
Preparation of SiCp/Cu composites by Ti-activated pressureless infiltration
ZHANG Lin(章 林), QU Xuan-hui(曲选辉), DUAN Bo-hua(段柏华),
HE Xin-bo(何新波), QIN Ming-li(秦明礼), LU Xin(路 新)
State Key Laboratory for Advanced Metals and Materials, School of Materials Science and Engineering,
University of Science and Technology Beijing, Beijing 100083, China
Received 6 December 2007; accepted 28 April 2008
Abstract:
Sessile drop technique was used to investigate the influence of Ti on the wetting behaviour of copper alloy on SiC substrate. A low contact angle of 15? for Cu alloy on SiC substrate is obtained at the temperature of 1 100 ℃. The interfacial energy is lowered by the segregation of Ti and the formation of reaction product TiC, resulting in the significant enhancement of wettability. Ti is found to almost completely segregate to Cu/SiC interface. This agrees well with a coverage of 99.8%Ti at the Cu/SiC interface predicted from a simple model based on Gibbs adsorption isotherm. SiCp/Cu composites are produced by pressureless infiltration of copper alloy into Ti-activated SiC preform. The volume fraction of SiC reaches 57%. The densification achieves 97.5%. The bending strength varies from 150 MPa to 250 MPa and increases with decreasing particle size.
Key words:
SiC/Cu composites; metal matrix composites; pressureless infiltration; wettability; mechanical property;
1 Introduction
High volume fraction (55%-70%) SiCp/Cu composites have attracted much attention in recent years, which exhibit high thermal conductivity, low thermal expansion, excellent mechanical properties and improved wear resistance[1]. These make SiCp/Cu composites potential candidates for thermal management application and wear resistance application[2].
The main techniques to prepare SiCp/Cu composites with high SiC content are hot pressing using coated powder and liquid metal infiltration[3-4]. Pressureless infiltration is probably the most attractive route to fabricate SiCp/Cu composites. It has the ability to prepare complex shaped parts with high content of ceramic phase[5-6]. However, the realization of pressureless infiltration is very difficult in this system, owing to the poor wettability between SiC and Cu. RADO et al[7] observed that the contact angle is 137? for pure Cu on sintered α-SiC. Little investigation has been reported on pressureless infiltration of SiCp/Cu composites.
The addition of active elements is the most widely used method to enhance the wettability between ceramic and liquid metal. The active elements (Ti, Cr and Zr) are characterized by their high chemical affinity to oxygen, carbon or boron. In the metal/ceramic systems of Cu-Cr/C[8], Ag-Cu-Ti/AlN[9], Ag-Cu-Ti/SiC[10], Ni-Ti/ Al2O3[11], Cu-Ti/AlN[9], Cu-Ti/Y2O3[12], Cu-Ti/SiC [13], Cu-Ti/Al2O3[14], Cu-Cr/ZrO2[15], Cu-Cr/SiC[16] and Cu-Ti/B4C[17], the wettability can be significantly improved by the addition of active elements. The addition of Ti to Ag or Cu alloys involves the formation of Ti compounds (TiOx, TiC or TiN on oxides, carbides and nitrides, respectively)[11]. A segregated layer of Cr was observed at the Cu-Cr/ZrO2 substrate interface[15]. A small addition of Ti to Ag or CuAg alloys causes the decrease in the contact angle on Al2O3 from values much higher than 90? to 10?-20?[10]. The improvement in wetting by the addition of active elements can be explained by two reasons. On one hand, the continuous layers of newly formed compounds are more easily wetted by the metal[10]; On the other hand, the beneficial effect of active elements can also be related to the adsorption process. The active element accumulated at the solid/liquid interface lowers the interfacial energy and the contact angle[11].
In this work, SiCP/Cu composites are fabricated by Ti-activated pressureless infiltration. The effects of Ti on the wetting behaviour, interfacial energy and interfacial composition are studied. And their microstructure and mechanical properties are characterized.
2 Experimental
The wetting experiments were performed using the sessile drop technique. Copper alloy with the size of 4 mm×3 mm×1 mm was used. The SiC substrate with the size of 15 mm×12 mm×5 mm was polished and degreased. In order to simulate the influence of active element Ti on the wetting behaviour, a piece of Ti of about 0.1 mm thickness was placed between the copper alloy and the SiC substrate. The experiment was carried out in vacuum in the temperature range from 975 ℃ to 1 110 ℃. The contact angle and the drop base radius were measured by high temperature photography.
