] PARK I I, ABIKO T, OKABE T H. Production of titanium powder directly from TiO2 in CaCl2 through an electronically mediated reaction (EMR) [J]. Journal of Physics and Chemistry of Solids, 2005, 66: 410-413.
[15] LIU Mei-feng, GUO Zhan-cheng, LU Wei-chang. Process of direct electrochemical reduction of TiO2 [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1752-1757. (in Chinese)
[16] LIANG Ying-jiao, CHE Yin-chang, LIU Xiao-xia, LI Nai-jun. Handbook of thermodynamic data for inorganic material [M]. Shenyang: Northeastern University Press, 1993: 43-380. (in Chinese)
熔盐电脱氧法制取Ti的反应机理
王 斌, 刘奎仁, 陈建设
东北大学 材料与冶金学院,沈阳 110004
摘 要:通过低电压电解实验、不同阴极电解对比实验和反电动势测定实验,并结合循环伏安法研究了熔盐电脱氧法制取金属Ti过程中阴极的还原过程。结果表明,还原过程是分步进行的。TiO2先被还原为Ti3O5或Ti2O3,再进一步被还原为Ti3O、Ti2O、TiO或金属Ti。另外,阴极进行的主要电极反应是TiO2的直接电还原反应,同时在阴极也存在一定的钙热还原反应,只是反应强度较弱。另外,分别以钼丝和二氧化钛为研究电极,进行了循环伏安曲线测定。
关键词:钛;电脱氧;反应机理;熔盐
(Edited by YANG Hua)
Foundation item: Project (2006AA068128) supported by the Hi-tech Research and Development Program of China
Corresponding author: LIU Kui-ren; Tel: +86-24-83686997; E-mail: liukr@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)61016-9


