
J. Cent. South Univ. (2020) 27: 762-771
DOI: https://doi.org/10.1007/s11771-020-4329-7
Influence of heat treatment on microstructure and electrochemical behaviors of Mg-Zn binary alloys prepared by gas-phase alloying technique
MA Jun(马俊)1, NIU Li-bin(牛立斌)1, 2, YAN Yu-ting(闫俞廷)1,GAO Chong(高冲)1, WANG Xiao-gang(王晓刚)1, 3
1. College of Materials Science and Engineering, Xi’an University of Science and Technology,Xi’an 710054, China;
2. Shaanxi Key Laboratory of Nano-materials and Technology, Xi’an University of Architecture and Technology, Xi’an 710054, China;
3. Shaanxi Research Center of Energy Conservation and Polygeneration Engineering Technology for Silicon and Magnesium Industry, Xi’an 710055, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Mg-Zn binary alloys fabricated by the gas-phase alloying technique under vacuum condition were investigated in the state of initial state and after heat treatment for the microstructure and electrochemical behaviors. Different from the traditional Mg-Zn alloys preparation methods, alloys prepared by gas-phase alloying have a large number of intermetallic compounds, such as MgZn, Mg7Zn3 and MgZn2. After solution treatment, the boundary of the eutectic disappeared and the size of α-Mg increased from 100 μm to 150 μm. At the same time, the value of the resistance of charge transfer increased, which indicates that the resistance of the charge transfer and the corrosion resistance of the alloys increased. After artificial aging treatment, the distribution of α-Mg was more uniform and its size was reduced to about 50 μm, and there was new eutectic structure formed. The newly formed eutectic structure forms galvanic cells with the alloy matrix, which makes the corrosion resistance of the alloy weaken.
Key words:
gas-phase alloying; Mg-Zn alloy; heat treatment; Ringer’s solution; electrochemical behavior;
Cite this article as:
MA Jun, NIU Li-bin, YAN Yu-ting, GAO Chong, WANG Xiao-gang. Influence of heat treatment on microstructure and electrochemical behaviors of Mg-Zn binary alloys prepared by gas-phase alloying technique [J]. Journal of Central South University, 2020, 27(3): 762-771.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4329-71 Introduction
Due to the significant advantages of low density, high specific strength, non-toxicity and non-adverse effects in the bone system [1], Mg-based alloys are considered to have potential as biomedical implantation [2, 3]. It is well known that, Zn is a common alloying element in magnesium alloys with the solubility limit of 6.2 wt% and can effectively improve the mechanical properties of magnesium [4]. Zn also exists in all human body tissues and is one of the most abundant nutritionally essential elements in human body [5].
The corrosion behavior of magnesium alloys is an important aspect of these alloys research. The corrosion performance of magnesium alloys is obviously improved by alloying or changing treatment process [6]. However, the traditional preparation methods always introduced harmful impurities during the formation process, and the corrosion performance is very sensitive to the second phase impurities. Because their low solid-solubility and easily presence of second phases that act as local cathodes cause micro- galvanic acceleration of corrosion in α-Mg, and provide effective cathodic sites [7], the corrosion performance of the magnesium alloy is very sensitive to the impurities. At the same time, the studies of the corrosion properties of alloys are mainly based on traditional preparation methods such as casting and powder metallurgy, while there are few studies on Mg-Zn alloys containing higher mass fractions of intermetallic compounds prepared by vacuum evaporation.
There are some researches on the method of fabricating new materials or coatings by vapor condensation. SINGHAL et al [8] produced nano- powders of oxides and studied the nano-particle aggregation using vapor phase synthesis method called combustion flame-chemical vapor condensation. SIMCHI et al [9] studied the mechanisms of nano-particle prepared in gas phase state, which is useful to the studies of the nano-particle characteristics. AKBARI et al [10] prepared the alloy nano-particles in the Mg-Al system by plasma arc evaporation technique. ZHONG et al [11] prepared the Zn-Mg coatings through a combined PVD and reaction-diffusion method, which exhibited a better corrosion resistance than the galvanized iron (GI) coating.
Due to the higher saturation vapor pressure for Mg and Zn elements and lower melting point, it is desirable and favorable to prepare Mg-Zn alloys through gas-phase alloying technique. The morphologies, chemical compositions, corrosion performance of specimens were investigated in order to support the foundation of Mg-Zn alloys application.
2 Experimental procedures
The equipment for preparing Mg-Zn alloy is self-designed, which can achieve a certain degree of vacuum, and the conceptual diagram of experimental device is shown in Figure 1. The commercially pure Mg (500 g) and Zn (500 g) were evaporated by electric resistance furnace in chambers 1 and 2, respectively. Some baffles were fixed at the chamber 3, which can increase the collision frequency of the two metal vapors and improve the nucleation quality of the mixing stage [12].
