Trans. Nonferrous Met. Soc. China 30(2020) 2207-2216
Preparation and characterization of polyvinylchloride membrane embedded with Cu nanoparticles for electrochemical oxidation in
direct methanol fuel cell
Rohitash KUMAR1, Aftab Aslam Parwaz KHAN2, Anish KHAN2, Abdullah M ASIRI2, Yusra HAMID3
1. Department of Petroleum Studies, Aligarh Muslim University, Aligarh 202002, India;
2. Center of Excellence for Advanced Materials Research and Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, P. O. Box 80203, Saudi Arabia;
3. Department of Industrial Chemistry and Chemical Engineering, Politecnico di Milano, Milano 20133, Italy
Received 4 August 2019; accepted 28 June 2020
Abstract:
Copper nanoparticles were prepared by the chemical reduction method. These copper particles were embedded into the polyvinylchloride (PVC) matrix as support and used as an electrode (PVC/Cu) for the oxidation of methanol fuel for improving the current response. The PVC/Cu electrodes were characterized by thermal gravimetric analysis (TGA) for thermal stability of the electrode, X-ray diffraction (XRD) for identification of copper nanoparticles in the electrode, Fourier transform infrared spectroscopy (FTIR) to identify the interaction between PVC and Cu and scan electron microscopy (SEM) with EDAX for the morphology of the electrode. The electrocatalytic activity of the electrode was characterized by the cyclic voltammetry, linear sweep voltammetry, and chronoamperometry techniques. An increase in the electrode activity was observed with the increase of copper quantity from 0.18 g (PVC/Cu-0.18 g) to 0.24 g (PVC/Cu-0.24 g) and the maximum was found at 0.24 g of copper in the electrode. Also, it was observed that the electrode achieved the maximum catalytic current in 0.5 mol/L CH3OH + 1 mol/L NaOH solution. FTIR identified that water molecules, C—H group, copper nanoparticle and its oxide were available in the electrode. SEM images with EDAX showed that copper particles were properly embedded in the polyvinylchloride matrix.
Key words:
copper nanoparticles; polyvinylchloride; direct methanol fuel cell; electrochemical methods;
1 Introduction
The potential future energy systems in India for sustainable energy and environmental management, today, depend upon the direct use of production, solar and storage energy carriers like hydrogen, along with the use of cutting-edge systems of energy conversion in a considerably more environment friendly manner when contrasted with the extensive use of fossil fuels. Out of these, hydrogen is probably going to be a definitive energy carrier later as it is renewable and non- contaminating compared to other energy carriers like diesel and gasoline. Hydrogen is a perfect energy carrier from a subjective perspective [1,2]. It tends to be changed into heat and mechanical energy through burning and to electrical energy with pure water as the main product in fuel cells. The carbon dioxide for the whole energy process relies upon the fuel or the kind of essential energy used for the hydrogen production. Hydrogen can be formed from an assortment of renewable energy sources, to be specific, electrolytic hydrogen delivered from photovoltaic sources/wind, and biomass derivative hydrogen through gasification. However, the absence of a vaporous fuel framework and the low density of volumetric energy make hydrogen hard to present as a fuel. Accordingly, fluid hydrogen transporters (rather than H2 gas) may give a solution for these issues. The present hydrogen production carriers utilize non-renewable energy sources and gaseous petrol specifically, even though hydrogen carriers can be produced from biofuels, which can be produced and expended without modifying the carbon dioxide balance. Sustainable biofuels incorporate an entire range of biotechnology derivative fuels, including liquid H2 carriers like methanol, ethanol, ammonia, and formic acid. Subsequently, it very well may be said securely that methanol might be the choice of fuel for the application of vehicles fuel cell [3]. Be that as it may, the primary disadvantage with hydrogen carrier is the cost of synthesizing it from either renewable energy or fossil fuel sources transforming it to hydrogen. The biofuels, like hydrogen, are additionally fit for conveying power straightforwardly in a polymer of electrolyte membrane fuel cell without the requirement for reforming. The utilization of methanol straightforwardly in fuel cells was first researched in 1960 after the invention of the proton-conducting membrane [4]. This direct methanol fuel cell rearranges equipment and reaction qualities and has gigantic potential for versatile applications. It is likewise being considered for use in compact gadgets with long charging batteries time. Further, failure to display moving parts increases reliability and reduces maintenance cost. In view of the above factor, no noise emission is required in the operation of the fuel cells [4]. Fuel cells are considered as a transition strategy that converts electricity into chemical resources. The fuel cells comprise phosphoric acid fuel cell (PAFC), alkaline fuel cell (AFC), direct methanol fuel cell (DMFC) and polymer electrolyte fuel cell (PEFC) at low temperatures: the electrolyte cells are periodically regulated by form and temperature. Instead of gaseous hydrogen in the PEFC, the DMFC differs between PEFC and the reactant, which is a liquid water and CH3OH combination. The fuel cells are classified into high-temperature molten carbonate fuel cell (MCFC) and solid oxide fuel cell (SOFC) [5-7]. The use of a wide range of ordinary fuel, such as natural gas, hydrogen and alcohol can be adaptable to fuel cells. When pure hydrogen is used directly as a fuel, only water is produced, and no contamination occurs. The DMFC generates CO2 additionally. However, owing to higher performance, the volumes of CO2 are ultimately smaller in contrast with ignition engines. The fuel cell capabilities of the Carnot cycle are not limited, and fuel cells are obviously more efficient than the conventional burning motors.