For the preform preparation, green and abrasive grade α-SiC particles with two kinds of particle size (63 and 24 μm) were used. The purity of active metal Ti was 98.5%. The binder was the solution of 10% (mass fraction) polyvinyl alcohol (PVA). Firstly, SiC powders were mixed with 1% binder and 10%-15%Ti (mass fraction). Secondly, the mixture was molded under the pressure of 100 MPa. Subsequently, the preforms were dried at 80 ℃ for several hours. The copper alloys used for infiltration were prepared using induction melting method, whose chemical compositions were Cu-3Si- 2Al-1Ti (alloy A) and Cu-5Al-2Fe-2Ni (alloy B). Pressureless infiltration was carried out in vacuum (1×10-3 Pa). A plate of copper alloy was placed on the top of the preform and the system was held isothermally for 60 min at 1 150 ℃.
The microstructure and fractural surfaces of composite were observed on a LEO1450 scanning electron microscope(SEM). XRD analysis was carried out on a Siemens D5000 X-ray diffractometer using Cu radiation. The bending strength was determined using three-point-bending tests with a span of 30 mm on an Instron 5569 universal testing system.
3 Results and discussion
3.1 Wetting behaviour
During infiltration process, Ti particles are distributed on the surface of SiC particle. The effect of Ti on the wetting behaviour can be simulated by the method, where a piece of Ti is placed between the copper piece and the SiC substrate in the sessile drop experiment. Fig.1 shows the variation of the contact angle (θ) and the droplet base radius (R) in the temperature range from 970 ℃ to 1 115 ℃. The contact angle decreases significantly from 75? to less than 20? in the temperature range from 970 ℃ to 1 010 ℃. The corresponding spreading rate (dR/dt) in this stage is very fast. When the temperature increases to higher than 1 010 ℃, the contact angle remains approximately constant. The final contact angle is as low as 15?. The spreading radius increases with temperature to an equilibrium value. The initial radius is 3 mm, while the final radius reaches 5.2 mm, indicating that the wettability is improved significantly.
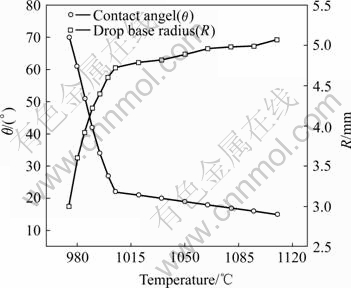
Fig.1 Change of contact angle and drop base radius with temperature
Fig.2(a) shows the shape of pure copper without the addition of Ti plate at the temperature of 1 300 ℃. A non-wetting contact angle of approximate 142? is obtained. It is clear that the copper alloy can not spread on the substrate even at high temperature. Figs.2(b)-(d) correspond to the experiments, where Ti is placed between the copper alloy and the substrate. Fig.2(b) shows the shape of the copper alloy on SiC at the beginning of the collapse of the copper plate at 970 ℃. This temperature is close to the eutectic temperature of 960 ℃. Therefore, it can be deduced that the composition of the liquid should locate in the Ti2Cu+TiCu interval by reference to the binary diagram[14]. The liquid forms firstly at the copper/titanium interface. Then the copper and titanium quickly dissolve into the liquid. It is observed that the complete dissolution of the droplet needs only 20 s. When the temperature increases to 985 ℃, the drop completely melts and low contact angle of 45? is achieved (Fig.2(c)). It is evident that the presence of Ti results in a major improvement in wettability. The copper alloy can completely spread on the substrate at the temperature of 1 100 ℃, as shown in Fig.2(d).