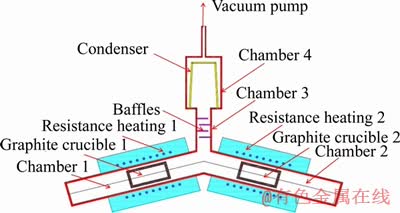
Figure 1 Conceptual diagram of alloys prepared device
Specimens for heat treatment had the same dimensions of 3 cm×3 cm×1 cm (length×width× thickness) which were cut from the condensed alloy. Samples without any treatment were considered initial state in the following paper. Subsequently solution treatment (T4) consisted of annealing at 340 °C for 12/24 h to homogenize the microstructure and quenching in warm water. Specimens for artificial aging (T6) treatment were annealed at 340 °C for 24 h and quenched in warm water and then annealed at 175 °C for 16 h.
The X-ray diffraction (XRD) data were recorded on a PW 1730 X-ray diffractmeter with monochromatic CuKα radiation at 40 kV and 40 mA. Microstructure and elemental analyses were conducted by using a TESCAN VEGA 3 scanning electron microscope (SEM) and energy dispersive X-ray spectroscope (EDS), respectively.
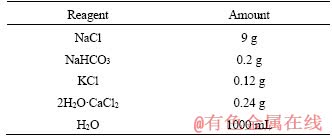
The corrosion behaviors were tested in Ringer’s simulated body fluid (SBF). The ion composition of SBF is listed in Table 1. Electrochemical tests were performed in SBF using a classical three-electrode cell with a platinum plate as counter electrode, and a saturated calomel electrode as reference electrode and the sample with an exposed area of 1×3 cm2 (1 cm in width, 3 cm in length) as working electrode. The initial delay of 300 s was set to ensure a stable system before undertaking the experiment. The potentiodynamic curves were obtained using an EG & G potentiostat model 273. The measurements started from -200 mV. Open circuit potential (OCP) was kept at a constant scan rate of 0.5 mV/s and terminated until a final current density of approximate 10 mA/cm2. The electrochemical tests results of polarization were fitted by Tafel method.
Table 1 Reagent for preparation of Ringer’s SBF
3 Results and discussion
3.1 Theoretical analysis and calculation
The microstructure of condensed production is determined by the chemical component, collision frequency, thermal velocity and kinetic energies of nucleus vapor atoms. When a single type of gas is involved in the formation of a critical nucleus, nucleation is homo-molecular. When two types of gases are involved in the formation of a critical nucleus, nucleation is hetero-molecular. In this study, the chemical component, namely, the Mg/Zn atoms ratio is one of the most important factors in nucleation course. According to Refs. [13, 14], the vapor rate of certain metal is described by Eq. (1). The saturated vapor pressure of Mg and Zn metals can be calculated through Eqs. (2) and (3), and the results were shown in Table 2.
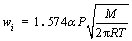 (1)
(1)
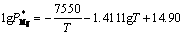 (2)
(2)
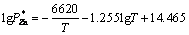 (3)
(3)
where wi is vapor rate for certain metal; α is the condensation constant for most crystalline metals approaches unity; M is the molar mass of metal (g/mol);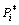 is the saturated vapor pressure (Pa); T is Kelvin’s temperature (K). Then, the vapor ratio for Mg/Zn can be determined using the following Eq. (4).
is the saturated vapor pressure (Pa); T is Kelvin’s temperature (K). Then, the vapor ratio for Mg/Zn can be determined using the following Eq. (4).
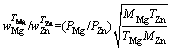 (4)
(4)
Therefore,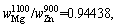
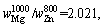
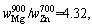
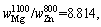
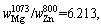
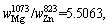
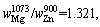 where superscript and subscript in
where superscript and subscript in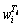 are the deputies of the evaporating temperature and certain metal,respectively. When the vapor rate of Mg-Zn is close to 1, the vapor perturbation will be reduced, which is beneficial to the cooling and solidification of Mg-Zn vapor. Then in this study, we chose the evaporation temperature of 1073 and 873 K for the commercial Mg and Zn, respectively.
are the deputies of the evaporating temperature and certain metal,respectively. When the vapor rate of Mg-Zn is close to 1, the vapor perturbation will be reduced, which is beneficial to the cooling and solidification of Mg-Zn vapor. Then in this study, we chose the evaporation temperature of 1073 and 873 K for the commercial Mg and Zn, respectively.