Practically, the fuel cell efficiency is still electrically safe. For example, great efficiency is required in cars even a long way from peak capacity, and this makes fuel pipes truly suitable. Fuel cells also can co-generate electricity and heat in view of exothermic chemical and electrochemical reactions. Fuel cell devices, from one watt to megawatts, can also be scaled here and there efficiently. The fuel cells are also used as a power source for portable electronic devices on one side and as large power stations on the other side. The shortage of moving parts increases efficiency and reduces repair costs by cuts in servicing. The activity of fuel cells is quiet, and no noise emission is detected due to the reasons described above. The infrastructure costs currently constitute the main obstacle to DMFC marketing [8,9]. Although most of the necessary technologies have already been launched, several questions have been made regarding the use of fuel cells such as alternative storage and its cell of fuel, heat treatment and the optimization of each of the reactant’s transport and infrastructure. Performance of the direct methanol fuel cell depends on the catalyst (oxidation reaction at anode electrode, and reduction reaction at cathode electrode), electrolyte (proton exchange membrane) and electrode design/ high-current-density operation. However, it has been observed that catalyst plays an important role. Several studies are available on platinum (Pt) as a catalyst, but it is very costly [10-12]. JIANG et al [13] have tested the Pt-catalysts for methanol oxidation in methanol fuel cells with biomass. The strong catalytic activity of the anodic was recently noticed because of intrinsic activity of Cu for this reaction, which was prepared by FAITH et al [14] as 3D copper nanodomes as anodium substrate for direct fuel cells. SONG et al [15] used Pd/Co2O3 composites for catalytic oxidation of methanol in DMFC. The catalytic activity was compared and found that oxidation with Co2O3 alone was lower than the Pd/Co2O3 composites. NAZAL et al [16] studied the precious metal-free Ni/Cu/Mo supported multi-walled carbon nanotubes as the methanol oxidation catalysts and found that catalysts containing Ni showed higher rate while copper showed a lower rate of catalytic turnover. These researches emphasize that nonprecious metals can also be alternative to the precious metals, e.g., gold and platinum as the methanol oxidation catalyst. DURAIRAJ and VAITHILINGAM [17] worked towards the development of methanol oxidation catalyst using zeolite-based nickel deposited conducting polymer/CuS catalyst. They concluded that the prepared catalyst is a cheap, potential and non-precious metal electrode used in DMFC. PATTANAYAK et al [18] reported the non-metallic (Cu2O/Ppy-GO) catalytic behavior as an anode catalyst for DMFC oxidation. The copper-based catalytic converter is superior to Pt-Ru/C in terms of DMFC efficiency. Hence, it is desirable that copper (Cu) which is much cheaper than platinum (Pt) may be tried to see its performance. Despite the great industrial importance of DMFC, no systematic work seems to be available in the literature using Cu catalyst anode.
2 Experimental
2.1 Chemicals
All the reagents used to carry out the experiments were of analytical reagent grade and used without any purification. Cupric nitrate trihydrate (Cu(NO3)2·3H2O) (purity of 95.10%) was obtained from Fisher Scientific, India. Sodium borohydride (NaBH4) (purity 96.00%) was obtained from Thomas Baker, India. Sodium hydroxide (NaOH) and sulphuric acid (H2SO4) (purity of 97.00%) were obtained from Rankem, India. Polyvinylchloride (PVC) (relative molecular mass of 48000) and tetrahydrofuran (THF) (purity of 99.5%) were obtained from Otto Kemi, India and Fisher Scientific, India, respectively. Methanol (CH3OH) (purity of 99.00%) was obtained from Sigma Aldrich, USA.
2.2 Preparation of reagent solutions and synthesis of Cu nanoparticles
0.2 mol/L cupric nitrate (Cu(NO3)2·3H2O) and 0.05 mol/L sodium borohydride (NaBH4) solutions were prepared in demineralized water (DMW). Copper nanoparticles were prepared by sodium borohydride (NaBH4) reduction method [19]. According to this method, sodium borohydride (NaBH4) solution was added dropwise into cupric nitrate (Cu(NO3)2·3H2O) solution with continuous magnetic stirring at a temperature of 40 °C and pH of the solution was adjusted to 12 by adding H2SO4 or NaOH solutions. The formation of copper nanoparticles was confirmed by the change in colour of the solution from blue to brown. The chemical reaction for the reduction of copper nanoparticles by using sodium borohydride solution (NaBH4) solution is given by
4Cu2++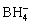 +8OH-→4Cu+
+8OH-→4Cu+ +4H2O (1)
+4H2O (1)
The precipitates of copper nanoparticles so- formed are allowed to settle down at room temptation (25±2) °C for 24 h. The liquid was removed, and the receptacle was washed in an electric oven several times with demineralized water to remove excess reagents and dried at 45° C.
