- Abstract:
- 1 Introduction▲
- 2 Experimental ▲
- 3 Results and discussion▲
- 3.1 Effect of pH on extraction ratio of zinc
- 3.2 Effect of volume fraction of extractant D2EHPA on extraction ratio of zinc
- 3.3 Effect of phase ratio on extraction ratio of zinc
- 3.4 Effect of phase contact time on extraction ratio of zinc
- 3.5 Effect of stirring speed on extraction ratio of zinc
- 3.6 Effect of concentration of metal ions on extraction ratio of zinc
- 3.7 Effect of inorganic agents in aqueous phase on extraction ratio of zinc
- 3.8 Effect of saponifiers on extraction ratio of zinc
- 3.9 Effect of stripping speed on extraction ratio of zinc
- 4 Conclusions▲
- References
- Figure
- Fig.1 Schematic diagram of constant interfacial area cell: 1—Constant temperature water bath; 2—Constant interfacial area cell; 3—Thermometer; 4—Stirring paddle; 5—Sample; 6—Organic phase; 7—Aqueous phase
- Fig.2 Effect of pH on extraction ratio of zinc
- Fig.3 Effect of volume fraction of extractant D2EHPA on extraction ratio of zinc
- Fig.4 Effect of phase ratio (Vo/Va) on extraction ratio of zinc
- Fig.5 Effect of phase contact time on extraction ratio of zinc
- Fig.6 Effect of stirring speed on extraction ratio of zinc
- Fig.7 Effect of concentration of ferric ion on extraction ratio of zinc at different zinc ion concentrations
- Fig.8 Effect of concentration of organic agent in aqueous phase on extraction ratio of zinc
- Fig.9 Effect of saponification concentrations of Na2CO3 and NaOH on extraction ratio of zinc
J. Cent. South Univ. Technol. (2010) 17: 760-764
DOI: 10.1007/s11771-010-0553-x![]()
Solvent extraction of zinc from zinc sulfate solution
LONG Huai-zhong(龙怀中)1, CHAI Li-yuan(柴立元)1, QIN Wen-qing(覃文庆)2, TANG Shuang-hua(唐双华)2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China
Abstract:
The extraction of zinc from zinc sulfate solution was investigated, using 20% saponified D2EHPA as an extractant and 260# sulfonate kerosene as a diluent. The solution was stirred for 8 min at phase ratio (Vo/Va) of 1.0?1.0, initial pH of 2.0 and stirring speed of 200 r/min. The results show that 75% zinc can be extracted from the zinc sulfate solution when the concentration of zinc is 18.7 g/L after being settled for 10 min. 88.60% zinc can be stripped by 196 g/L sulfuric acid, and zinc ion can be separated from ferric ion.
Key words:
1 Introduction
At present, the purification of zinc hydrometallurgy lixivium is based on the chemical precipitation and replacement methods, which not only consume a large amount of chemical reagents, but also are difficult to achieve high degree of purification. Because of large difference between the leaching process of the zinc oxide ore and the traditional zinc calcining in the cycle of leaching solution and solution chemical conditions, the traditional purification process is not suitable for the disposal of zinc oxide ore leaching solution [1-2]. However, the extractive purification of zinc from zinc oxide ore leaching solution is a better selection [3-5].
The studies about solvent extraction of zinc from sulfate solution were mostly carried out on the background of removing trace impurity zinc [6-7]. Because of the lack of effective zinc extraction reagents and relatively low prices of zinc metal, the applications of solvent extraction of zinc from sulfate solution are limited [8-9]. Organic phosphorus extractants are widely used in the solvent extraction of zinc from zinc sulfate solution [10-13]. However, one disadvantage of this process is that ferric ions can be extracted simultaneously [14-15]. In this work, the solvent extraction of zinc from zinc sulfate solution was studied using bi-(2-ethylhexyl) phosphoric acid (D2EHPA) as an extractant and 260# sulfonate kerosene as a diluent.
2 Experimental
2.1 Materials
A low-grade zinc oxide ore was from the Mengzi Ore in Yunnan Province, China. The chemical composition of the leaching solution of zinc oxide ore is listed in Table 1. The concentrations of zinc ion and ferric ion in the leaching solution are 18 700.00 and 30.00 mg/L, respectively.
Table 1 Chemical composition of leaching solution of zinc oxide ore (mg/L)