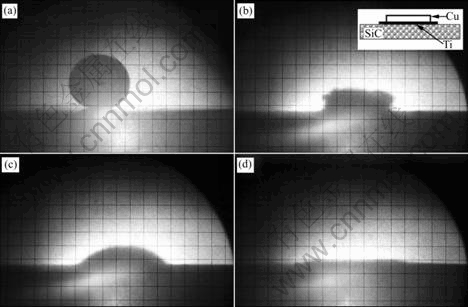
Fig.2 Shapes of liquid droplet on substrate: (a) Pure copper at 1 300 ℃; (b) Ti-activated at 970 ℃; (c) Ti-activated at 985 ℃; (d) Ti-activated at 1 100 ℃
3.2 Microstructure
Fig.3(a) shows the typical microstructure of SiCp/Cu composites with the particle size of 63 μm. It is revealed that the distribution of SiC particles is not very uniform. Large block of white phase (copper) is observed, which is related to the fact that SiC particles are difficult to homogenously mix with the small amount of binder. The aggregation of the binder will bring about the large matrix region. The bond between SiC and Cu matrix looks good and strong and no separated interface is observed. Residual pores can be introduced during pressureless infiltration, which mainly arise from the nonuniform distribution of Ti particles. The regions lack of Ti can not be infiltrated completely. Figs.3(b) and (c) show the high magnification microstructures of SiCp/Cu composites infiltrated with Cu-3Si-2Al-1Ti. The most important feather is that a thin grey reaction layer is found between SiC and copper matrix. The increase of Ti concentration from 10% to 15% leads to a greater interfacial segregation, as shown in Fig.3(c). Nearly all of the Ti aggregate to the boundary of SiC particles because Ti can not be detected in the matrix. Fig.3(d) shows the microstructure of SiCp/Cu composites infiltrated with Cu-5Al-2Fe-2Ni. The EDS analysis of different regions in the microstructure is shown in Table 1. The grey phase (region B) in the matrix is rich in Fe and Si. For the composites infiltrated with Cu-3Si-2Al-1Ti, there is no grey phase observed in the matrix. The results indicate that Fe is difficult to distribute homogenously in the matrix, while the distribution of Si, Al and Ni is uniform. The SiC particles can not remain in the original irregular shape, but saw-like shape is observed, indicating that the surfacial layer of SiC particles is dissolved or take part in the formation of the interfacial layer. The high Si content (10.38%) of the matrix (region C) reveals the dissolution of SiC particle. It is clear that the interfacial layer (region D) contains high content of Ti (86.3%). The line scan results shown in Fig.3(e) further reveal that the contents of Ti and C are high in the interface region. The EDS pattern of the interfacial layer is shown in Fig.3(f).
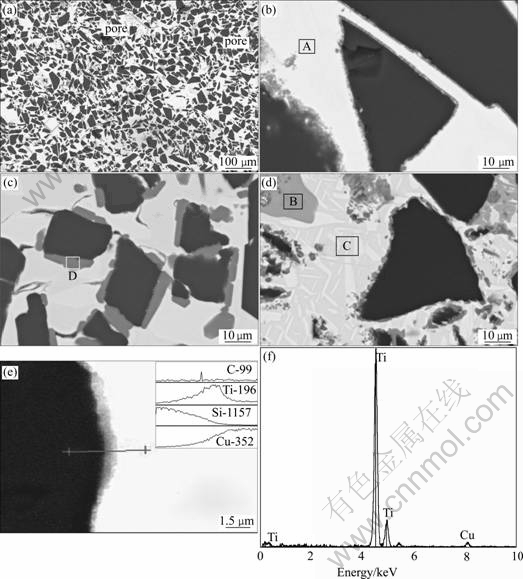
Fig.3 Microstructures and EDS analysis of SiCp/Cu composites: (a) Low magnification microstructure; (b) Infiltrated with alloy A (10%Ti); (c) Infiltrated with alloy A (15%Ti); (d) Infiltrated with alloy B; (e) Cu/SiC interface and composition profiles; (f) EDS pattern of interfacial layer
Table 1 EDS analysis result of different regions (mole fraction, %)
Fig.4 presents the XRD patterns of SiCp/Cu composites. The results indicate that totally four phases are identified for the composite infiltrated with Cu-5Al-2Fe-2Ni, i.e. SiC, AlCu4, TiC and Fe. Fe phase is not present in the composite infiltrated with Cu-3Si-2Al-1Ti.
