
J. Cent. South Univ. (2021) 28: 386-397
DOI: https://doi.org/10.1007/s11771-021-4610-4
Removal of AlN from secondary aluminum dross by pyrometallurgical treatment
WANG Jian-hui(王建辉)1, 2, ZHONG Ye-qing(钟叶清)1, 2, TONG Yue(仝悦)1, 2,XU Xun-lin(徐勋林)1, 2, LIN Gao-yong(林高用)1, 2
1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Nonferrous Materials Science and Engineering (Ministry of Education),Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2021
Abstract:
A new process of AlN removal from secondary aluminum dross (SAD) by pyrometallurgical treatment with added cryolite was applied for solving the problem of recycling the secondary aluminum dross. The response surface methodology (RSM) was used to design experiments and optimize parameters. The results show that adding the appropriate amount of cryolite can effectively promote the oxidation of AlN in the SAD, and too much cryolite will reduce the promotion effect. The effects of roasting temperature and cryolite on the denitrification rate are the most significant (p<0.0001) followed by holding time. Predicted values of the denitrification rate are found to be in good agreement with experimental values (R2=0.9894 and  =0.9775), which confirms the validity of the model employed. The optimum conditions of roasting temperature of 750 °C, holding time of 194 min, mass fraction of cryolite of 17.7% are obtained according to the quadratic model. Under these conditions, the maximum actual denitrification rate reaches 94.71% and the AlN content in the SAD is only 0.55 wt%. The unfired brick with compressive strength of 18.62 MPa (GB/T 2542-2012) was prepared based on the roasted SAD.
=0.9775), which confirms the validity of the model employed. The optimum conditions of roasting temperature of 750 °C, holding time of 194 min, mass fraction of cryolite of 17.7% are obtained according to the quadratic model. Under these conditions, the maximum actual denitrification rate reaches 94.71% and the AlN content in the SAD is only 0.55 wt%. The unfired brick with compressive strength of 18.62 MPa (GB/T 2542-2012) was prepared based on the roasted SAD.
Key words:
Cite this article as:
WANG Jian-hui, ZHONG Ye-qing, TONG Yue, XU Xun-lin, LIN Gao-yong. Removal of AlN from secondary aluminum dross by pyrometallurgical treatment [J]. Journal of Central South University, 2021, 28(2): 386-397.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-021-4610-41 Introduction
The increase in the generation of solid waste from the metallurgical and mining industries has become a global environmental problem [1]. As one of them, aluminum dross (AD) is a kind of hazardous solid waste generated during aluminum smelting and processing. It is usually divided into primary aluminum dross (PAD) and secondary aluminum dross (SAD) according to different sources [2, 3]. PAD comes from the process of producing primary aluminum by electrolysis of alumina, which contains 15%-75% of metallic aluminum and has high aluminum recovery value [4]. SAD is generated by remelting PAD or other aluminum scraps to recover aluminum [5]. Generally, 8%-15% of SAD will be generated per ton of aluminum refining [6]. As one of the most widely used metals, aluminum has an annual output of tens of millions of tons. Aluminum has unlimited recyclability and high durability, and nearly 75% of the aluminum currently produced is still being recycled, so the amount of SAD produced every year is also a huge number. However, more than 90% of SAD will be directly stacked or landfilled without treatment [7], and a small part will be used to produce alumina, water purification agent or refractory and so on [8].
Stabilization/solidification (S/S) is defined as a typical technology that aims to convert hazardous wastes into insoluble forms by physical and chemical methods [9-11]. Therefore, the preparation of unfired bricks with SAD as the main raw material is an effective treatment method. A large number of alumina, silica in SAD with adding cement, lime, gypsum and other activators can form calcium silicate hydrates (CSH) and calcium aluminates hydrates (CAH) with high-strength network structure [12-14], so as to solidify the heavy metal ions and realize zero hazard. Although there are a lot of researches on the solid waste unfired brick at home and abroad, most of them focus on fly ash, red mud and slag [15-17], while the research on aluminum dross is relatively less. Different from other solid wastes, SAD contains a lot of AlN, which hampers the large-scale application of this technology.
AlN is generated by the reaction of molten Al and N2 at high temperature [18], which is very unstable and has leaching toxicity. When it contacts with water, it will react to produce a kind of colorless, unpleasant odorous gas, NH3, and the reaction will be particularly strong under alkaline conditions [19]. Long-term exposure to ammonia will lead to rhinitis, pharyngitis, sore throat and other health problems, and even death. In addition, it also brings a lot of difficulties to the recycling of SAD. Therefore, denitrification pretreatment of SAD is required.