In this experiment, the extractant was D2EHPA and the diluent was 260# sulfonate kerosene. Other reagents included H2SO4, NaOH, FeSO4?7H2O, (NH4)2SO4, KCl, K2HPO4, Fe2(SO4)3?7H2O, and ZnSO4? 6H2O.
2.2 Principle of extraction
Alkyl phosphate extractant D2EHPA often exists in dimer form in kerosene solution. Zinc can be extracted using D2EHPA (expressed as HR) by the following reactions:
![]()
![]()
![]() (1)
(1)
![]()
![]()
![]() (2)
(2)
where H2R2 is the dimer molecule of HR; and m is the total number of molecule of HR. This test was carried out in a constant interfacial area cell. The schematic diagram of the constant interfacial area cell is shown in Fig.1. The leaching liquor was added in the constant interfacial area cell, and the concentrations of zinc ion and ferric ion were 18 700.00 and 30.00 mg/L, respectively. Then, the organic phase was slowly added to the aqueous phase along the inner wall, and the mixer was immediately turned on to control the stirring speed. After stirring for a given period the aqueous phase and organic phase were poured into a separating funnel and settled for 10 min. Finally, the aqueous phase was analyzed.

Fig.1 Schematic diagram of constant interfacial area cell: 1—Constant temperature water bath; 2—Constant interfacial area cell; 3—Thermometer; 4—Stirring paddle; 5—Sample; 6—Organic phase; 7—Aqueous phase
3 Results and discussion
3.1 Effect of pH on extraction ratio of zinc
H+ ions are released in the extraction process using acidic extractant D2EHPA, so the extraction process is greatly affected by pH. The effect of pH on the extraction ratio of zinc was investigated when being extracted for 8 min at volume fraction of extractant D2EHPA of 10%, phase ratio (Vo/Va) of 1.0?1.0, and stirring speed of 200 r/min. The results are shown in Fig.2.
It can be seen from Fig.2 that the extraction ratio of zinc increases with increasing pH. The extraction ratio of zinc is low because of the protonation of the extractant D2EHPA when pH is less than 2.0. The extraction ratio of zinc increases to 19.00% at pH of 2.0. When pH ranges from 2.0 to 3.5, the extraction ratio of zinc increases to 21.60%. Therefore, suitable pH of the extraction process is 2.0.
3.2 Effect of volume fraction of extractant D2EHPA on extraction ratio of zinc
The effect of the volume fraction of extractant D2EHPA on the extraction ratio of zinc was investigated under the conditions of pH of aqueous phase of 2.0, phase ratio (Vo/Va) of 1.0?1.0, stirring speed of 200 r/min and extraction time of 8 min. Fig.3 shows the effect of the volume fraction of extractant D2EHPA on the extraction ratio of zinc.
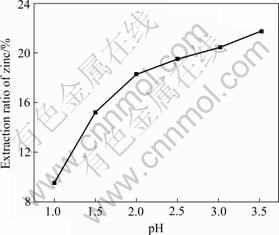
Fig.2 Effect of pH on extraction ratio of zinc

Fig.3 Effect of volume fraction of extractant D2EHPA on extraction ratio of zinc
It can be seen from Fig.3 that the extraction ratio of zinc increases with increasing volume fraction of extractant D2EHPA. The extraction ratio reaches 33.89% when the volume fraction of the extractant D2EHPA is 30%. The curve slope in Fig.3 is about 0.81 at the volume fraction of extractant D2EHPA less than 20%, while the curve slope decreases to 0.32 when the volume fraction of extractant D2EHPA increases to 30%. Generally, the greatest volume fraction of extractant D2EHPA should be less than 30%, otherwise the organic phase will become viscous, resulting in difficult phase separation and extractant D2EHPA waste. Therefore, the optimum volume fraction of extractant D2EHPA is 20%.
3.3 Effect of phase ratio on extraction ratio of zinc
Fig.4 shows the effect of phase ratio on the extraction ratio of zinc under the conditions of pH of aqueous phase of 2.0, volume fraction of extractant D2EHPA of 20%, phase contact time of 8 min, and stirring speed of 200 r/min.

