J. Cent. South Univ. Technol. (2009) 16: 0108-0111
DOI: 10.1007/s11771-009-0018-2![]()
Influence factors analysis of removing heavy metals from multiple metal-contaminated soils with different extractants
XIA Wen-bin(夏文斌), LI Xin(李 欣), GAO Hui(高 慧), HUANG Bao-rong(黄宝荣),
ZHANG Hui-zhi(张慧智), LIU Yun-guo(刘云国), ZENG Guang-ming(曾光明), FAN Ting(樊 霆)
(College of Environmental Science and Engineering, Hunan University, Changsha 410082, China)
Abstract:
Some key factors on the heavy metals removal efficiencies were studied when soil washing technology was used in the remediation of soils contaminated by multiple heavy metals. The results show that the dissolubilities of Cu and Zn are promoted by humic acids, but Pb and Cd are inhibited by humic acids; heavy metals in the clay are more difficult to be extracted than silt; the strong acidic soils can cause the protonation of EDTA and weaken its extracting ability; EDTA is effective for extracting Pb and Cd, while oxalate (OX) is effective for extracting Cu and Zn; and biosurfactant can be used as additive to improve the removal of some particular heavy metals.
Key words:
heavy metal; soil remediation; extraction; rhamnolipid;
1 Introduction
Contamination of soil by heavy metals, mainly due to acid mine drainage, tailings embankments, mining rock dumps and metallurgical waste piles, poses a serious threat to the environment, and their accumulation in the environmental compartments could lead to toxic effects on biotic life [1]. LIU et al [2] reported that even a low concentration of heavy metals in the soil had a potential impact on the environmental quality and human health via ground water and surface water.
Coping with metal-contaminated soils is often a major issue in cleanup of hazardous waste sites. Soil washing, a technique that is based on washing the contaminated soil in situ or ex situ, with water, inorganic acids such as sulfuric acid and hydrochloric acid, organic acids such as acetic acid and citric acid, and chelating agents such as EDTA, is gaining more popularity for soil remediation [3-6].
Many experiments have been carried out in order to study the effectiveness of different materials for the removal of heavy metals in contaminated soils [3]. EDTA has been widely proposed and studied, because of its attributes of high efficiency of metal extraction, weak adsorption on soils, and effective recovery and reuse [3, 5]. However, the low selectivity of EDTA may cause a high consumption of this reagent due to the enhancement in the mobilization of all the exchangeable cations present in the solid matrix [7]. Therefore, comparisons with oxalate (OX), citric acid (CA), and the mixture of citric acid and rhamnolipid (CAR) using as extractant agents were also performed in this study.
It has been reported that metal removal efficiency by EDTA depends on many factors such as the liability of HMs in soil, the concentration of EDTA, electrolytes, pH and the soil matrix [8]. Thus determining the influence of these factors on the removal of multiple metals is quite important for soil remediation in practice, especially the remediation of mine-tailing soils. In this work, four factors including the concentration of humic acid (HA), the soil pH and texture, the variation of extractants and the addition of rhamnolipid were considered to investigate their effects on the removal efficiencies of Pb, Cd, Cu, and Zn from the mine-tailing soils by EDTA.
2 Materials and methods
2.1 Soil samples and analysis
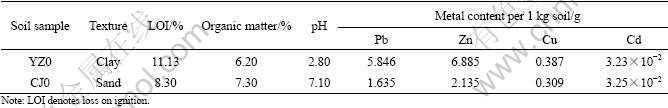
Surface soils (0-20 cm) were collected from two heavily contaminated tailing areas: Yongzhou Lead-Zinc Mine and Chejiang Copper Mine in Hunan province. The soil samples were air-dried and ground to pass through a 2-mm sieve, then homogenized and stored in jars signed YZ0 and CJ0, respectively.
Soil pH was determined using a pH-meter (pHS- 3C). Organic matter was measured with standard methods [9]. All the reagents used were of analytical grade. Each experiment was performed in triplicate and the mean values were taken into account.
In the immobilization experiments, 125 g of air- dried soil samples that have not passed through the sieve were mixed with different masses of humic acids (HA) (0.5, 1.5 and 2.5 g, respectively), and the HA was biological reagent. Then distilled water was added to submerge the soil samples and the acid/soil slurries were agitated on a rotary shaker for 24 h at 200 r/min. After being deposited for one week, these mixtures were air-dried and ground to pass through a 2-mm sieve. In this way, six soil samples containing different contents of humic acids signed YZ1, YZ2, YZ3, and CJ1, CJ2, CJ3 were prepared, respectively. The physical-chemical characteristics of YZ0 and CJ0 are listed in Table 1.