At present, hydrometallurgical processes such as water, alkali, and acid leaching are the main ways to dispose of SAD, which usually involves four steps: leaching, solid-liquid separation, gas treatment and evaporation/crystallization [6, 8, 20]. DAVID et al [21] proposed an investigation of hydrogen generation by the reaction of mechanically milled aluminum dross with tap water. MURAYAMA et al [22] prepared Mg-Al, Ca-Al and Zn-Al type layered double hydroxides (abbreviated as LDHs) from secondary waste aluminum dross. Although these processes can achieve the removal of AlN and the harmlessness of the dross, it is complicated and the supporting equipment needs to be developed, and the waste gas and waste liquid generated during the treatment process will cause secondary pollution if it is not handled properly, which has some defects. Compared with the hydrometallurgical processes, there has been less discussion about the pyrometallurgical processes of SAD. As there are a lot of metal aluminum, alumina and other metal oxides in AD, it is usually used to prepare refractory materials, such as nano alumina, sialon ceramics, magnesia alumina spinel. RAMASWAMY et al [23] prepared the refractory composites with aluminium dross (after leaching out AlN) and un-stabilized zirconia (monoclinic ZrO2) as raw materials by conventional process (calcination, ball milling, compaction and sintering (1550 °C/6 h)). ZHANG et al [24] used NaOH solution to extract soluble aluminum SAD and hydrolyze AlN in it; the remaining slag with adding MgO was sintered in the temperature range 1373-1773 K; MgAl2O4 spinel with a compressive strength as high as 69.4 MPa was produced from the sample sintered at 1673 K for 3 h.It can be seen that the pyrometallurgical processes have a common problem of high temperature (>1000 °C), which leads to large energy consumption and high costs, and the SAD still needs to be pretreated. The general consensus is that the starting temperature of AlN oxidation is between 800 and 900 °C [25] and a higher temperature is needed for complete oxidation. However, it was found in our laboratory that AlN could be effectively oxidized by roasting SAD with adding cryolite at about 700 °C, which realizes the removal of it. Besides, the activity of the dross can be improved by roasting [26, 27]. Although the SAD after denitrification contains cryolite, it is transformed from hazardous to renewable, which can still be used as the raw material of unfired bricks confirmed by experiments.
To date, the study on the oxidation of AlN is mainly focused on AlN powder and AlN ceramics, which is quite different from AlN in SAD. In this study, response surface methodology (RSM) [28, 29] was used to study the influence of three factors including roasting temperature, holding time and mass fraction of added cryolite on the denitrification rate. Analysis of variance (ANOVA) was used to obtain a quadratic model, and the accuracy of the model was verified to determine the optimum process parameters. In addition, the preliminary study was carried out on the preparation of unfired bricks with roasted SAD as the main raw material.
2 Materials and methods
2.1 Materials
The SAD used in the experiments is a malodorous, dark black powder, which was collected from an aluminum plant in Jiangxi Province, China. More than 99% of the dross can pass through a standard sieve with a pore size of 150 μm. Prior to the experiment, the dross was dried at 120 °C for 5 h at least, and then quickly stored in a desiccator for future use. The contents of Al, Si, Mg and other main elements in the SAD are shown in Table 1. The mineral phases of the dross are Al, α-Al2O3, MgAl2O4, and AlN, salts, calcium- containing compounds and silicon compounds are the remaining constituents of the dross as shown in Figure 1. The mass fraction of AlN in the SAD is 14.53% determined by neutralization titrimetric method (the average of three experiments).
Table 1 Chemical composition of SAD (wt%)
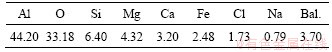
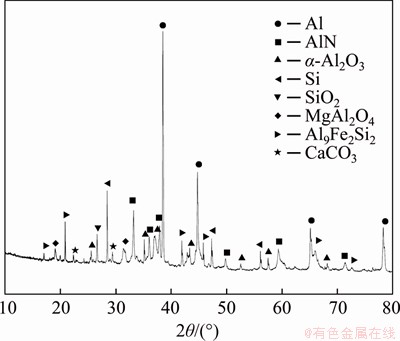
Figure 1 XRD pattern of SAD
2.2 Experimental procedure and sample analysis
5 g of SAD and cryolite with the corresponding mass fraction (powdery and analytically pure) were mixed evenly in the agate grinding bowl firstly, and then placed in the cylindrical corundum crucible with outer diameter of 35 mm. Although Al2O3 is easy to melt in cryolite, the experimental temperature has not reached this temperature. The Al2O3 content in the corundum crucible used is more than 99%, and its performance is stable. Therefore, no other impurities will be brought into the experimental sample, and no chemical reaction will be issued with the experimental sample at the experimental temperature. Secondly, the corundum crucible with the sample was transferred to the muffle furnace and roasted to the set time in the air. Finally, the roasted products which were sintered were ground evenly again to make the particle size less than 150 μm. The content of AlN in the SAD was determined by neutralization titrimetric method. The specific operation flow of neutralization titrimetric method is as follows.