Table 2 Results of saturated vapor pressures of magnesium and zinc in certain temperatures
3.2 Microstructure of initial state Mg-Zn alloys
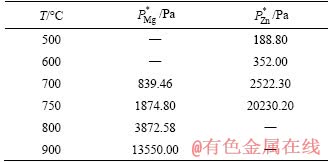
XRD patterns of the Mg-Zn binary alloys are shown in Figure 2. Mg-Zn alloys in the initial state were composed of α-Mg solid solution, MgZn, Mg7Zn3 and MgZn2. Since the boiling points of magnesium and zinc are lower under vacuum conditions, some high melting point impurities will remain in the evaporation chamber, giving rise to improving the purity of alloy.
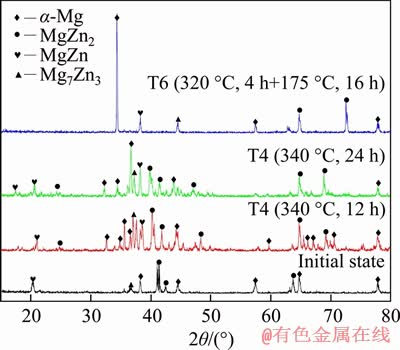
Figure 2 XRD results of Mg-Zn binary alloy in different treatment process
Figure 3 shows the microstructure of Mg-Zn binary alloys at the initial state. The microstructure of Mg-Zn alloys at the initial state was mainly composed of dark gray oval-like structure and eutectic mixture of bright gray polygons and the eutectic structures had clear boundary. A magnified observation of the polygonal eutectic structure in Figure 3(b) revealed that the morphology was black-white layered overlapping with different shapes, which accounts for about one-third of the entire field of view (Figure 3(c)). The point EDS analysis of the eutectic mixture was carried out to obtain the atomic ratio of elements at different points as shown in Table 3. According to the atomic ratio of elements, it was presumed that points A and B were MgZn and Mg7Zn3 intermetallic compounds, respectively, and thus the polygonal eutectic structure was composed of MgZn and Mg7Zn3. During the solidification process, due to the similar structure of MgZn and Mg7Zn3, MgZn intermetallic compounds precipitated on the side of the Mg7Zn3 microstructures and the precipitation of the MgZn phases promotes the growth of Mg7Zn3, which makes MgZn and Mg7Zn3 form overlapping microstructure [15, 16].

Figure 3 SEM morphology of initial state Mg-Zn binary alloy at different magnifications
Table 3 EDS results of initial state Mg-Zn alloy

A large number of black, elliptical structures with smooth edges were distributed around the eutectic structure, accounting for about half of the total volume. EDS analysis of the point C revealed that the atomic ratio of Mg/Zn was 96:4, and it was presumed that the black structure was α-Mg. According to the solidification theory, the polygonal eutectic mixture is a primary phase with segregation [17]. When the alloy crystallizes, a large amount of heat was released, which caused the Mg element to be segregated in the lamellar structure and finally formed elliptical structure. Different from the traditional alloy preparation methods, Mg-Zn alloys prepared by vacuum evaporation had a large amount of Zn vapor entering the condenser and mixing with Mg vapor during evaporation under vacuum condition, resulting in a large amount of intermetallic compounds distributed in α-Mg solid solution around.
3.3 Microstructure of heat treatment state Mg-Zn alloys
The phase composition of the Mg-Zn alloy after heat treatment of T4 and T6 was the same as the initial state, which was composed of α-Mg, MgZn, Mg7Zn3 and MgZn2 phases. While compared with the initial state, the peaks of representing MgZn2 and MgZn phases after solution treatment increased significantly, indicating that the crystal structure and growth orientation of MgZn2 and MgZn phases changed during solution treatment. After T6 treatment, the peaks of each phase changed significantly, but the phase composition of the alloy did not change, which reveals that the growth orientation of the second phases were more uniform after aging treatment.
Figure 4 shows the microstructure of the Mg-Zn binary alloy at different magnifications after T4 treatment. After the solution treatment, the boundary of the polygonal structure disappeared, and the lamellar eutectic structure almost completely changed to the same morphology. At the same time, some white oval structure appeared at the alloy matrix. The color of α-Mg changed from black to gray, and its average size was increased from 100 μm to about 150 μm. The results of EDS analysis of the alloy are shown in Table 4. EDS results revealed that the gray elliptical structure (point A1) was still α-Mg, the newly formed white elliptical structure (point B1) was MgZn intermetallic and the lamellar eutectic structure was transformed into Mg7Zn3 intermetallic compound.
When solution treatment at 340 °C, the solid solubility of Zn atoms in α-Mg reached a maximum of 6.2 wt%, and the microstructure and composition of intermetallic compounds were also changed. When some Zn atoms diffused into the α-Mg microstructure, the lamellar eutectic structure was changed, and the MgZn phase with higher Zn content was transformed into the Mg7Zn3 phase with lower Zn content [18]. At the same time, as the solution treatment time increased, some of the Zn atoms will also be segregated, resulting in white MgZn intermetallic compounds formed [19].