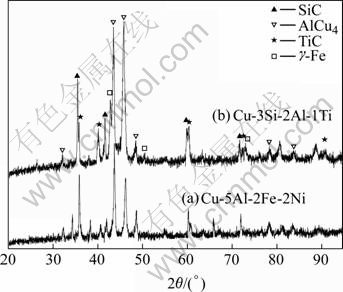
Fig.4 XRD patterns of SiCp/Cu composites infiltrated with Cu-5Al-2Fe-2Ni (a) and Cu-3Si-2Al-1Ti (b)
Fig.5 shows the TEM image of the interfacial region and the diffraction pattern of the reaction layer between SiC and Cu. The reactive layer can be clearly seen and the thickness is about 0.3 μm. The diffraction pattern of the medium layer between SiC and Cu coincides with that of TiC. Therefore, it is considered that element Ti in the liquid metal segregates to the solid/liquid interface and promotes the chemical reaction during the wetting process.

Fig.5 TEM image of interface region and electron diffraction pattern of interfacial layer
3.3 Interfacial energy and interfacial composition
The surface tension of liquid Ti (1 650 mJ/m2) is higher than that of the liquid Cu (1 300 mJ/m2)[18]. Therefore, element Ti is not tensioactive at the liquid free surface. However, Ti is tensioactive at the Cu/SiC interface due to its high affinity to C. From the dynamic point of view, two stages are involved in the wetting process. Firstly, Ti diffuses in liquid copper and segregates to metal/ceramic interface. Secondly, Ti involves in the reaction and results in the formation of interfacial products. The influence of Ti on the interfacial energy and interfacial composition can improve the understanding of the wetting mechanism.
The work of adhesion, W, of a metal/ceramic interface is a common metric of mechanical interfacial strength. W is defined by
W=γSV+γLV-γSL (1)
where γSV and γLV are the surface energies of the ceramic and metal phases, respectively; γSL is the interfacial energy of metal/ceramic. W can be obtained from the contact angle and the surface energy of metal phase:
W=(1+cos θ)γLV (2)
The change of the solid/liquid interfacial energy (Δγ) with the concentration of the active element in the liquid (xB) can be expressed by a simple model[19]:
Δγ=-RTA[ln(1+KxB)-xB] (3)
![]() (4)
(4)
![]() (5)
(5)
where Δγ is the change in interfacial energy due to the segregation of active element B at the interface; A is the number of interface sites per unit area; ΔGseg is the Gibbs free energy of segregation; xS is the interfacial mole fraction of solute B; xb is the bulk mole fraction of B. xS can be calculated from Eqn(5). In the calculation, A is taken to be the number of Cu sites in a Cu(111) interfacial plane: 2.93×10-5 mol/m2[20], γSV is taken as 1 800 mJ/m2 for α-SiC[19], xB is taken as mole fraction of 6.7% (corresponding to mass fraction of 10%Ti), and T is taken as 1413 K.
Δγ is calculated from the interfacial energy data based on the experimental value of θ. The interfacial energy decrease of 2168.4 mJ/m2 due to the addition of Ti is observed, as shown in Table 2. A value of 8663.5 for K is obtained from Eqn.(3). The value of K obtained yields a free energy of segregation of -106.5 kJ/mol by Eqn.(4). Using the value of K in Eqn.(5), we can compute the Ti interfacial mole fraction of 99.8%.
Table 2 Contact angle, surface energy, work of adhesion, interfacial energy, interfacial energy decrease and Ti interfacial concentration

During pressureless infiltration, Ti segregates to the solid/liquid interface and decreases the solid/liquid interfacial energy. Subsequently, Ti reacts with the superfacial layer of SiC particle, resulting in the dissolution of SiC. The Si diffuses into the copper and C remains at the boundary of SiC due to the fact that the dissolution content of C in copper is extremely low[22]. Finally, Ti combines with C and forms a continuous layer of TiC. The equilibrium between solid TiC and the liquid Cu-Ti-C solution can be evaluated by considering the chemical reaction:
[Ti]Cu + [C]=TiC (6)
The change of the Gibbs free energy associated with this reaction is derived by combining the thermodynamic data of the following reaction:
Ti(β)+C(graphite)=TiC (7)
Ti(β)=Ti(liquid) (8)
The data for reactions (7) and (8) are expressed as[22]
![]() /(J?mol-1)=-186.480+14.32T (9)
/(J?mol-1)=-186.480+14.32T (9)
![]() /(J?mol-1)=-16.218-8.36T (10)
/(J?mol-1)=-16.218-8.36T (10)
According to reactions (6)-(10), the equilibrium constant for reaction (6) is written as
![]() (11)
(11)
Considering the three-phase equilibrium of TiC- liquid Cu-graphite, and assuming that carbon activity is equal to unity, the Ti activity calculated for 1 150 ℃ from Eqn.(11) is 5.56×10-7. It is clear that in the presence of TiC, the solubility of Ti in liquid Cu is very low, which is confirmed by the EDS analysis of the composition of the matrix.