2.3 Preparation of PVC embedded with copper nano-particles for membrane electrode
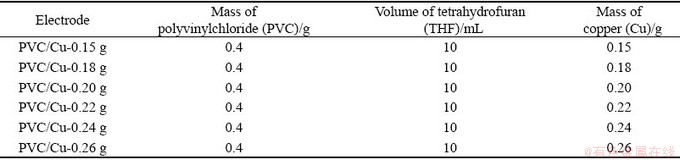
For the fabrication of catalyst membrane electrode, 0.4 g of PVC was slowly dissolved in 10 mL of tetrahydrofuran (THF) followed by constant stirring for 4 h using a magnetic stirrer at room temperature of (25±3) °C. Various quantities of copper (Cu) were added into PVC solution and mixed thoroughly using a magnetic stirrer. For the different electrodes of PVC/Cu, different amounts of polyvinylchloride (PVC), tetrahydrofuran (THF) and copper (Cu) were added, as indicated in Table 1.
Table 1 Conditions for preparation of different electrodes of PVC/Cu
Finally, the solution was cast in a borosil petri dish (diameter of 5.588 cm). The membrane casting disc was covered by using Whatman filter paper for the slow evaporation of the solvent and left to dry at room temperature up to 24 h. Six sheets of master membranes of different thicknesses were received. Then, the membrane was removed from the disc and cut into 3 cm2 (3 cm in length and 1 cm in width) electrode. The computer-controlled potentiostat/galvanostat (302N Autolab Switzerland) was used for all studies in electrochemical measuring. Experiments were performed in a single three-electrode system compartment cell. The working electrode was PVC/Cu, the platinum wire serves as an electromagnetic electrode and the Ag/AgCl worked as a referring electrode. Before performing all experiment, the working electrode was dipped in de-mineralized water (DMW) for electron mobility.
3 Results and discussion
3.1 Potentiostat/galvanostat
All electrochemical measurements were carried out using the potentiostat/galvanostat conducted with computer control (302N Autolab, Switzerland). The electrodes were cleaned until they were put in traditional cell, utilizing sterile water and ultrasonic cleaning (Elmasonic, Germany).
3.2 Cyclic voltammetry
The voltammograms were recorded in 1 mol/L NaOH solution for the different quantities of copper electrode (PVC/Cu) varying from 0.15 to 0.26 g Cu, as shown in Fig. 1. In these experiments, the potential range has been fixed between -1 to +1 V at scan rate of 100 mV/s. From the characteristics of the plot, it can be seen that all electrodes (B, C, D and E) show the redox behavior but the intensity of the oxidation-reduction peaks increases with an increase in the quantity of copper in the electrode. This may be due to the increase in the active surface area of the electrodes.
On examination, maximum redox current was observed at 0.24 g of the copper electrode, as exhibited by the Electrode E (Fig. 1), which may have got saturated. Further, with the increase or decrease in copper quantity, i.e. greater than 0.24 g or less than 0.18 g, the oxidation-reduction peaks disappeared, as exhibited by the Electrodes F and A in Fig. 1, respectively. The behavior of Electrode A (when the copper quantity was less than 0.18 g) may be explained by the fact that the copper quantity was not sufficient to enhance the rate of oxidation [20]. The characteristic of the Electrode F (when the copper quantity was more than 0.24 g) can be explained by the fact that the electrode may saturate at 0.24 g. Figure 2 exhibits the behaviour of Electrodes A, B, C, D, E and F when 0.5 mol/L methanol has been added into 1 mol/L NaOH in the cell. On observation, there are no oxidation peaks on all the curves. This means that irreversible reaction takes place as followed by SOFIA et al [20]. This may be due to the electrochemical formation of Cu(III) species, as observed by SOFIA et al [20]. The anodized current also increased with the increase in the quantity of copper electrode, as seen in Electrodes B, C , D and E.