Table 1 Physical-chemical characteristics of tailing soil
2.2 Extraction experiments
1 g of each soil sample was weighed using analytical balance and was placed in a 250 mL high- density polyethylene (HDPE) bottle. 50 mL of 0.05 mol/L EDTA, 0.05 mol/L oxalate (OX), 0.05 mol/L citric acid (CA), mixture of 0.05 mol/L citric acid and 0.05 mol/L rhamnolipid (CAR) were added separately to each soil sample to extract heavy metals. After being shaken for 6 h, each sample was centrifuged at approximately 15 000 r/min for 30 min. The liquid portion of the sample was decanted into a 125 mL HDPE bottle. The concentration of dissolved heavy metal in each sample was then determined using atomic absorption spectrophotometer. Heavy metal removal efficiency is calculated by the following equation:
η=(Mdiss/Mtotal)×100% (1)
where η is the removal efficiency, Mdiss is the dissolved heavy metal mass by extractant in each gram of soil, and Mtotal is total heavy metal mass in each gram of soil.
3 Results and discussion
3.1 HA concentrations in soils
Humic acid is known to be one of the major facilitators of the transport of metal ions in the environment. HA may be strongly adsorbed on soil particle surfaces and could influence the activity of heavy metals in the soil. Several studies have demonstrated that HAs can form stable complexes with many heavy metal ions, thus influencing their extraction efficiencies from soils [10]. Therefore, humic acid rich materials are adequate for the field-scale remediation of heavy metal polluted soils [11], it has been developed as a valuable soil remediation materials because of its low price and few by-products.
The removal efficiencies of metals from the two groups of soil samples with different HA concentrations are shown in Table 2.
Table 2 Heavy metals removal efficiencies obtained using different extractants on different soils
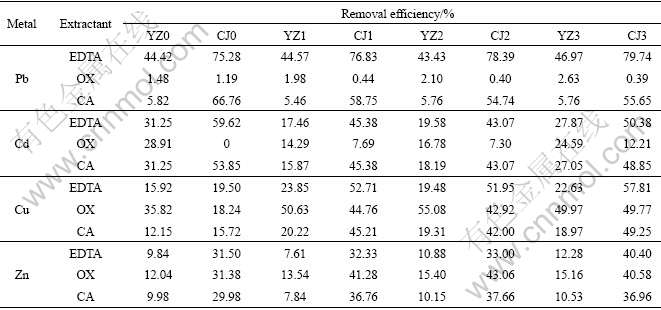
It can be seen from Table 2 that the extraction efficiencies of Cu and Zn using different extractants are increased with the increase of concentration of HA, which indicates that the concentrations of Cu and Zn in the extractable fraction of HA-treated soils are higher than those in control soils. However, the effects of HA in the two groups of soil are contrary with respect to Pb extraction, which are promoted by the addition of HA in Soil YZ and are inhibited in Soil CJ except using EDTA as extractant. This difference is likely to be related to the competitive adsorption of Pb among soil particles, HA and other metals. Increasing content of HA does not promote the extraction efficiencies of Cd in both two groups of soil samples, which is mainly caused by the formation of inert cadmium matter.
3.2 Soil texture and pH
As seen in Table 2, extractable efficiencies of almost all the metals do not exceed 50% in Soil YZ, and the maximum efficiency only reaches 55.08% in Soil YZ2 using OX as extractant, which is much lower than the average level of 77.56% in Soil CJ.
It is also found that the removal efficiencies of metals in Soil YZ are far lower than those in Soil CJ when the same extractant is used. It is likely to be related to the soil properties. The clay content in Soil YZ is too high to make the metals migrate to other media according to Ref.[12]. Usually, the higher the proportion of the clay and silt content in soil, the harder the metal extraction, because extracted HMs could easily be adsorbed by iron-manganese oxides located on the surface of those soil particles [13].
Soil pH may also contribute to the variation of extraction efficiencies of HMs by EDTA. Because of the strong chelating ability of EDTA, high extraction efficiencies of Pb are usually obtained in previous researches [14]. However, in this study, only 44.85% (average level) of Pb is extracted from Soil YZ by EDTA. One probable reason for this low efficiency is that the pH of Soil YZ is relatively low compared with that of Soil CJ. The strong acidic soils would cause the protonation of EDTA and weaken its extracting ability [15]. Many previous studies also showed that pH influenced the extraction of HMs by EDTA only in the acidic range (pH<5) [16].