0.300 g of SAD was added to the conical flask containing 20% NaOH solution for distillation, and the distilled solution was absorbed with the nearly saturated boric acid solution, which was titrated by HCl solution (c of concentration) with methyl red-bromocresol green as an indicator. The change in the colour at the end-point of the titration was from bright green to wine-red, and the volume of HCl consumed was recorded as V1. The blank experiments (without adding SAD) were completed in the same way, and the volume of HCl consumed was recorded as V2. All reagents used were analytical and deionized water was made by our own laboratory. The experimental equipment used is shown in Figure 2. The mass fraction of AlN in the SAD is mathematically expressed as Eq. (1):
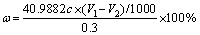 (1)
(1)
In order to accurately calculate the mass of AlN in the roasted SAD, it was necessary to record the mass change of the sample as △m (including oxidation mass gain and cryolite mass). The mass of AlN in the roasted SAD is mathematically expressed as Eq. (2):
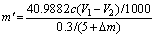 (2)
(2)
The mass of AlN in the SAD before roasting is shown as Eq. (3):
m=5ω (3)
The denitrification rate is shown as Eq. (4):
 (4)
(4)
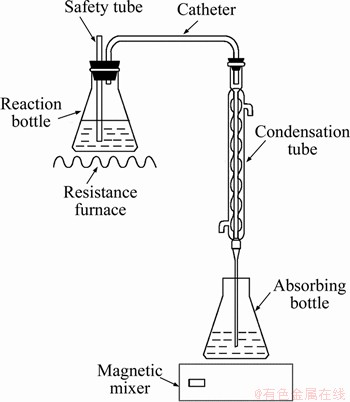
Figure 2 Experimental equipment of neutralization titrimetric method
The mass fraction of AlN in the roasted SAD is shown as Eq. (5):
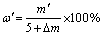 (5)
(5)
XRD analysis was carried out using an X-ray diffractometer (smartlab9K) with Cu-Kα (λ=1.54056 A) radiation in the 2θ degree range of 10°-80° operating at 40 kV and 40 mA to determine the phases of the sample. Elemental analysis of the sample was performed using an X-ray fluorescence analysis (AxiosmAX). The morphology of the sample was observed by a scanning electron microscope (SU8010). Thermogravimetric- differential scanning calorimetry analysis (TG-DSC) was conducted by a comprehensive thermal analyzer (STA449F3). Approximately 10 mg dross sample was heated from 30 to 800 °C in an Al2O3 crucible with a heating rate of 10 °C/min in air atmosphere. The compressive strength of the unfired brick was determined by an electronic universal testing machine (WDW-100C).
2.3 Experimental design and data analysis
Central composite design (CCD) [30] was used to optimize the process parameters on denitrification rate. Factors studied included roasting temperature (X1, °C), holding time (X2, min), and mass fraction of cryolite (X3, wt%). Denitrification rate was used as the response target value. The runs included in CCD are 8 factorial runs, 6 axial runs, and at least one centre point [31] as shown in Figure 3. In order to examine the experimental error and the reproducibility of the dates, in the present study, 4 centre points were used and a total of 18 experiments were calculated according to Eq. (6):
N=2k+2k+nc (6)
where k is the number of factors; nc is the number of centre points.
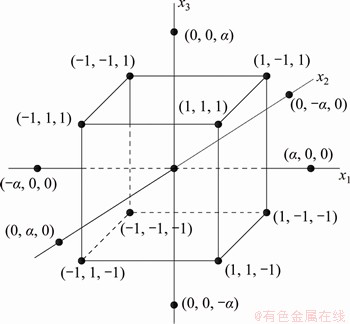
Figure 3 Central composite design (CCD) for three factors
The independent variables were coded as ±1, 0, ±α in CCD, where +1 and -1 represent the eight factorial points at their low and high levels, respectively, and α was the distance of the axial point from centre which makes the design rotatable. The value of α was calculated by Eq. (7) [32], which was 1.682.
α=2k/4 (7)
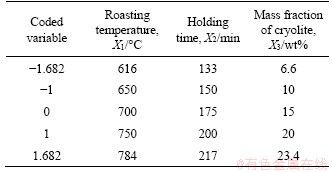
Considering the operability of the experiments, the upper and lower extreme values of roasting temperature, holding time and mass fraction of cryolite were 784 and 616 °C, 217 and 133 min, 23.4% and 6.6%, respectively. The actual variables of CCD and corresponding coded variables are shown in Table 2.
The responses were determined using the optimal quadratic model predictor given as Eq. (8):
 (8)
(8)
where y is the predicted response; β0 is the coefficient for the intercept; βi is the linear regression coefficient; βii is the squared regression coefficient; βij is the interaction regression coefficient; xi, xj are encoded variables; ε is the error term [33].
Table 2 Coded variables and actual variables for CCD
3 Results and discussion
3.1 Comparative analysis before and after roasting
The SAD with added 15% cryolite before roasting is shown in Figure 4(a). After roasting at 784 °C for 175 min, the denitrification rate of it reached the highest, 93.54%, in all runs of experiments. The macroscopic appearance shows that the colour changed from dark-black to grey- white as shown in Figure 4(b), which is the same as that after water treatment [34]. In addition, all the samples were sintered in different degrees, from powder to bulk.