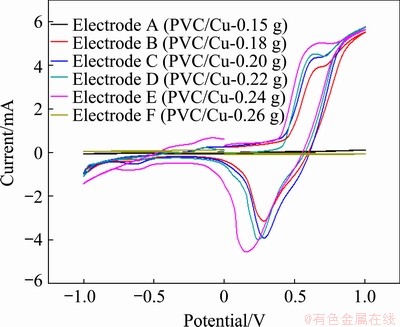
Fig. 1 Cyclic voltammetry in 1 mol/L NaOH solution with different quantities of copper electrodes
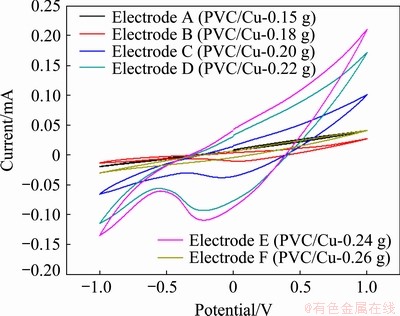
Fig. 2 Cyclic voltammetry in 0.5 mol/L methanol + 1 mol/L NaOH solution with different quantities of copper electrodes
In a catalytically enhanced reaction, the increase in catalyst quantity should induce a more significant number of active sits for improving the response of the global reaction, as indicated by Electrodes B, C, D and E. Additionally, it is observed that an increase or decrease in copper quantity, i.e., greater than 0.24 g or less than 0.18 g, results in the disappearance of reduction peaks, as exhibited by Electrodes F and A in Fig. 2 respectively. The behavior of Electrode A (when the copper quantity was less than 0.18 g) may be well explained by the fact that the copper quantity was not enough to enhance the rate of methanol oxidation. The characteristic of Electrode F (when the copper quantity was more than 0.24 g) can be related to the fact that it is quite likely that the undesired products (COHads, HCOads, HCOOads) inhibit the production of CO2 and H- ion and also poison the catalyst or may be due to saturation condition. Therefore, the electrodes which have the copper quantity range from 0.18 to 0.24 g were selected for further study.
Figure 3 represents the influence of the increase of the methanol concentration (i.e., from 0.5 mol/L to 1 mol/L) in the electrochemical cell. By comparing the anodic peaks in Figs. 2 and 3, it is observed that for the same quantity of copper (0.24 g) in electrode, anodic current in 1 mol/L methanol + 1 mol/L NaOH was less than that in 0.5 mol/L methanol + 1 mol/L NaOH. This result may be due to the higher methanol crossing over and this cross-reacts directly with oxygen at the cathode and reduces the response of the global reaction. Keeping in view the analysis carried on the data in Figs. 2 and 3, it is decided that the further study will be carried out for copper quantity in electrode ranging from 0.18 to 0.24 g and a solution having 0.5 mol/L methanol in 1 mol/L NaOH.
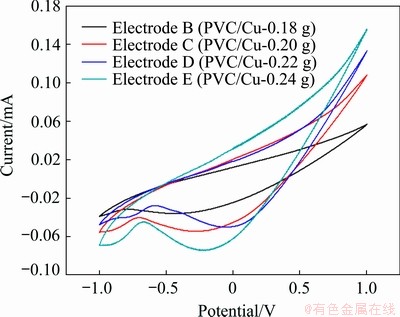
Fig. 3 Cyclic voltammetry in 1 mol/L methanol +1 mol/L NaOH solution with different quantities of copper electrodes
3.3 Linear sweep voltammetry
Linear sweeping voltammetry is a method of voltammetry in which the current is measured in a working electrode. The potential of the operating electrode to the reference electrode is, therefore, traced linearly over time. The voltage was set between 0 and 1 V in these tests (Fig. 4), with the electrode being positioned at 0.5 mol/L methanol + 1 mol/L NaOH solution at a time. It was observed that the catalytic current was higher when the copper catalyst quantity was higher in the electrode. This is due to the fact that the active surface area increases with the increase of copper catalyst amount in the electrode.
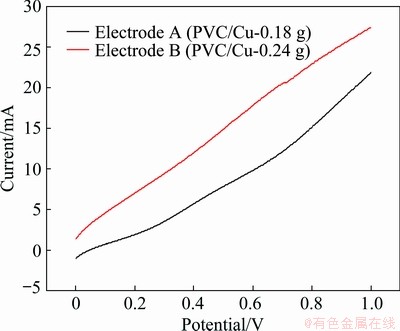
Fig. 4 Liner sweep voltammetry in 0.5 mol/L methanol + 1 mol/L NaOH solution
3.4 Chronoamperometry
Both electrodes were tested with chronoamperometry for their success in the methanol oxidation reaction after long activity. Figure 5 shows the chronoamperograms of 0.5 mol/L methanol + 1 mol/L NaOH solution for approximately 3000 s of PVC/Cu-0.18 g and PVC/Cu-0.24 g electrodes. After 2200 s activity, the steady current intensity was found, which is expected to be attributed to the saturation kinetics of the electrode. It can also be noted that PVC/Cu-0.24 g electrode maintained the highest current density up to 3000 s.
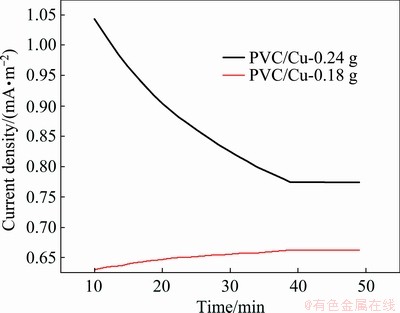
Fig. 5 Chronoamperometric responses in 0.5 mol/L methanol + 1 mol/L NaOH solution
4 Characterizations
4.1 Thermogravimetric analysis (TGA)
The thermo-gravimetric curves of PVC/Cu for two electrodes are shown in Figs. 6 and 7. It is observed that the increase in the copper quantity in the electrode increases the thermal stability of the electrode. Figures 6 and 7 show that both electrodes have undergone thermal degrade in three stages, and the condensation of the water molecule adsorbed on the material surface causes the first stage mass loss of the electrode to 200 °C. The second degradation step was within the temperature range of 200-300 °C, which can be explained by emission of chloride hydrogen as well as electrode degradation [21]. In the temperature range from 310 to 600 °C, the third deterioration was attributed to the chain width of the carbon separating backbone and the oxidation of the instability char [21]. Further, a horizontal section represents the oxide formation on both curves.