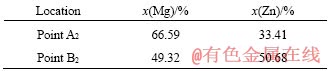
After artificial aging treatment, the microstructure of the alloy is shown in Figure 5. The size of α-Mg obviously reduced to about 50 μm and those were aggregated to form dendritic structure (Figure 5(b)). In Figure 5(b), it can be observed that the eutectic microstructure which disappeared after the solution treatment was reprecipitated around α-Mg. By analyzing the EDS data (Table 5), the gray matrix (point A2) was the Mg7Zn3 phase, and the newly formed eutectic structure (point B2) was the MgZn. This phenomenon may be caused by the fact that during artificial aging, Zn atoms in the supersaturated α-Mg will precipitate and form MgZn phases at the grain boundaries. At the same time, the intermetallic compound around α-Mg also has a part of Mg atoms precipitated to form new α-Mg solid solution, and thus alternately grow to form eutectic structures as shown in Figure 5(b).
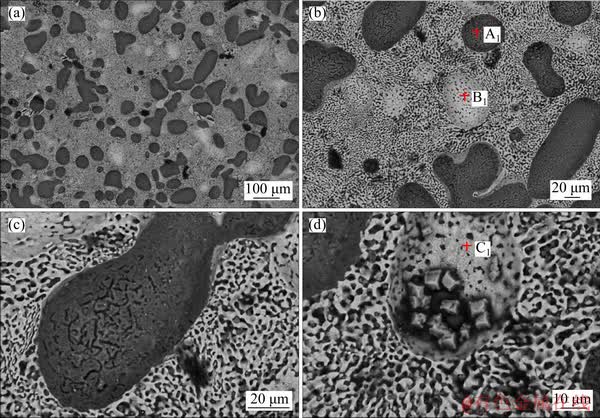
Figure 4 SEM micrographs of the T4 (340 °C, 24 h) state Mg-Zn binary alloy at different magnifications
Table 4 EDS results of Mg-Zn alloys at T4 (340 °C, 24 h) state

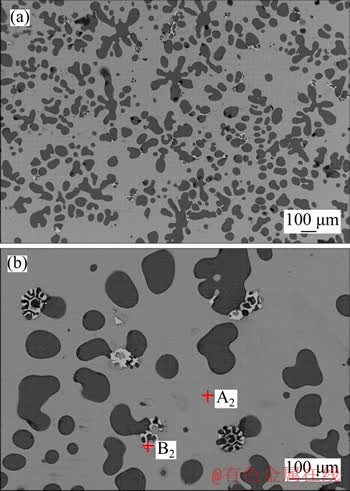
Figure 5 SEM micrographs of T6 state Mg-Zn alloy
Table 5 EDS analysis of Mg-Zn alloys at T6 state
3.4 Electrochemical corrosion behaviors of Mg- Zn alloys
The potentiodynamic polarization curves of Mg-Zn binary alloys in Ringer’s simulated body fluid under different states are shown in Figure 6. The upper part of the polarization curve is the anode and the lower part is the cathode, and it can be inferred from the curves that the cathode is in activated dissolved state. Compared with the alloy in the initial state, the polarization curve of the Mg-Zn binary alloy after T4 treatment moved to the right and after T6 treatment moved to the left. The dynamic potential polarization curves of Mg-Zn alloys in Ringer’s solution with different heat treatment are shown in Table 6 after Tafel fitting. ba is the Tafel slope of the anode, and bc is the Tafel slope of the cathode. Compared with the initial value of -1468.8 mV, the open corrosion potential (Ecorr) of Mg-Zn alloys increased to -1267.1 mV after solution treatment, which means that with the increase of solution treatment time, the corrosion resistance of Mg-Zn alloys in Ringer’s solution was significantly improved. However, after artificial aging treatment, the open corrosion potential of the alloy was reduced to -1535.4 mV, which explains that the corrosion resistance of the alloy deteriorated. The initial state alloy corrosion current density (Jcorr) was 308.44 μA/cm2. And after solution treatment at 340 °C for 24 h, the corrosion current density was reduced to 134.47 μA/cm2. But after aging treatment, the corrosion current density was significantly increased to 685.00 μA/cm2.