Fig.4 Effect of phase ratio (Vo/Va) on extraction ratio of zinc
It can be seen from Fig.4 that the extraction ratio of zinc increases with increasing phase ratio. When the volume fraction of extractant D2EHPA is 20% and phase ratio Vo/Va is 1.0:1.0, the extraction ratio is 36.10%. With increasing phase ratio, the viscosity of organic phase is accelerated, which leads to difficult phase separation and even the appearance of the third phase.
3.4 Effect of phase contact time on extraction ratio of zinc
Fig.5 shows the curve of extraction ratio of zinc vs phase contact time under the conditions of equilibrium pH of aqueous phase of 2.0, volume fraction of extractant D2EHPA of 20%, phase ratio (Vo/Va) of 1.0?1.0, and stirring speed of 200 r/min.
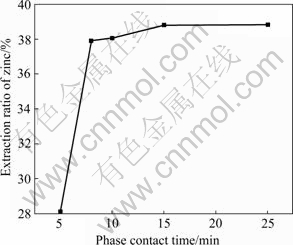
Fig.5 Effect of phase contact time on extraction ratio of zinc
It can be seen from Fig.5 that the extraction ratio of zinc is 37.88% when the phase contact time is 10 min. Afterwards, the extraction ratio of zinc increases slightly. So, the optimal phase contact time is 8 min.
3.5 Effect of stirring speed on extraction ratio of zinc
The effect of stirring speed on the extraction ratio of zinc was investigated under the conditions of equilibrium pH of aqueous phase of 2.0, volume fraction of extractant D2EHPA of 20%, and phase ratio (Vo/Va) of 1.0?1.0. The results are shown in Fig.6.

Fig.6 Effect of stirring speed on extraction ratio of zinc
It can be seen that the stirring speed has some impact on the reaction in the liquid-liquid extraction process, and the optimum value is 200 r/min.
3.6 Effect of concentration of metal ions on extraction ratio of zinc
The effect of the concentration of metal ions on the extraction ratio of zinc was investigated under the conditions of equilibrium pH of 2.0, volume fraction of extractant D2EHPA of 20%, phase ratio (Vo/Va) of 1.0?1.0, phase contact time of 8 min, and stirring speed of 200 r/min. The results are shown in Fig.7.
It can be seen from Fig.7 that the extraction ratio of zinc decreases with increasing concentration of zinc and ferric ions. The extraction of metal ion using organic phase D2EHPA as solvent is cation exchange reaction. The metal ions and H+ ion exchange according to Eqs.(1) and (2). Therefore, the number of H+ ions participating in exchange keeps constant when the concentration of extractant D2EHPA is invariable. So, zinc extracted from the solution is invariable because the number of H+ ions participating in exchange keeps constant with increasing concentration of metal ions. Therefore, the extraction ratio of zinc declines.

Fig.7 Effect of concentration of ferric ion on extraction ratio of zinc at different zinc ion concentrations
3.7 Effect of inorganic agents in aqueous phase on extraction ratio of zinc
The effect of components of aqueous phase on extraction ratio of zinc is shown in Fig.8. The extraction conditions are described as follows: equilibrium pH of aqueous phase of 2.0, volume fraction of extractant D2EHPA of 20%, phase ratio (Vo/Va) of 1.0?1.0, phase contact time of 8 min, and stirring speed of 200 r/min.