3.3 Types of extractant
The removal efficiencies of the extracted metals for the three extractants are compared and the results are also shown in Table 2. The extractability of the extractants for Pb and Cd is in the order: EDTA>CA> OX; while for Zn it is in the order: OX>EDTA>CA. Comparatively, OX is the best extractant for Cu removal, especially in Soil YZ samples. The maximum removal efficiency reaches 55.08%. Although EDTA presents high Cu removal efficiencies in Soil CJ (57.81%), it is invalid in Soil YZ samples. The results indicate that the removal efficiencies of heavy metals depend significantly on the kinds of extractants. EDTA is more effective in extracting Pb and Cd than other two extractants. The extraction efficiencies reach 79.74% and 59.62%, respectively. EDTA could release the metals that have formed a complex or have been adsorbed by organic substances, which could enhance the solubility and mobility of metals in the soil. Thus it may hold potential in the remediation of soils singly contaminated with Pb or Cd. However, OX shows higher extraction ability for Cu and Zn, indicating that it is a competent for the remediation of soils contaminated with Cu and Zn.
3.4 Biosurfactant
Rhamnolipid like other surfactants is an amphiphilic compound with both hydrophobic and hydrophilic portions. The molecular structure of rhamnolipid makes it capable of enhancing soil flushing efficiency and suitable for removing heavy metals from soil. Rhamnolipid is also a biosurfactant that can be used to reduce the surface properties through lowering the interfacial tension and can reduce the work of adhesion between the metal and soil, which enables the lifting of the metal from the soil surface according to Ref.[17]. However, using biosurfactant as metal extractant in the soil remediation is not cost-effective and feasible because pure biosurfactant is too expensive to apply in the large-scale soil treatment. Therefore, the extraction ability of CA and CAR (mixture of 0.05 mol/L citric acid and 0.05 mol/L rhamnolipid) was compared to research whether the added rhamnolipid could promote the metal removal efficiencies. The results are shown in Table 3.
Table 3 Removal efficiencies of lead, cadmium, copper and zinc with CAR and CA
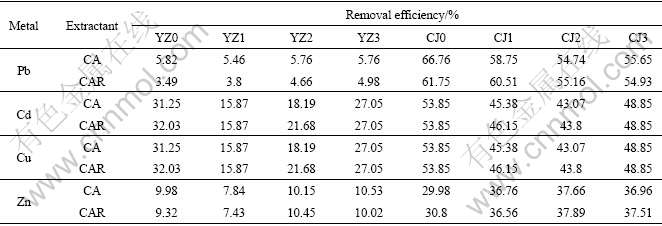
As seen in Table 3, the effects of rhamnolipid are different towards different soil textures and metals. This difference lies on whether it could lower the interfacial tension of the particles of soil samples and reduce the adhesion between metals and the particles to a critical point threshold, which enable the lifting of metals from soil surface. Although there are some fluctuating in the removal efficiencies of Pb, Cd and Zn in all soil samples and that of Cu in Soil CJ samples, the removal efficiencies are not increased obviously when rhamnolipid is added into citric acid as a mixed extractant. While with respect to Cu, removal efficiencies of which in Soil YZ samples are enhanced when CAR is used as the mixed extractant, indicating that adding biosurfactant surfactin into an ordinary extractant may be feasible for promoting the removal of some particular metals from soil.
4 Conclusions
(1) The effect of HA on the extraction of HMs from soils is strongly dependent on the inherent soil properties and the source of heavy metal contamination in soil. And the stimulative effect of HA is probably related to the content of extractable fraction of metals in the HA-treated soils.
(2) Heavy metals in clay are more difficult to be extracted out than those in silt probably because extracted HMs could easily be readsorbed by iron-manganese oxides located on the surface of clay particles, and the combination is too strong to make the metals migrate to other media.
(3) Soil pH value may contribute to the variation of extraction efficiencies of HMs by EDTA. The strong acidic soils cause the protonation of EDTA and weaken its extracting ability.
(4) EDTA is effective in extracting Pb and Cd, while OX is effective for extraction of Cu and Zn. Therefore, these extractants could be used in the remediation of soils contaminated with the corresponding metals.
(5) Adding biosurfactant surfactin into an ordinary extractant may lower the interfacial tension and reduce the work of adhesion between the metal and soil, being feasible for promoting the removal of heavy metals from soils.