4.2 X-ray diffraction (XRD)
XRD measurements were performed on Lab X XRD-6100 diffractometer and the radiation in the range of 2θ=20°-80° was used.
Figure 8 shows the XRD patterns of copper nanoparticles synthesized by the chemical reduction method. Figure 8 shows two peaks of 2θ values around 43.5o and 74o which are assigned to (111) and (220) indices of face center cubic (FCC) lattice of metallic copper. SUBRAMAN et al [22] stated that the crystal designed coppers (111) and (110) were used to boost the catalytic activity of methanol oxidation reaction relative to the crystal plane copper (100). The anisotropic laminated structure, as can be seen in Fig. 8, enables a totally open, active (111) and (220) crystal plane for a catalytic action to oxidize methanol. At 2θ around 42.6°, 62.6° and 36.4°, peaks of cupreous oxide were also observed, which may be due to the air oxidation of copper during drying [23].
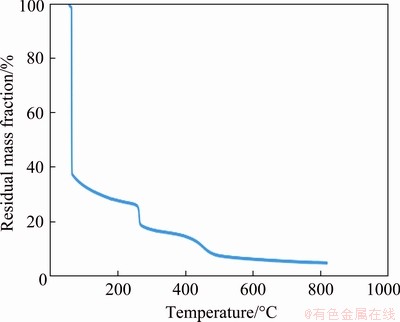
Fig. 6 Thermogravimetric curve for PVC/Cu-0.18 g electrode
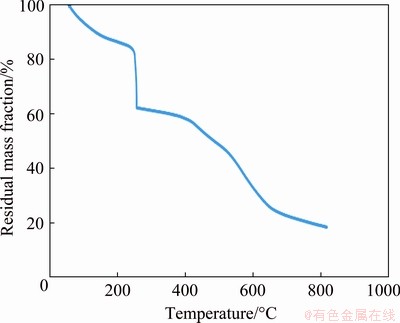
Fig. 7 Thermogravimetric curve for PVC/Cu-0.24 g electrode
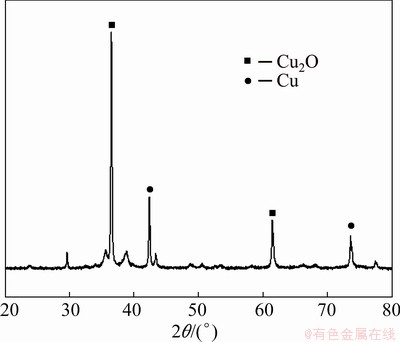
Fig. 8 XRD pattern for copper nanoparticles
If Figs. 9 and 10 are compared, in Fig. 10, copper and cuprous oxide are uniformly dispersed in the polyvinylchloride membrane. This helps to enhance the activity of oxidation reaction.
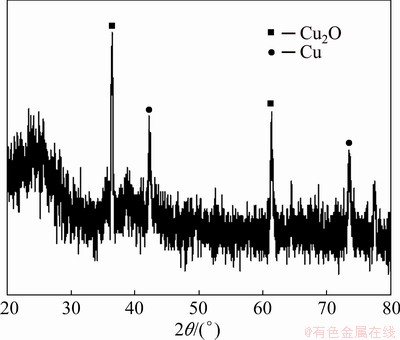
Fig. 9 XRD pattern of PVC/Cu-0.18 g electrode
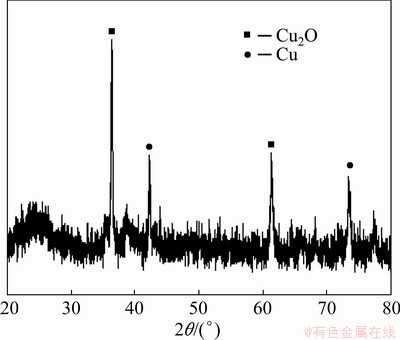
Fig. 10 XRD pattern of PVC/Cu-0.24 g electrode
4.3 Fourier transform infrared spectroscopy (FTIR)
FTIR was performed in the wavenumber range from 3500 to 400 cm-1 using Perkin Elmer spectrum Version 10.4.00 equipment.