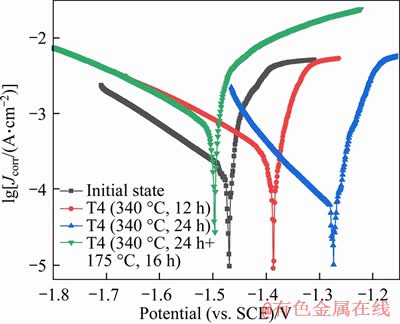
Figure 6 Dynamic potential polarization curves of Mg-Zn alloy in Ringer’s solution at different states
Table 6 Fitting results of polarization curves Mg-Zn alloy in Ringer’s solution at different states
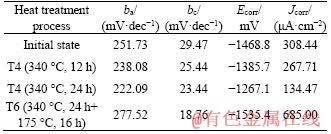
Figure 7 shows the electrochemical impedance Nyquist diagram of Mg-Zn alloys in Ringer’s solution under different heat treatment. The electrochemical impedance spectroscopy of the Mg-Zn binary alloys consisted of three arcs: the capacitive arc in the high frequency region, the capacitive arc in the intermediate frequency region, and the inductive arc in the low frequency region [20, 21], which indicates that the corrosion process of the alloy in Ringer’s solution has gone through three stages. It is known from the corrosion mechanism of the magnesium alloy that the capacitive arc in the high frequency region indicated the process of charge or the substance participating in the reaction passing through the corrosion product layer [20]. The capacitive arc of the intermediate frequency region was related to the diffusion of ions through the corrosion product layer. The inductive arc observed in the low frequency region was related to the adsorption process of ions and electrons. The Nyquist plot analysis of the impedance of Mg-Zn alloy in different heat treatment in Ringer’s solution was obtained. After T4 treatment, the radius of the capacitive reactance arc in the high frequency region and the inductive arc in the low frequency region were continuously expanded, presuming that all heat treatment alloys matrix were in activated dissolved state, and corrosion products were formed. After T6 treatment, the radius of the capacitive arc and the inductive arc in the Nyquist diagram was significantly reduced, even smaller than that in the initial state, which means that the corrosion resistance of the Mg-Zn binary alloy was significantly reduced after T6 treatment.
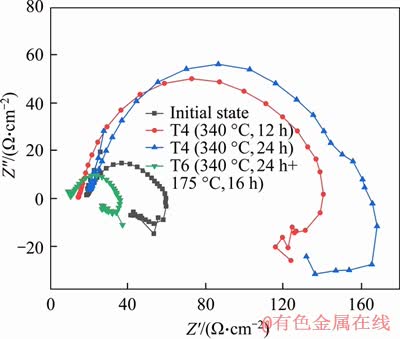
Figure 7 Nyquist diagram of impedance of Mg-Zn alloy at different states
The electrochemical impedance spectroscopy of the alloys was analyzed using an equivalent circuit model (Figure 8). In this model, Rs represented the resistance of the corrosion solution [22], and Rct was the resistance of charge transfer. The electrochemical impedance Nyquist curve of the alloy is a regular semi-circular shape, so CPE1 was used instead of Cdl in this model [20]. In addition, CPE2 and the resistor Rf were used to analyze the capacitance and resistance of the product layer formed by the binary alloy at the later stage of corrosion [23, 24], and considered the influence of the inductance, the inductor L and its resistance RL were introduced [21].

Figure 8 Equivalent circuit model of corrosion behavior of Mg-Zn alloy
From the electrochemical impedance spectroscopy equivalent circuit fitting data in Table 7, it can be seen that the value of Rct increased from 128.70 Ω in the initial state to 216.31 Ω after solution treatment. After artificial aging treatment, the value of Rct was significantly reduced to only 26.55 Ω. The radius of the capacitive arc in the high frequency region increased after T4 treatment, which means that the impedance increased. The increase of impedance is closely related to the corrosion product. It is known from Eq. (5) of magnesium alloy that the corrosion product is mainly Mg(OH)2 precipitate [25]. As the corrosion reaction proceeds, the amount of precipitate of Mg(OH)2 will gradually increase, which will protect Mg-Zn alloy matrix. The corrosion products layer resistance value Rf is known from the data in Table 7, the value of Rf was increased from 47.5 Ω in the initial state to 269.6 Ω after T4 treatment, indicating that the corrosion resistance of the alloy was improved. However, the value of Rf decreased to 58.6 Ω after T6 treatment, and the resistance of the corrosion product layer of the alloy was not changed much compared with the initial state, which indicates the corrosion product had less protection to the alloy matrix.
Mg+2H2O→Mg(OH)2+H2 (5)
Combined with the previous analysis, the elliptical α-Mg phases aggregated and grew to form dendritic structures and there were reprecipitated eutectic structures around the α-Mg matrix after T6 treatment. The α-Mg matrix formed galvanic cells with the surrounding eutectic structure and galvanic corrosion occurs [26]. When the eutectic structure was completely corroded, the corrosion product layer was easily detached, and the Mg matrix cannot be protected. This phenomenon can also be found by the change of Rct decreasing from 216.31 Ω at T4 to 26.55 Ω at T6. The rising pH value caused the Mg matrix to be corroded more severely, resulting in corrosion resistance of the Mg-Zn binary alloys lower.