Fig.8 Effect of concentration of organic agent in aqueous phase on extraction ratio of zinc
The extraction ratio of zinc can be affected by the anions in aqueous phase. When using D2EHPA as an extractant, the common anions impacting on the extraction ratio could be arranged as: ![]() >
>![]() >Cl->
>Cl->![]() [13]. The investigation on the effects of KCl, KH2PO4 and (NH4)2SO4 on the extraction of zinc was carried out. The results show that the order of the three kinds of salt is KCl<KH2PO4<(NH4)2SO4 according to their impact on the extraction ratio of zinc. The extraction ratio of zinc decreases first and then increases with increasing Cl- concentration. KH2PO4 and (NH4)2SO4 accelerate the extraction of zinc because
[13]. The investigation on the effects of KCl, KH2PO4 and (NH4)2SO4 on the extraction of zinc was carried out. The results show that the order of the three kinds of salt is KCl<KH2PO4<(NH4)2SO4 according to their impact on the extraction ratio of zinc. The extraction ratio of zinc decreases first and then increases with increasing Cl- concentration. KH2PO4 and (NH4)2SO4 accelerate the extraction of zinc because ![]() and
and ![]() in the extraction system have a buffer action to pH of extraction. The equilibrium pH of extraction increases with increasing concentration of KH2PO4 or (NH4)2SO4, which is conducive to the extraction ratio of zinc.
in the extraction system have a buffer action to pH of extraction. The equilibrium pH of extraction increases with increasing concentration of KH2PO4 or (NH4)2SO4, which is conducive to the extraction ratio of zinc.
3.8 Effect of saponifiers on extraction ratio of zinc
Saponification is a process in which the metal ions replace H+ ions through acid-alkali neutralization reaction. Common saponifiers of D2EHPA are NaOH, NH4OH and Na2CO3, and the organic phase is the oil-water emulsion after saponification [14-15].
The effect of the organic phase saponified by different concentrations of NaOH and Na2CO3 on the extraction ratio of zinc was studied. The extraction conditions are as follows: concentration of zinc of 18.7 g/L, equilibrium pH of aqueous phase of 2.0, volume fraction of extractant D2EHPA of 20%, phase ratio (Vo/Va) of 1.0?1.0, phase contact time of 8 min, stirring speed 200 r/min, and settling time of 10 min. The results are shown in Fig.9.

Fig.9 Effect of saponification concentrations of Na2CO3 and NaOH on extraction ratio of zinc
It can be seen from Fig.9 that the extraction ability of extractant D2EHPA saponified is enhanced. And the extraction ratio of zinc reaches 75% when the extractant is saponified by Na2CO3. So, the extraction reaction is the exchange process of sodium ions with metal ions, which accelerates the extraction reaction in the alkaline environment.
3.9 Effect of stripping speed on extraction ratio of zinc
The stripping was carried out using sulfuric acid solution as an extractant at a certain concentration. In this experiment, the organic phase contains 20% D2EHPA- 260# oil solution, the concentration of zinc ion is 18.7 g/L and that of ferric ion is 30 mg/L in the organic phase, and the phase ratio is 1.0?1.0. After stirring for 8 min under the stirring speed of 200 r/min and then settling for 10 min, the sample was analyzed. The results are listed in Table 2.
It is indicated that zinc can be extracted absolutely by the sulfuric acid with high concentration. The stripping yield of zinc is 87.55% (mass fraction) when the concentration of sulfuric acid solution is 120 g/L. Ferric ion is not extracted, retaining in the organic phase even in the sulfuric acid with high concentration. When the concentration of sulfuric acid is 196 g/L (2 mol/L), 88.60% zinc ions and only 1.76% ferric ions are stripped. However, the stripping yield of zinc is reduced when the concentration of sulfuric acid is 200 g/L, which makes zinc dissolve into the organic phase again, forming an extracted species as [nH2SO4·nZnSO4·m(HR)2].
Table 2 Effect of sulfuric acid concentration on stripping yield of zinc