To understand the interaction between PVC and Cu, the FTIR spectra were recorded. As seen in Figs. 11 and 12 for FIIR spectra of PVC/Cu-0.18 g and PVC/Cu-0.24 g electrodes, the characteristic peak occurring at wavenumber of 3434.12 cm-1 is due to the water molecules. The peak in wavenumber range between 2924.80 and 934.88 cm-1 is due to C—H group and that in wavenumber range between 1045 to 467.5 cm-1 is due to copper nanoparticle and its oxide [24]. The high and wide peak with a center at 3440 cm–1 corresponds to a H—OH bending at 1598 cm–1. The peak of 615 cm–1 is due to the Cu—O vibration in the Cu2O [25].
The peaks present by PVC are C—H vibration at 2924.80 cm-1, CH2 mode at 1322.4 cm-1, and C—H mode at 1165.5 cm-1, as shown in Figs. 11 and 12.
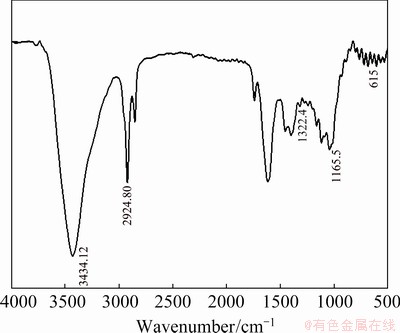
Fig. 11 FTIR spectrum of PVC/Cu-0.18 g electrode
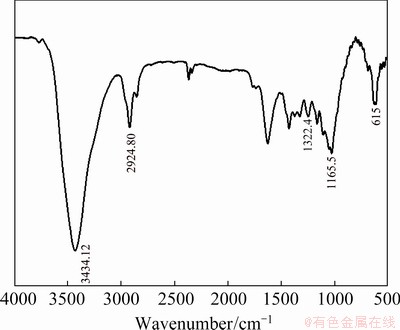
Fig. 12 FTIR spectrum of PVC/Cu-0.24 g electrode
4.4 Scanning electron microscopy (SEM) with EDAX
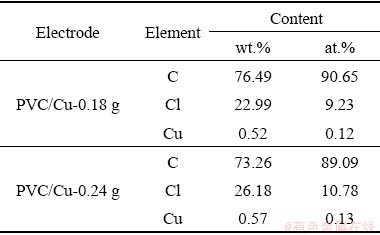
SEM images of electrodes for PVC/Cu-0.18 g and PVC/Cu-0.24 g electrodes are shown in Figs. 13(a, b) and 14(a, b), respectively. Both electrodes achieved different morphologies for different quantity of electrode. Figures 13 and 14 show that particles of the copper are appropriately embedded in the polyvinylchloride matrix. Figures 13(c) and 14(c) illustrate the EDAX analysis of the electrodes, which is used to analyze the elemental composition of the electrodes. The compositions of the electrodes are present in Table 2.
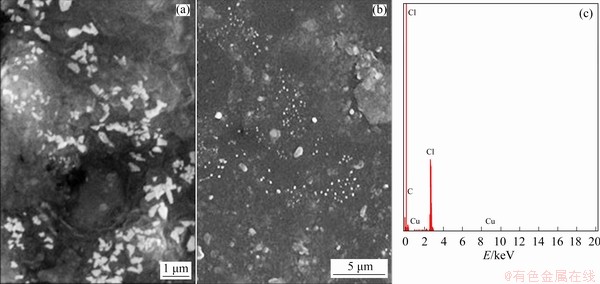
Fig. 13 SEM images (a, b) and EDAX spectrum (c) of PVC/Cu-0.18 g electrode
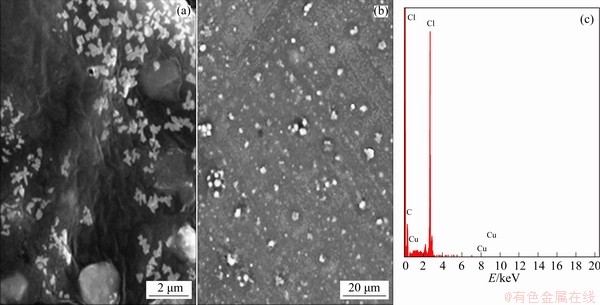
Fig. 14 SEM images (a, b) and EDAX spectrum (c) of PVC/Cu-0.24 g electrode
Table 2 Elemental compositions of electrodes
5 Conclusions
Copper nanoparticles were prepared by chemical reduction method. These copper particles were embedded into the polyvinylchloride (PVC) matrix and used as an electrode (PVC/Cu) for the oxidation of methanol fuel for improving the current response. Catalyst size was found in the nano range. The results show that the catalytic activity of the electrode is due to the presence of copper particles. However, the presence of a little amount of copper oxide decreases the catalytic activity of the electrode. So, it is advisable that a method of the preparation of catalyst should be developed in future, which gives a little or no amount of copper oxide. SEM with EDAX shows that the catalyst particles are embedded adequately in the PVC matrices. TGA indicates that electrodes are thermally stable at reaction temperature but the one having a higher quantity of copper is more stable. The electrode shows an increase in the quantity of catalyst (i.e., from 0.18 to 0.24 g Cu) with an increase in the current response, where the maximum current is obtained at 0.24 g catalyst electrode in the solution of 0.5 mol/L methanol + 1 mol/L NaOH.