Table 7 Equivalent circuit fitting results of electrochemical impedance spectroscopy of alloys at different heat treatment states
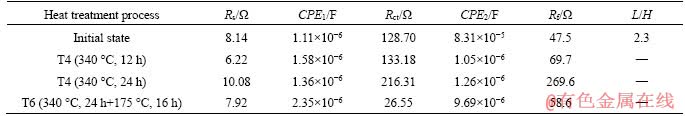
Figure 9 shows the corrosion morphology of the alloys at different heat treatment states after electrochemical corrosion in Ringer’s solution. It can be seen from these figures that the surface of the alloys were corroded and corrosion products existed. There was obvious differences that the corrosion surfaces morphology of initial state and T4 alloys was local corrosion, and the corrosion products of the alloy at the state of T4 were less than those of the initial state alloy and mainly concentrated on the α-Mg matrix (Figures 9(a)-(c)). Alloys at the states of T6 showing general corrosion and corrosion products were distributed on the entire alloys surface (Figure 9(d)).
The initial state alloy matrix contained eutectic structures, which were lamellar structure composed of MgZn and Mg7Zn3 phases. During the corrosion process, due to the different self-corrosion potential of MgZn and Mg7Zn3 phases, the galvanic corrosion of Mg7Zn3 and MgZn phases was formed [27]. As the corrosion progressed, the eutectic structures will fall off and form corrosion pits. After aging treatment, α-Mg phases aggregated and formed dendritic structure surrounded by reprecipitated Mg7Zn3 intermetallic compounds, which makes the tendency of galvanic corrosion ratio increase. After solution treatment, the content of eutectic structures of the alloy was reduced, which can effectively reduce the segregation of Zn element, and make the phases distribution of the alloy more uniform, and reduce the tendency of local corrosion.

Figure 9 Corrosion morphology of Mg-Zn alloys in different states after electrochemical corrosion in Ringer’s solution:
4 Conclusions
1) The initial state Mg-Zn alloys prepared by vacuum evaporation consisted of α-Mg, MgZn, Mg7Zn3, and MgZn2 phases and a large number of intermetallic compounds are distributed around the α-Mg matrix.
2) After solution treatment, the lamellar eutectic structures change to the same morphology and the size of α-Mg phase increases from 100 μm to 150 μm. After artificial aging treatment, the α-Mg distribution is more uniform and the size is reduced to about 50 μm, and there are new eutectic structures attached to its growth.
3) After T4 treatment, the value of Rct increased, which indicates that the resistance of the charge transfer and the corrosion resistance of the alloys increased. After T6 treatment, both the value of CPE1 and Rct were significantly reduced, meaning that the resistance of the charge transfer process is inhibited and the corrosion resistance of the alloy is weakened.
References
[1] PAN Fu-sheng, YANG Ming-bo, CHEN Xian-hua. A review on casting magnesium alloys: Modification of commercial alloys and development of new alloys [J]. Journal of Materials Science & Technology, 2016, 32(12): 1211-1221. DOI: 10.1016/j.jmst.2016.07.001.
[2] RADHA R, SREEKANTH D. Insight of magnesium alloys and composites for orthopedic implant applications–A review [J]. Journal of Magnesium and Alloys, 2017, 5(3): 286-312. DOI: 10.1016/j.jma.2017.08.003.
[3] YANG Guo-qing, PENG Xiao-dong, YANG Yan, LI Meng-luan, WEI Guo-bing, SHAO Hong-yan, WANG Bao. Microstructure and mechanical properties of as-cast and extruded Mg-8Li-3Al-0.7Si alloy [J]. Journal of Central South University, 2018, 25(4): 764-771. DOI: 10.1007/ s11771-018-3781-0.
[4] NAVE M D, DAHLE A K, STJOHN D H. The role of zinc in the eutectic solidification of magnesium-aluminium-zinc alloys [M]. The Minearls, Metals and Materials Society, 2000. DOI: 10.1002/9781118808962.ch34.
[5] WAN Peng, TAN Li-li, YANG Ke. Surface modification on biodegradable magnesium alloys as orthopedic implant materials to improve the bio-adaptability: A review [J]. Journal of Materials Science & Technology, 2016, 32(9): 827-834. DOI: 10.1016/j.jmst.2016.05.003.
[6] YOU Zhi-yong, ZHANG Yu-hua, CHENG Wei-li, ZHANG Jin-shan, WEI Ying-hui. Effect of heat-treatment on the microstructures and mechanical properties of Mg-10Zn-5Al-0.1Sb-xCu magnesium alloy [J]. Journal of Wuhan University of Technology-Mater Sci Ed, 2013, 28(4): 834-839. DOI: 10.1007/s11595-013-0778-2.