XRD patterns of the raw SAD and roasted SAD (784 °C, 175 min, 15%) are presented in Figure 5. It can be observed that the Al-containing phases of the initial dross are mainly Al, AlN, α-Al2O3, MgAl2O4 and Al9Fe2Si. After roasting, the Al phase disappeared, and meanwhile, the α-Al2O3 diffraction peaks become stronger and new phase mullite solid solution (Al1.4Si0.3O2.7) appeared. The vertical lines in Figure 5 mark the positions of the AlN diffraction peaks. The diffraction angles corresponding to a, b, c, d, and e lines are 33.2°, 36.0°, 37.9°, 49.8°, 59.4° and 71.3°, respectively. After roasting, the diffraction peaks of AlN at 33.2°, 36.0° are obviously decreased, and other diffraction peaks disappear. The XRD analyses show that all Al and most of AlN were oxidized to Al2O3 after roasting, which also avoids the reaction of SAD with water to generate H2 and the Al2O3 produced is a good raw material for unfired bricks.
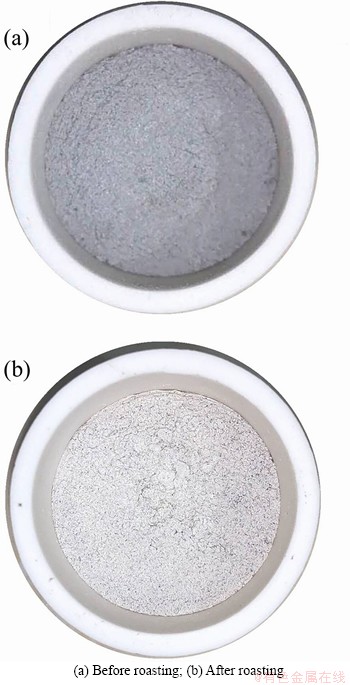
Figure 4 Pictures of SAD with added 15% cryolite:
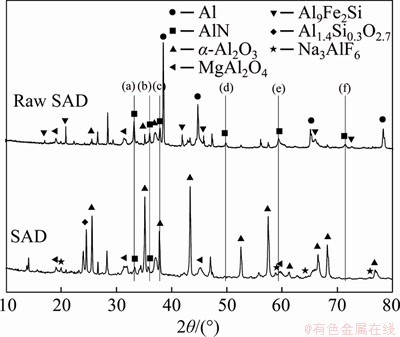
Figure 5 XRD patterns of raw SAD and roasted SAD (784 °C, 175 min, 15%)
3.2 Thermodynamic and thermal behavior analysis
Experimental results show that cryolite can effectively promote the oxidation of AlN. ZHOU et al [35] put forward an explanation about the mechanism that during the roasting process, the fluorine atoms of cryolite and oxygen atoms of O2 combined into the gaseous species of OxF2x, which had higher activity than O2, so it was easier to oxidize AlN and generate NyFx and Al2O3. The reaction of NyFx and O2 generated OxF2x again so that the reaction continued. However, due to the special composition of NyFx and OxF2x gases, and the small amount of their formation, it is difficult to detect them, which needs to be confirmed by a large number of experiments in the future. The total reaction equation is as follows:
 (9)
(9)
The reaction products of AlN and O2 at high temperature may also include NO, N2O, NO2, N2O4, N2O3, N2O5. The reaction equations are as follows:
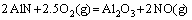 (10)
(10)
 (11)
(11)
 (12)
(12)
 (13)
(13)
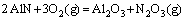 (14)
(14)
 (15)
(15)
In the temperature range of 500 to 900 °C, the Gibbs free energy change of each reaction was calculated by HSC Chemistry. The thermodynamic diagram of △G-T is shown in Figure 6.
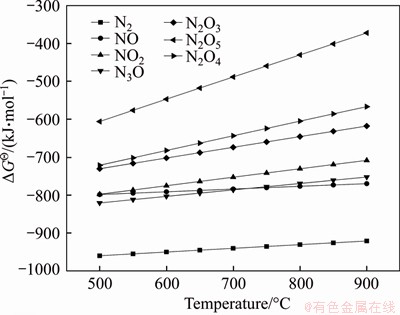
Figure 6 △G-T thermodynamic diagram of AlN-O2 reaction
It can be seen that the thermal stability of AlN in the air is not good. The Gibbs free energy changes of Eqs. (9)-(15) are all negative in the experimental temperature range, which shows that these reactions can be spontaneous in thermodynamics. The Gibbs free energy of Eq. (9) is minimized, which indicates that AlN and O2 are most likely to form N2 in the temperature range of 500 to 900 °C according to the principle of the minimum Gibbs free energy. A large number of studies also have shown that the oxidation of AlN mainly involves the exchange of oxygen and nitrogen [36-38].
Figure 7 shows the thermogravimetry and differential scanning calorimetry (TG-DSC) analysis of the samples. Stippling line and solid line represent the SAD (called sample 1) and the SAD added with 15% cryolite (called sample 2), respectively. The TG curves show that in the roasting process, the weight of both of them decreases first and then increases, which involves a series of complex physical/chemical reactions. As the composition of SAD is extremely complex, the weight loss may be due to the desorption of physically adsorbed water in the sample, the decomposition of other impurities or some surface materials, and the partial dehydroxylation of alumina [39]. For weight gain, it is mainly due to the oxidation of Al and AlN. The difference is that although the weight of sample 1 begins to increase at 550 °C with a small increase, and the total weight loss is 4.94%, and no obvious exothermic peak appears in DSC curve. A weak endothermic peak appears at about 572 °C, which is due to the endothermic melting of Al in SAD. The weight of sample 2 begins to increase at 520 °C with large increase, earlier than sample 1, corresponding to two exothermic peaks in the temperature range of 520-640 °C and 715-760 °C, respectively. The total weight increases to 6.87% finally. It can be concluded by comparison that AlN is oxidized significantly in the roasting process after adding cryolite, and from the starting temperature of the exothermic peak, it means that AlN may be oxidized at about 536 °C.