References
[1] LINDSTROM B, PETTERSSON L J. Hydrogen generation by steam reforming of methanol over copper-based catalysts for fuel cell applications [J]. International Journal of Hydrogen Energy, 2001, 26: 923-933.
[2] KASPER T M, TORBEN R J, ETSUO A, HAIWEN L. Hydrogen—A sustainable energy carrier [J]. Progress in Natural Science: Materials International, 2017, 1: 34-40.
[3] IAIN S, DANIEL S, ANTHONY V A, PAUL B, PAUL E D, PAUL E, NILAY S, KATE R W. The role of hydrogen and fuel cells in the global energy system [J]. Energy and Environmental Science, 2019, 12: 463-491.
[4] SELAND F. Electrochemical oxidation of methanol and formic acid in fuel cell processes [D]. NTNU,2005: 204.
[5] WEBE A, IVERS T E. Materials and concepts for solid oxide fuel cells (SOFCs) in stationary and mobile applications [J]. Journal of Power Sources, 2004, 127: 273-283.
[6] GIDDEY S, BADWAL S P S, KULKARNI A, MUNNINGS C. A comprehensive review of direct carbon fuel cell technology [J]. Progress in Energy Combustion Science, 2012, 38: 360-399.
[7] BADWAL S P S, GIDDEY S, MUNNINGS C, KULKARNI A. Review of progress in high temperature solid oxide fuel cells [J]. Journal of the Australian Ceramic Society, 2014, 50: 23-37.
[8] ZHANG X. Preparation and characterization of proton exchange membranes for direct methanol fuel cells [D]. Department of Chemical Engineering, Universitat Rovira i Virgili (URV), 2005.
[9] DEBE M K. Electrocatalyst approaches and challenges for automotive fuel cells [J]. Nature, 2012, 486, 7401: 43-51.
[10] SYED J Z, MUKHTAR B, AMIR A A, AMMAR B Y, IMRAN M. Mesoporous carbon supported Pt/MO2(M=Ce, Pr, Nd, Sm) heteronanostructure: Promising non-Ru methanol oxidation reaction catalysts for direct methanol fuel cell application [J]. Journal of Electroanalytical Chemistry, 2017, 794: 86-92.
[11] MEIXIA, XIN W, LEI Z, ALI A, IKWHANG C, CHONG Q, YANGCHENG J, JIANHUANG Z, FAISAL A. Cu@Ptcatalysts prepared by galvanic replacement of polyhedral copper nanoparticles for polymer electrolyte membrane fuel cells [J]. Electrochimica Acta, 2019, 306: 167-174.
[12] YUAN W, TANG Y, YANG X, LIU B, WAN Z. Manufacture, characterization and application of porous metal-fiber sintered felt used as mass-transfer controlling medium for direct methanol fuel cells [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 2085-2093.
[13] JIANG Q L, PENG Z D, XIE X F, DU K, HU G R, LIU Y X. Preparation of high active Pt/C cathode electrocatalyst for direct methanol fuel cell by citrate-stabilized method [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 127-132.
[14] FATIH B, HANDAN Y, RAMAZAN S. Fabrication of three-dimensional copper nanodomes as anode materials for direct methanol fuel cells [J]. International Journal of Hydrogen Energy, 2019, 44: 14235-14242.
[15] SONG Y, DUAN D, SHI W, WANG H, SUN Z. Facile synthesis of 3D nanoporous Pd/Co2O3composites with enhanced catalytic performance for methanol oxidation [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 676-686.
[16] NAZAL M K, OLAKUNLE O S, AHMED A, MERZOUGUI B, ABUALKIBASH A, SULTAN A, YOUSAF A B, ZAIDI S J. Precious metal free Ni/Cu/Mo trimetallic nanocomposite supported on multi-walled carbon nanotubes as highly efficient and durable anode-catalyst for alkaline direct methanol fuel cells [J]. Journal of Electroanalytical Chemistry, 2018, 823: 98-105.
[17] DURAIRAJ S, VAITHILINGAM S. Hydrothermal assisted synthesis of zeolite based nickel deposited poly(pyrrole-co- fluoro aniline)/CuS catalyst for methanol and sulphur fuel cell applications [J]. Journal of Electroanalytical Chemistry, 2017, 787: 55-65.
[18] PATTANAYAK P, NILKAMAL P, KUMAR P, KUNDU P P. Fabrication of cost-effective non-noble metal supported on conducting polymer composite such as copper/polypyrrole graphene oxide (Cu2O/PPy-GO) as an anode catalyst for methanol oxidation in DMFC [J]. International Journal of Hydrogen Energy, 2018, 43: 11505-11519.
[19] LIU Qing-ming, ZHOU De-bi, YAMAMOTO Y, ICHINO R, OKIDO M. Preparation of Cu nanoparticles with NaBH4 by aqueous reduction method [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 117-123.