[7] ATRENS A, LIU Ming, ABIDIN N I Z. Corrosion mechanism applicable to biodegradable magnesium implants [J]. Materials Science and Engineering B, 2011, 176(20): 1609-1636. DOI: 10.1016/j.mseb.2010.12.017.
[8] SINGHAL A, SKANDAN G, WANG A, GLUMCA N, KEAR B H, HUNT R D. On nanoparticle aggregation during vapor phase synthesis [J]. Nanostructured Materials, 1999, 11(4): 545-552. DOI: 10.1016/S0965-9773(99)00343-8.
[9] SIMCHI A, AHMADI R, REIHANI S S, MAHDAVI A. Kinetics and mechanisms of nanoparticle formation and growth in vapor phase condensation process [J]. Materials & Design, 2007, 28(3): 850-856. DOI: 10.1016/j.matdes.2005. 10.017.
[10] AKBARI M K, DERAKHSHAN R, MIRZAEE O. A case study in vapor phase synthesis of Mg–Al alloy nanoparticles by plasma arc evaporation technique [J]. Chemical Engineering Journal, 2015, 259(290): 918-926. DOI: 10.1016/j.cej.2014.08.053.
[11] ZHONG Ge-xi, ZHANG Qi-fu, JIANG She-ming, YU Gang-qiang. Effect of the process parameters on the microstructure and corrosion resistance of Zn-Mg alloy coatings prepared via acombined PVDand reaction-diffusion process [J]. Surface and Coatings Technology, 2016, 306(25): 418-427. DOI: 10.1016/j.surfcoat.2016.05.017.
[12] ZHANG Ren-yi, KHALIZOV A, WANG Lin, HU Min, XU Wen. Nucleation and growth of nanoparticles in the atmosphere [J]. Chemical Reviews, 2012, 112(3): 1957-2011. DOI: 10.1021/cr2001756.
[13] JIANG Yu, LIU Ming, YANG Yan-min, GUAN Deng-gao, CHEN Jia-zhao, TU Min-jing. The hydrogen mechanism study in preparing nanonickel by plasma arc spraying method [J]. Rare Met Mater Eng, 2005, 34: 475-478. DOI: 10.1021/ja00226a070.
[14] DAI Yong-nian, YANG Bin. The vacuum metallurgy of nonferrous metals [M]. Beijing: Metallurgical Industry Press, 2000. (in Chinese)
[15] VERISSIMO NATHALIA C, BRITO C, AFONSO CONRADO R, SPINELLI JOSE E, CHEUNG N, GARCIA A. Microstructure characterization of a directionally solidified Mg-12wt.% Zn alloy: Equiaxed dendrites, eutectic mixture and type/morphology of intermetallic [J]. Materials Chemistry and Physics, 2018, 204: 105-131. DOI: 10.1016/j.matchemphys.2017.10.032.
[16] VIDA T A, BRITO C, LIMA T S, SPINELLI J E, CHEUNG N, GARCIA A. Near-eutectic Zn-Mg alloys: Interrelations of solidification thermal parameters, microstructure length scale and tensile/corrosion properties [J]. Current Applied Physics, 2019, 19(5): 582-598. DOI: 10.1016/j.cap.2019.02.013.
[17] POUND G M. Selected values of evaporation and condensation coefficients for simple substances [J]. Journal of Physical & Chemical Reference Data, 1972, 1(1): 135-146. DOI: 10.1063/1.3253096.
[18] NEMEC M, JAGER A, TESAR K, GARTNEROVA V. Influence of alloying element Zn on the microstructural, mechanical and corrosion properties of binary Mg-Zn alloys after severe plastic deformation [J]. Materials Characterization, 2017, 134: 69-75. DOI: 10.1016/j.matchar. 2017.10.017.
[19] YAN Yang, CAO Han-wen, KANG Yi-jun, YU Kun, XIAO Tao, LUO Jie, DENG You-wen, FANG Hong-jie, XIONG Han-qing, DAI Yi-long. Effects of Zn concentration and heat treatment on the microstructure, mechanical properties and corrosion behavior of as-extruded Mg-Zn alloys produced by powder metallurgy [J]. Journal of Alloys and Compounds, 2017, 693: 1277-1289. DOI: 10.1016/j.jallcom.2016.10.017.
[20] JAMESH M, KUMAR S, NARAYANAN T S N S. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution–long term evaluation by EIS [J]. Corrosion Science, 2011, 53(2): 645-654. DOI: 10.1016/ j.corsci.2010.10.011.
[21] CAO Fu-yong, SHI Zhi-ming, HOFETETTER J, UGGOWITZER P J, SONG Guang-ling, LIU Ming, ATRENS A. Corrosion of ultra-high-purity Mg in 3.5% NaCl solution saturated with Mg(OH)2 [J]. Corrosion Science, 2013, 75: 78-99. DOI: 10.1016/j.corsci.2013.05.018.