In conclusion, during infiltration process, the chemical gradient at the interfacial region provides the thermodynamic and kinetic condition for the interfacial reaction. Interfacial energy is further reduced due to the interaction between Ti and C atom. This is the reason that small amount of active element (Ti) can trigger the transformation from unwetting to wetting. Despite the concentration of the alloying elements in the matrix is low, the amount of alloying elements taking parts in the interfacial reaction is very high, which results in the reduction of Gibbs free energy and thus the spontaneous infiltration.
3.4 Mechanical properties
The compression strength and bending strength of the SiCp/Cu composites are shown in Fig.6.
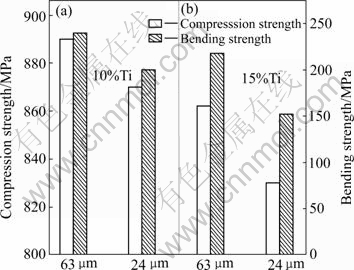
Fig.6 Compression strength and bending strength of SiCp/Cu composites
It can be seen that the content of active element (Ti) and the particle size in the preform have noticeable effect on the mechanical properties of SiCp/Cu composites. It is clear that the compression strength and bending strength of 10%Ti composites are higher than those of 15%Ti composites. This can be attributed to the increase of thickness of the interfacial layer. The compression and bending strength of the composite infiltrated with 63 μm preform are higher than those of the composites infiltrated with 24 μm preform. This can be explained by the fact that large particles are more prone to fail than the small ones.
Fig.7 shows the fracture surface of SiCp/Cu composites. Nearly all of the SiC particles correspond to the failure mode of transparticle fracture. Pure interface debonding is not observed, indicating that the bonding strength of the SiC particles to the matrix exceeds the matrix yielding stress. The interfacial layer is clearly seen on the boundary of SiC particles and forms a continuous layer. The joining of the matrix and the ceramic phase is good.

Fig.7 SEM fracture surface(a) and deformation behaviour(b) of SiCp/Cu composites
4 Conclusions
1) Ti can strongly improve the wettability of copper alloy on SiC. Low contact angle of 15? is obtained at the temperature of 1 100 ℃. The interfacial energy is lowered by the segregation of Ti and the formation of reaction product TiC, resulting in the enhancement of wettability.
2) Ti is found to almost segregate to Cu/SiC interface. This agrees well with a coverage of 99.8% Ti at the Cu/SiC interface predicted from a simple model based on Gibbs adsorption isotherm. In addition, Ti reacts with C to form TiC.
3) SiCp/Cu composites with 57% (volume fraction) reinforcement content are fabricated by Ti-activated pressureless infiltration process, and the densification reaches 97.5%. The bending strength varies between 152 MPa to 240 MPa and increases with decreasing particle size.
References
[1] SHU K M, TU G C. The microstructure and the thermal expansion characteristics of Cu/SiCp composites [J]. Material Science and Engineering A, 2003, 349: 236-247.
[2] KRAUSS G, KUBLER J, TRENTINI E. Preparation and properties of pressureless infiltrated SiC and AlN particulate reinforced metal ceramic composites based on bronze and iron alloys [J]. Materials Science and Engineering A, 2002, 337(1/2): 315-322.
[3] SUNDBERG G, PAUL P, SUNG C M. Fabrication of CuSiC metal matrix composites [J]. Journal of Materials Science, 2006, 41(2): 485-504.
[4] YIH P, CHUNG D D L. Silicon carbide whisker copper matrix composites fabricated by hot pressing copper coated whiskers [J]. Journal of Material Science, 1996, 31: 399-406.
[5] LEMSTER K, GRAULE T, KUEBLER J. Processing and microstructure of metal matrix composites prepared by pressureless Ti-activated infiltration using Fe-base and Ni-base alloys [J]. Materials Science and Engineering A, 2005, 393(1/2): 229-238.