[20] SOFIA C, CHASSAING E, ROSSO M, GONZALEZ G A. Enhanced electrochemical oxidation of methanol on copper electrodes modified by electrocorrosion and electrodeposition [J]. Materials Chemistry and Physics, 2014, 143: 1012-1017.
[21] XU Jian-zhong, LIU Cai-hong, QU Hong-qiang, MA Hai-yun, JIAO Yun-hong, XIE Ji-xing. Investigation on the thermal degradation of flexible poly(vinyl chloride) filled with ferrites as flame retardant and smoke suppressant using TGA-FTIR and TGA-MS [J]. Polymer Degradation and Stability, 2013, 98: 1506-1514.
[22] VENKATASUBRAMANIAN R, HE Ji-bao, JOHNSON M W, STERN I, KIM Dae-ho, PESIKA N S. Additive-mediated electrochemical synthesis of platelike copper crystals for methanol electrooxidation [J]. Langmuir, 2013, 29: 13135-13139.
[23] XIA Lun-peng, GUO Peng, WANG Yan, DING Shi-qi, HE Jian-bo. Multi-laminated copper nanoparticles deposited on conductive substrates for electrocatalytic oxidation of methanol in alkaline electrolytes [J]. Journal of Power Sources, 2014, 262: 232-238.
[24] SURESH, Y, ANNAPURNA S, SINGH A K, BHIKSHAMAIAH G. Green synthesis and characterization of tea decoction stabilized copper nanoparticles [J]. Int J Innov Res Sci Eng Technol, 2014, 3: 11265-11270.
[25] SWARNKAR R K, SINGH S C, GOPAL R. Effect of aging on copper nanoparticles synthesized by pulsed laser ablation in water: structural and optical characterizations [J]. Bulletin of Materials Science, 2011, 34: 1363-1369.
直接甲醇燃料电池用含纳米铜聚氯乙烯电化学氧化膜的制备与表征
Rohitash KUMAR1, Aftab Aslam Parwaz KHAN2, Anish KHAN2, Abdullah M ASIRI2, Yusra HAMID3
1. Department of Petroleum Studies, Aligarh Muslim University, Aligarh 202002, India;
2. Center of Excellence for Advanced Materials Research and Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, P. O. Box 80203, Saudi Arabia;
3. Department of Industrial Chemistry and Chemical Engineering, Politecnico di Milano, Milano 20133, Italy
摘 要:采用化学还原法制备纳米铜颗粒,然后将其嵌入聚氯乙烯(PVC)基体中得到PVC/Cu膜,作为电极材料以提高甲醇燃料氧化的电流响应。用热重分析(TGA)表征 PVC/Cu电极的热稳定性,用X射线衍射(XRD)检测电极中的铜纳米颗粒,用傅里叶变换红外光谱(FTIR)鉴定PVC与铜之间的相互反应,用扫描电镜(SEM)和能谱 (EDAX)分析该电极的显微形貌,并用循环伏安法、线性扫描伏安法和计时电流法表征该电极的电催化活性。结果表明,随着铜含量从0.18 g(PVC/Cu-0.18 g)增加到0.24 g(PVC/Cu-0.24 g),电极的活性增加,且在铜含量为0.24 g时达到最大值。电极在0.5 mol/L CH3OH + 1 mol/L NaOH溶液中达到最大催化电流。FTIR结果显示,电极中含有水分子、C—H 基团、铜颗粒和铜的氧化物。SEM和EDAX结果表明,铜颗粒在聚氯乙烯基体中嵌入良好。
关键词:铜纳米颗粒;聚氯乙烯;直接甲醇燃料电池;电化学方法
(Edited by Bing YANG)
Corresponding author: Aftab Aslam Parwaz KHAN; E-mail: draapk@gmail.com
DOI: 10.1016/S1003-6326(20)65373-0
Abstract: Copper nanoparticles were prepared by the chemical reduction method. These copper particles were embedded into the polyvinylchloride (PVC) matrix as support and used as an electrode (PVC/Cu) for the oxidation of methanol fuel for improving the current response. The PVC/Cu electrodes were characterized by thermal gravimetric analysis (TGA) for thermal stability of the electrode, X-ray diffraction (XRD) for identification of copper nanoparticles in the electrode, Fourier transform infrared spectroscopy (FTIR) to identify the interaction between PVC and Cu and scan electron microscopy (SEM) with EDAX for the morphology of the electrode. The electrocatalytic activity of the electrode was characterized by the cyclic voltammetry, linear sweep voltammetry, and chronoamperometry techniques. An increase in the electrode activity was observed with the increase of copper quantity from 0.18 g (PVC/Cu-0.18 g) to 0.24 g (PVC/Cu-0.24 g) and the maximum was found at 0.24 g of copper in the electrode. Also, it was observed that the electrode achieved the maximum catalytic current in 0.5 mol/L CH3OH + 1 mol/L NaOH solution. FTIR identified that water molecules, C—H group, copper nanoparticle and its oxide were available in the electrode. SEM images with EDAX showed that copper particles were properly embedded in the polyvinylchloride matrix.