[22] SONG Ying-wei, HAN En-hou, SHAN Da-ying, YIM C D, YOU B S. The effect of Zn concentration on the corrosion behavior of Mg–xZn alloys [J]. Corrosion Science, 2012, 65: 322-330. DOI: 10.1016/j.corsci.2012.08.037.
[23] JIA Hong-ming, FENG Xiao-hui, YANG Yuan-sheng. Effect of crystal orientation on corrosion behavior of directionally solidified Mg-4wt.% Zn alloy [J]. Journal of Materials Science & Technology, 2018, 34(7): 1229-1235. DOI: 10.1016/j.jmst.2017.06.009.
[24] YANG Jiang, PENG Jian, NYBERG E A, PAN Fu-sheng. Effect of Ca addition on the corrosion behavior of Mg–Al–Mn alloy [J]. Applied Surface Science, 2016, 369: 92-100. DOI: 10.1016/j.apsusc.2016.01.283.
[25] XU Hong, WU Zhi-quan, WANG Xiao-ru, ZHANG Xin, REN Ji-ping, SHI Yang, WANG Ze-pu, WANG Li-wei, LIU Chang-hua. Corrosion mechanism and corrosion model of Mg-Y alloy in NaCl solution [J]. Journal of Wuhan University of Technology-Mater Sci Ed, 2016, 31(5): 1048-1062. DOI: 10.1007/s11595-016-1489-2.
[26] SONG Ying-wei, HAN En-hou, SHAN Da-ying, YIM C D, YOU B S. The role of second phases in the corrosion behavior of Mg–5Zn alloy [J]. Corrosion Science, 2012, 60: 238-245. DOI: 10.1016/j.corsci.2012.03.030.
[27] MANDAL M, MOON A P, DEO G, MENDIS C L, MONDAL K. Corrosion behavior of Mg–2.4Zn alloy micro-alloyed with Ag and Ca[J]. Corrosion Science, 2014, 78: 172-182. DOI: 10.1016/j.corsci.2013.09.012.
(Edited by ZHENG Yu-tong)
中文导读
热处理对气相合金化Mg-Zn二元合金组织和电化学行为的影响
摘要:采用气相合金化技术,在真空条件下制备了Mg-Zn二元合金,研究了合金在初始状态和热处理后的组织和电化学行为。与传统的Mg-Zn合金制备方法不同,气相合金化制备的合金中含有大量的MgZn、Mg7Zn3 和 MgZn2金属间化合物,α-Mg的含量较低。固溶处理后,共晶界面消失,α-Mg相尺寸由100 μm增加到150 μm,同时在Ringer’s试液中的电化学测试结果显示电荷转移的电阻值增加,表明合金的电荷转移电阻和耐蚀性增加。经人工时效处理后,α-Mg的分布更加均匀,α-Mg相的尺寸减小到50 μm左右,并且形成了新的依附于其生长的共晶组织。由于新形成的共晶组织与合金基体形成腐蚀原电池,使合金的耐蚀性减弱。
关键词:气相合金化;Mg-Zn合金;热处理;Ringer’s试液;电化学行为
Foundation item: Project(2015DFR50990-01) supported by the International Cooperation Project of Ministry of Science and Technology of China; Project(2016KF-01) supported by the Shaanxi Key Laboratory of Nano-materials and Technology, China; Project(2015CXY-01) supported by the Cooperation Project on the Integration of Industry, Education and Research of Yulin Science and Technology Bureau, China
Received date: 2019-09-21; Accepted date: 2020-03-13
Corresponding author: NIU Li-bin, PhD, Associate Professor; Tel: +86-29-85587373; E-mail: dy059@126.com; ORCID: 0000-0003- 3164-0310
Abstract: Mg-Zn binary alloys fabricated by the gas-phase alloying technique under vacuum condition were investigated in the state of initial state and after heat treatment for the microstructure and electrochemical behaviors. Different from the traditional Mg-Zn alloys preparation methods, alloys prepared by gas-phase alloying have a large number of intermetallic compounds, such as MgZn, Mg7Zn3 and MgZn2. After solution treatment, the boundary of the eutectic disappeared and the size of α-Mg increased from 100 μm to 150 μm. At the same time, the value of the resistance of charge transfer increased, which indicates that the resistance of the charge transfer and the corrosion resistance of the alloys increased. After artificial aging treatment, the distribution of α-Mg was more uniform and its size was reduced to about 50 μm, and there was new eutectic structure formed. The newly formed eutectic structure forms galvanic cells with the alloy matrix, which makes the corrosion resistance of the alloy weaken.