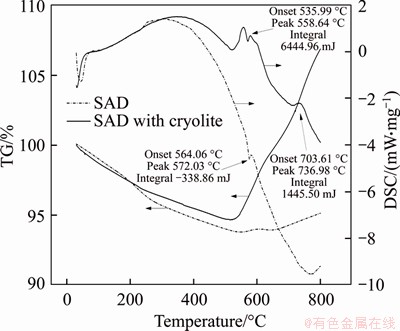
Figure 7 TG-DSC curves of SAD
3.3 Response analysis and interpretation
The experimental results are shown in Table 3. In the 18 runs of experiments, the lowest denitrification rate is 58.82%, and the highest is 93.54%.
The experimental results were analyzed by Design Expert software. The ANOVA result of the quadratic model for the denitrification rate is listed in Table 4. The model F-value of 83.17 implies that the model is significant. There is only a 0.01% chance that the large F-value could occur due to noise. P-value (Prob>F) less than 0.05 indicates that the experimental parameters have a significant impact on the experimental results, and less than 0.0001 indicates that the impact is extremely significant. Therefore, factors X1, X2, X3, X12, X32 are important model terms, and factors X1, X3 have a greater influence on experimental results than factor X2. The P-values of interaction terms X1X2, X1X3, X2X3 are all greater than 0.05, which indicates that the interactions of three factors, namely roasting temperature, holding time and mass fraction of cryolite, are not significant. The F-value of lack of fit is not significant, indicating that the model fits well in the entire regression area. The multiple quadratic regression equation regarding the denitrification rate was obtained as follows:

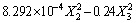 (16)
(16)
Furthermore, the error statistics of the fitted regression equation was analyzed. As shown in Table 5, CV (2.05%)<10%, which indicates that the experiment has high credibility and accuracy. The value of Adeq precision (28.529) is the ratio of effective signal to noise, which is greater than 4, indicating that the model can be used for prediction. The value of R2 (0.9894) is very close to 1, and the values of (0.9775) and
(0.9775) and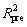 (0.9266) are very high and the difference is small (Radj2-Rpre2<0.2), which means that the fit of the model is good, and there is a high correlation between the observed and predicted values for the denitrification rate.
(0.9266) are very high and the difference is small (Radj2-Rpre2<0.2), which means that the fit of the model is good, and there is a high correlation between the observed and predicted values for the denitrification rate.
Table 3 CCD arrangement and results
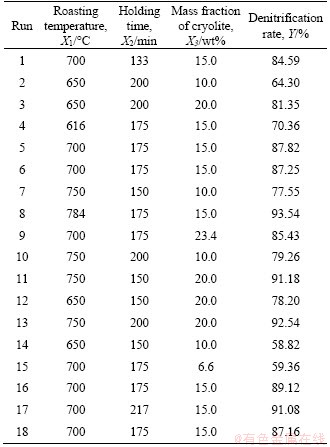
Table 4 ANOVA for denitrification rate
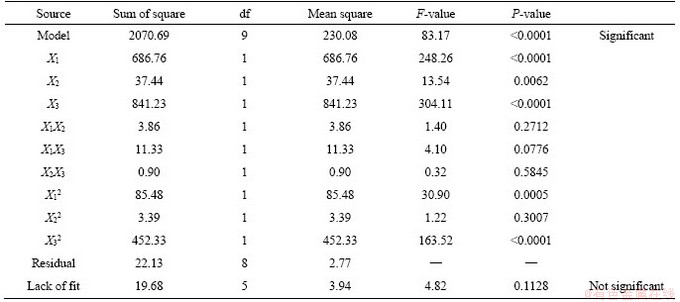
Table 5 Error statistical analysis

3.4 Response surfaces and contour plots
The response surface methodology overcomes the defect that the orthogonal experiment cannot give the intuitionistic figure. According to the 3D response surfaces and contour plots made by the quadratic equation model, the influence of the interaction of other two factors on denitrification rate can be investigated when one factor is fixed at the centre value as shown in Figure 8.