[6] PECH-CANUL M I, MAKHLOUF M M. Processing of Al-SiCp metal matrix composites by pressureless infiltration of SiCp preforms [J]. Journal of Materials Synthesis and Processing, 2000, 8(1): 35-53.
[7] RADO C, DREVET B, EUSTATHOPOULOS N. Role of compound formation in reactive wetting: The Cu/SiC system [J]. Acta Materialia, 2000, 48(18/19): 4483-4491.
[8] VOITOVITCH R, MORTENSEN A, HODAJ F. Diffusion-limited reactive wetting: Study of spreading kinetics of Cu-Cr alloys on carbon substrates [J]. Acta Materialia, 1999, 47(4): 1117-1128.
[9] PALIT D, MEIER A M. Reaction kinetics and mechanical properties in the reactive brazing of copper to aluminum nitride [J]. Journal of Materials Science, 2006, 41(21): 7197-7209.
[10] EUSTATHOPOULOS N. Progress in understanding and modeling reactive wetting of metals on ceramics [J]. Current Opinion in Solid State and Materials Science, 2005, 9(4/5): 152-160.
[11] SAIZ E, CANNON R M, TOMSIA A P. Reactive spreading: Adsorption, ridging and compound formation [J]. Acta Materialia, 2000, 48(18/19): 4449-4462.
[12] BARZILAI S, AIZENSHTEIN M, FROUMIN N. Y2O3/(Cu-Me) systems (Me = Al, Ti): Interface reactions and wetting [J]. Journal of Materials Science, 2006, 41(16): 5108-5112.
[13] MARTINEZ V, ORDONEZ S, CASTRO F. Wetting of silicon carbide by copper alloys [J]. Journal of Materials Science, 2003, 38(19): 4047-4054.
[14] MEIER A, CHIDAMBARAM P R, EDWARDS G R. Generation of isothermal spreading data and interfacial energy data for liquid reactive metals on ceramic substrates [J]. Journal of Materials Science, 1995, 30(15): 3791-3798.
[15] NAKASHIMA K, MATSUMOTO H, MORI K. Effect of additional elements Ni and Cr on wetting characteristics of liquid Cu on zirconia ceramics [J]. Acta Materialia, 2000, 48(18/19): 4677-4681.
[16] XIAO P, DERBY B. Wetting of silicon carbide by chromium containing alloys [J]. Acta Materialia, 1998, 46(10): 3491-3499.
[17] AIZENSHTEIN M, FROUMIN N, FRAGE N. Interface phenomena in the B4C/(Me-Ti) systems (Me = Cu, Au and Sn) [J]. Journal of Materials Science, 2005, 40(9/10): 2325-2327.
[18] DELANNAY F, FROYEN L, DERUYTTERE A. The wetting of solids by molten metals and its relation to the preparation of metal-matrix composites [J]. Journal of Materials Science, 1987, 22(1): 1-16.
[19] WANG Z, WYNBLATT P. Wetting and energetics of solid Au and Au-Ge/SiC interfaces [J]. Acta Materialia, 1998, 46(14): 4853-4859.
[20] LI J G, COUDURIER L, EUSTATHOPOULOS N. Work of adhesion and contact-angle isotherm of binary alloys on ionocovalent oxides [J]. Journal of Materials Science, 1989, 24(3): 1109-1116.
[21] NOVAKOVIC R, RICCI E, MUOLO M L. On the application of modelling to study the surface and interfacial phenomena in liquid alloy-ceramic substrate systems [J]. Intermetallics, 2003, 11(11/12): 1301-1311.
[22] FROUMIN N, FRAGE N, POLAK M. Wetting phenomena in the TiC/(Cu-Al) system [J]. Acta Materialia, 2000, 48(7): 1435-1441.
Foundation item: Project(2006AA03Z557) supported by The High-tech Research and Development Program of China; Project(2006CB605207) supported by the National Basic Research Program of China; Project(I2P407) supported by MOE Program For Changjiang Scholars and Innovative Research Team in University
Corresponding author: ZHANG Lin; Tel: +86-10-62332727; E-mail: zhanglincsu@163.com
(Edited by YUAN Sai-qian)