References
[1] CICCU R, GHIANI M, SERCI A, FADDA S, PERETTI R, ZUCC A. Heavy metal immobilization in the mining-contaminated soils using various industrial wastes [J]. Miner Eng, 2003, 16(3): 187-192.
[2] LIU Yun-guo, ZHANG Hui-zhi, ZENG Guang-ming, HUANG Bao-rong, LI Xin, XU Wei-hua. Characteristics of tailings from the metal mines in Hunan Province, China [J]. Journal of Central South University of Technology, 2005, 12(2): 225-228.
[3] LESTAN D, LUO C L, LI X D. The use of chelating agents in the remediation of metal-contaminated soils: A review [J]. Environmental Pollution,2008, 153(1): 3-13.
[4] ZHANG W H, TSANG D C W, LO I M C. Removal of Pb by EDTA—washing in the presence of hydrophobic organic contami- nants or anionic surfactant [J] Journal of Hazardous Materials,2008, 155(3): 433-439.
[5] BARONA A, ARANGUIZ I, ELIAS A. Metal associations in soils before and after EDTA extractive decontamination: Implications for the effectiveness of further clean-up procedures [J]. Environmental Pollution, 2001, 113(1): 79-85.
[6] MAKKINA T, TAKANO H, KAMIYA T, ITOU T, SEKIYA N, INAHARA M, SAKURAI Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. [J]. Chemosphere,2008, 70(6): 1035-1043.
[7] KEDZIOREK M A M, BOURG A C M. Solubilization of lead and cadmium during the percolation of EDTA through a soil polluted by smelting activities [J]. Contam Hydrol, 2000, 40(4): 381-392.
[8] ELLIOTT H A, SHASTRI L. Extractive decontamination of metal-polluted soils using oxalate [J]. Water Air Soil Pollut, 1999, 110(3): 335-346.
[9] XI Dan-li. Environment monitoring of China [M]. Beijing: Science and Environment Literature Press, 1992: 56-79. (in Chinese)
[10] CHANG C S W, HUANG C C, WANG M C. Analytical and spectroscopic characteristics of refuse compost-derived humic substances [J]. International Journal of Applied Science and Engineering, 2003, 1(1): 62-71.
[11] CLEMENTE R, WALKER D J, ROIG A, BERNAL M P. Heavy metal bioavailability in a soil affected by mineral sulphides contamination following the mine spillage at Aznalco′llar (Spain) [J]. Biodegradation, 2003, 14(3): 199-205.
[12] LO I M C, YANG X Y. EDTA extraction of heavy metal from different soil fraction and synthetic soils [J]. Water, Air and Soil Pollution, 1999, 109(1): 219-236.
[13] ZHUANG J, YU G R, LIU X Y. Characteristics of lead sorption on clay minerals in relation to metal oxides [J]. Pedosphere, 2000, 10(1): 11-20.
[14] N??EZ-L?PEZ R A, MEAS Y, GAMA S C, BORGES R O, OLGUIN E J. Leaching of lead by ammonium salts and EDTA from Salvinia minima biomass produced during aquatic phytoremediation [J]. Journal of Hazardous Materials, 2008, 154(2): 623-632.
[15] ORTEGA L M, LEBRUN R, BLAIS J F, HAUSLER R, DROGUI P. Effectiveness of soil washing, nanofiltration and electrochemical treatment for the recovery of metal ions coming from a contaminated soil [J]. Water Research,2008, 42(8): 1943-1952.
[16] LO I M C, ZHANG W. Study on the optimal conditions for the recovery of EDTA from soil washing effluents [J]. Environ Eng, 2005, 131(11): 1507-1513.
[17] MULLIGAN C N, YONG R N, GIBBS B F, JAMES S, BENNETT H P J. Metal removal from contaminated soil and sediments by the biosurfactant surfactant [J]. Environment Science and Technology, 1999, 33(21): 3812-3820.
Foundation item: Project(20050532009) supported by the Doctoral Foundation of Ministry of Education of China; Projects(2006BAD03A1704, 2006BAD03A1706) supported by the National 11th-Five Technology Supporting Project
Received date: 2008-07-18; Accepted date: 2008-10-28
Corresponding author: LIU Yun-guo, Professor; Tel: +86-731-8649208; Fax: +86-731-8822829; E-mail: liuyunguo2005@yahoo.com.cn
(Edited by YANG You-ping)
- Influence factors analysis of removing heavy metals from multiple metal-contaminated soils with different extractants