Figure 8(a) shows the effect of the interaction between the roasting temperature (X1) and the holding time (X2) on the denitrification rate. The mass fraction of cryolite is fixed at 15%. It can be observed that the denitrification rate increases with the increase of roasting temperature and holding time, and the roasting temperature has a more significant effect on the denitrification rate. But the increase is getting smaller and smaller over time, which indicates that the oxidation rate of AlN decreases gradually. The denitrification rate calculated by the model has reached 75.39% with the roasting temperature of 650 °C, the holding time of 150 min are the mass fraction of cryolite of 15%, which means that the AlN oxidation reaction is mainly concentrated in the initial 150 min of the reaction. On the one hand, the phenomenon occurring is due to material consumption; on the other hand, it is attributed to the oxidation of AlN controlled by the surface reaction in the initial stage, and then controlled by diffusion [40]. This is quite different from the hydrolysis of AlN in SAD [2, 41]. At the initial stage of the reaction, the gas-solid reaction interface between AlN and O2 is large, so the degree of AlN oxidation is high. As the reaction progresses, the thickness of the aluminum oxide film formed on the AlN surface gradually increases, which hinders the contact between O2 and AlN, and the diffusion rate of the reaction gas decreases, resulting in a slower reaction. Therefore, during the actual industrial pyrometallurgical treatment of SAD, the roasting temperature can be increased appropriately to reduce the holding time and improve the working efficiency. Besides, to reduce the cost, we propose to use the waste heat of the cleaning furnace as the heat source, which is generated by baking the furnace for cleaning the slag at the bottom and wall of the furnace, and the temperature can reach 750-800 °C.
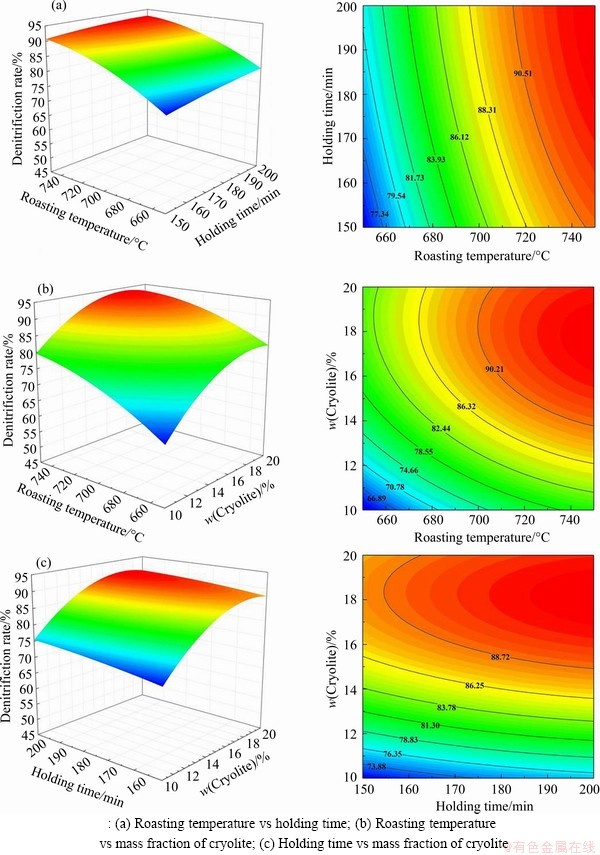
Figure 8 Response surface plots and contour plots:
Figure 8(b) shows the 3D response surface and contour plot of the roasting temperature (X1) and the mass fraction of cryolite (X3) on the denitrification rate. The holding time is fixed at 175 min. It can be seen that the mass fraction of cryolite has a significant effect on the denitrification rate. As the mass fraction of cryolite increases, the denitrification rate increases, but when the mass fraction of cryolite exceeds a certain range, the denitrification rate decreases instead, indicating that the mass fraction of cryolite has an optimum value for the denitrification rate. During the experiment, it was found that cryolite added to the SAD would be sintered after roasting, and with the increase of cryolite content, the sintering would become more serious. Figure 9 shows the SEM images of the samples with runs of 15, 5 and 9. The roasting temperature of the three samples is 700 °C, the holding time is 175 min, and the cryolite mass fractions are 6.6%, 15% and 23.4%, respectively. It can be seen that when the mass fraction of cryolite is 6.6%, the sintered surface is mainly composed of needle-like substances and has more pores. However, when the mass fraction of cryolite is increased to 15%, the sintered surface is composed of flakes. When the mass fraction is 23.4%, the flakes reduce and large-sized substances generate, resulting in the denser sintered surface. Generally, cryolite is used as a flux in the electrolytic aluminum industry, and may play a role as a sintering aid in the process of roasting SAD [42]. Therefore, as the mass fraction of cryolite increased, the sintering of SAD becomes more and more serious, which seriously affects the diffusion of O2 to the reaction interface. When the mass fraction of cryolite exceeds a certain optimum value, the hindering effect of sintering on the oxidation reaction is already greater than the promoting effect of cryolite on the oxidation reaction, resulting in a decrease in the denitrification rate. Consequently, SAD can be stirred properly in the roasting process to reduce sintering. Likewise, Figure 8(c) shows the effect of the interaction between the holding time (X2) and the mass fraction of cryolite (X3) on the denitrification rate, which also indicates that the mass fraction of cryolite has a more significant effect on the denitrification rate than the holding time.