In fact, the stripping product of ferric ions is complicated and the hydrophobic molecules are produced in the extraction process. So, the stripping can take place at the interface. The stripping product at the interface with disorder reduces its effective concentration. And the inorganic anion and H+ ions of aqueous solutions are highly hydrated, resulting in the reduction of the effective concentration at the interface. Therefore, the stripping yield of ferric ions using inorganic acid as a reagent is very low. At high concentration and low pH, it is difficult for the stripping product of the ferric ion to contact with the inorganic acid in aqueous phase because of solvent function which inhibits the stripping of ferric ion. Therefore, zinc ion and ferric ion can be separated through selective stripping although the ferric ion is extracted into organic phase with zinc ion together.
4 Conclusions
(1) The extraction of zinc from sulfate solution is investigated using D2EHPA and 260# sulfonate kerosene as an extractant and a diluent, respectively. The extraction ratio of zinc increases with increasing pH, volume fraction of extractant D2EHPA and phase ratio. The extraction yield of zinc in one-stage is 37.88% under the condition of equilibrium pH of aqueous phase of 2.0, volume fraction of extractant D2EHPA of 20%, phase ratio of 1.0?1.0, phase contact time of 8 min, stirring speed 200 r/min, and settling time of 10 min.
(2) Under the same conditions the extraction ratio of zinc reaches 75% using saponified D2EHPA as an extractant. Zinc ion can be separated from ferric ion using 196 g/L sulfuric acid solution as stripping reagent under this condition, and 88.60% zinc ion but only 1.76% ferric ion can be stripped.
References
[1] LAN Xing-hua. Solvent extraction of multiple nonferrous metals in the South Africa(Ⅳ) [J]. World Nonferrous Metals, 2007(1): 20-21.
[2] ZHANG Xiao-juan, LI Xin-gang, CAO Hong-bin, ZHANG Yi. Separation of copper, iron (Ⅲ), zinc and nickel from nitrate solution by solvent extraction using LK-C2 [J]. Separation and Purification Technology, 2010, 70(3): 306-313.
[3] LIU Ge, ZHANG Jin-zhu. Recent advance for separation of zinc from cadmium [J]. Hydrometallurgy of China, 2005, 24: 221-225. (in Chinese)
[4] LAN Xing-hua. Recent advance for solvent extraction of zinc [J]. World Nonferrous Metals, 2008(8): 28-32.
[5] FU Weng, CHEN Qi-yuan, WU Qian, HU Hui-ping, BAI Lan. Solvent extraction of zinc from ammoniacal/ammonium chloride solutions by a sterically hindered β-liketone and its mixture with tri-n-octylphosphine oxide [J]. Hydrometallurgy, 2010, 100(3/4): 116-121.
[6] WANG Sheng-dong, JIANG Xun-xiong, JIANG Kai-xi, FAN Yan-qing. Application of P204 in hydrometallurgy of ocean cobalt-rich crust [J]. Nonferrous Metals, 2006, 58(1): 69-71.
[7] VAHIDI E, RASHCHI F, MORADKHANI D. Recovery of zinc from an industrial zinc leach residue by solvent extraction using D2EHPA [J]. Minerals Engineering, 2009, 22(2): 204-206.
[8] CHEN Hao, ZHU Yun, HU Han. Zinc extraction with LIX54 in Zn-NH3-H2O system [J]. Nonferrous Metals, 2003, 55(3): 50-52.
[9] MA Rong-jun. Research progress of solvent extraction in hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 1979: 227-230. (in Chinese)
[10] GAO Zi-li, SUN Si-xiu, SHEN Jing-lan. Solvent extraction chemistry [M]. Beijing: Science Press, 1991: 66-69. (in Chinese)
[11] MELLAH A, BENACHOUR D. The solvent extraction of zinc, cadmium and chromium from phosphoric acid solutions by tri-n butyl phosphate in kerosene diluent [J]. Separation and Purification Technology, 2007, 56(2): 220-224.
[12] GU H, CHANG C M. Preliminary design of a solvent extraction processing for the galvanic striping of iron from D2EHPA [J]. Mineral and Metallurgical Processing, 2000, 17(1): 16-22.
[13] YANG Da-jin, XIE Gang, WANG Ji-kun. Study on solvent extraction of zinc sulfate solution [J]. Nonferrous Metals: Extractive Metallurgy, 2006(2): 9-24.
[14] ZENG Ping, WEI Hong-mei. Studies on the properties of the extraction of vanadium(Ⅳ) by saponified P204 [J]. Journal of Xiangtan University: Natural Science, 1995, 17(4): 64-68. (in Chinese)
[15] QIN Wen-qing, LI Wei-zhong, LAN Zhuo-yue, QIU Guan-zhou. Simulated small-scale pilot plant heap leaching of low-grade oxide zinc ore with integrated selective extraction of zinc [J]. Minerals Engineering, 2007, 20(7): 694-700.
Foundation item: Project(50774094) supported by the National Natural Science Foundation of China
Received date: 2010-04-01; Accepted date: 2010-05-20
Corresponding author: LONG Huai-zhong, Doctoral candidate; Tel: +86-731-88876765; E-mail: ysxblong@mail.csu.edu.cn
(Edited by CHEN Wei-ping)
- Solvent extraction of zinc from zinc sulfate solution