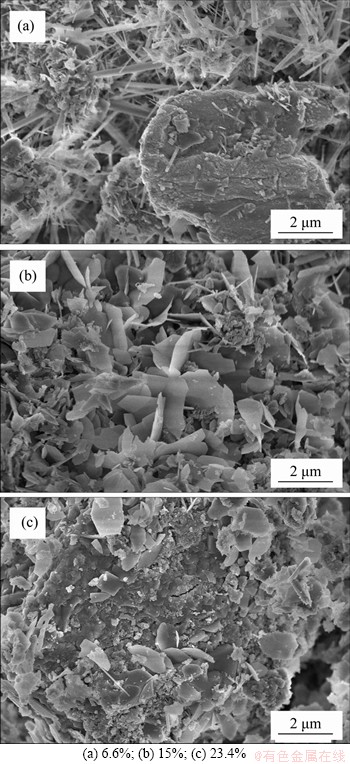
Figure 9 SEM images of sintered surface with different mass fraction of cryolite:
3.5 Determination of optimum conditions and preparation of unfired brick
The optimum levels of variables are found to be roasting temperature of 750 °C, holding time of 194 min, mass fraction of cryolite of 17.7% with a prediction of 94.59% for the denitrification rate using the optimization function of Design Expert software. In order to verify the reliability of the optimum process parameters predicted by the model, the experiment was carried out using the optimum levels. The actual denitrification rate measured is 94.71% and the AlN content in the SAD is only 0.55 wt% after roasting. The relative error between the actual value and the predicted value is only 0.13%, which shows that the model is reliable. The mass ratio of roasted SAD, standard sands, P.O 42.5 cement, hydrated lime and anhydrite is 100:25:10:8:5, and after mixing them evenly and adding 15% water, the unfired brick blank was prepared under the molding pressure of 25 MPa. After conventional curing for 7 d, we got the prepared unfired brick. The compressive strength of the prepared unfired brick reaches 18.62 MPa (GB/T 254-2012).
4 Conclusions
The results of this study demonstrate that the response surface method could efficiently optimize the process for removal of AlN from SAD by roasting. Analysis of ANOVA shows that the effects of roasting temperature and added cryolite on the denitrification rate are the most significant (p<0.0001) followed by holding time. According to the response surface model, the optimum process parameters are as follows: roasting temperature of 750 °C, holding time of 194 min, and mass fraction of cryolite of 17.7%. Under the optimum conditions, the predicted denitrification rate is 94.59%, confirmed by the experiment as 94.71%, and the AlN content in the SAD was only 0.55 wt% after roasting. The results show that AlN can be effectively removed from the SAD by pyrometallurgical treatment. Further research is required to explain the catalytic mechanism of cryolite for the oxidation of AlN and optimize the process parameters for the preparation of unfired bricks.
Contributors
The overarching research goals were developed by WANG Jian-hui, ZHONG Ye-qing, TONG Yue and LIN Gao-yong. WANG Jian-hui, ZHONG Ye-qing and XU Xun-lin provided the experimental data, and analyzed the experimental data. WANG Jian-hui and XU Xun-lin established the response surface model, and validated the model. The initial draft of the manuscript was written by WANG Jian-hui. All authors replied to reviewers’ comments and revised the final version.
Conflict of interest
WANG Jian-hui, ZHONG Ye-qing, TONG Yue, XU Xun-lin, and LIN Gao-yong declare that they have no conflict of interest.
References
[1] GAUTAM M, PANDEY D, AGRAWAL S B, AGRAWAL M. Metals from mining and metallurgical industries and their toxicological impacts on plants [M]//Plant Responses to Xenobiotics. Singapore: Springer, 2016: 231-272. DOI: 10.1007/978-981-10-2860-1_10.
[2] YANG Qun, LI Qi, ZHANG Guo-fan, SHI Qing, FENG Hai-gang. Investigation of leaching kinetics of aluminum extraction from secondary aluminum dross with use of hydrochloric acid [J]. Hydrometallurgy, 2019, 187: 158-167. DOI: 10.1016/j.hydromet.2019.05.017.
[3] MAHINROOSTA M, ALLAHVERDI A. A promising green process for synthesis of high purity activated-alumina nanopowder from secondary aluminum dross [J]. Journal of Cleaner Production, 2018, 179: 93-102. DOI: 10.1016/ j.jclepro.2018.01.079.
[4] GUO Xue-yi, LI Fei, TIAN Qing-hua, JI Kun. Recovery of aluminium from secondary aluminum dross using low-temperature alkaline smelting [J]. Journal of Central South University (Science and Technology), 2012, 43(3): 809-814. DOI: 10.7666/d.y1913986. (in Chinese)
[5] LOPEZ-DELGADO A, TAYIBI H. Can hazardous waste become a raw material? The case study of an aluminium residue: a review [J]. Waste Management & Research, 2012, 30(5): 474-484. DOI: 10.1177/0734242X11422931.
[6] MESHRAM A, SINGH K K. Recovery of valuable products from hazardous aluminum dross: A review [J]. Resources, Conservation and Recycling, 2018, 130: 95-108. DOI: 10.1016/j.resconrec.2017.11.026.
[7] HONG Jian-ping, WANG Jun, CHEN Hai-yan, SUN Bao-de, LI Jia-jing, CHEN Chong. Process of aluminum dross recycling and life cycle assessment for Al-Si alloys and brown fused alumina [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(11): 2155-2161. DOI: 10.1016/ S1003-6326(09)60435-0.
[8] GIL A, KORILI S A. Management and valorization of aluminum saline slags: Current status and future trends [J]. Chemical Engineering Journal, 2016, 289: 74-84. DOI: 10.1016/j.cej.2015.12.069.
[9] TIAN Q, NAKAMA S, SASAKI K. Immobilization of cesium in fly ash-silica fume based geopolymers with different Si/Al molar ratios [J]. Science of the Total Environment, 2019, 687: 1127-1137. DOI: 10.1016/ j.scitotenv.2019.06.095.
[10] GILLIAM T M, WILES C C. Stabilization and solidification of hazardous, radioactive and mixed wastes [M]. Boca Raton: CRC Press, 2005.
[11] TAJUDIN S A A, AZMI M A M, NABILA A T A. Stabilization/solidification remediation method for contaminated soil: A review [J]. IOP Conference, 2016, 136: 12043. DOI: 10.1088/1757-899X/136/1/012043.
[12] OJO E B, MUSTAPHA K, TEIXEIRA R S, SAVASTANO H. Development of unfired earthen building materials using muscovite rich soils and alkali activators [J]. Case Studies in Construction Materials, 2019, 11: e00262. DOI: 10.1016/ j.cscm.2019.e00262.
[13] PART W K, RAMLI M, CHEAH C B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products [J]. Construction & Building Materials, 2015, 77: 370-395. DOI: 10.1016/j.conbuildmat.2014.12.065.
[14] SHI C, DAY R L. Pozzolanic reaction in the presence of chemical activators: Part II—Reaction products and mechanism [J]. Cement and Concrete Research, 2000, 30(4): 607-613. DOI: 10.1016/s0008-8846(00)00214-3.
[15] MIQUELEIZ L, RAMIREZ F, OTI J E, SECO A, KINUTHIA J M, OREJA I, URMENETA P. Alumina filler waste as clay replacement material for unfired brick production [J]. Engineering Geology, 2013, 163: 68-74. DOI: 10.1016/j.enggeo.2013.05.006.
[16] MUNTOHAR A S. Engineering characteristics of the compressed-stabilized earth brick [J]. Construction and Building Materials, 2011, 25(11): 4215-4220. DOI: 10.1016/j.conbuildmat.2011.04.061.
[17] YI Yao-lin, GU Li-yang, LIU Song-yu. Microstructural and mechanical properties of marine soft clay stabilized by lime-activated ground granulated blastfurnace slag [J]. Applied Clay Science, 2015, 103: 71-76. DOI: 10.1016/ j.clay.2014.11.005.
[18] SLACK G A, MCNELLY T F. Growth of high purity AlN crystals [J]. Journal of Crystal Growth, 1976, 34(2): 263-279. DOI: 10.1016/0022-0248(76)90139-1.
[19] KRNEL K, KOSMAC T. Protection of AlN powder against hydrolysis using aluminum dihydrogen phosphate [J]. Journal of the European Ceramic Society, 2001, 21(10): 2075-2079. DOI: 10.1016/S0955-2219(01)00175-3.
[20] DAVID E, KOPAC J. The Assessment of the recycling process of aluminum hazardous waste and a new route of development [J]. Materials Today: Proceedings, 2019, 10: 340-347. DOI: 10.1016/j.matpr.2018.10.415.
[21] DAVID E, KOPAC J. Hydrolysis of aluminum dross material to achieve zero hazardous waste [J]. Journal of Hazardous Materials, 2012, 209-210: 501-509. DOI: 10.1016/j.jhazmat.2012.01.064.
[22] MURAYAMA N, MAEKAWA I, USHIRO H, MIYOSHI T, SHIBATA J, VALIX M. Synthesis of various layered double hydroxides using aluminum dross generated in aluminum recycling process [J]. International Journal of Mineral Processing, 2012, 110-111: 46-52. DOI: 10.1016/j.minpro. 2012.03.011.
[23] RAMASWAMY P, TILLETI P, BHATTACHARJEE S, PINTO R, AVIJIT GOMES S. Synthesis of value added refractories from aluminium dross and zirconia composites [J]. Materials Today: Proceedings, 2020, 22: 1264-1273. DOI: 10.1016/j.matpr.2020.01.419.
[24] ZHANG Yong, GUO Zhao-hui, HAN Zi-yu, XIAO Xi-yuan, PENG Chi. Feasibility of aluminum recovery and MgAl2O4 spinel synthesis from secondary aluminum dross [J]. International Journal of Minerals Metallurgy and Materials, 2019, 26(3): 309-318. DOI: 10.1007/s12613-019-1739-3.
[25] CAO Chang-wei, FENG Yong-bao, TAI Qiu, YANG Jian, LI Xiao-yun, LIANG Tian, LI Jun. Effects of isothermal annealing on the oxidation behavior, mechanical and thermal properties of AlN ceramics [J]. Ceramics International, 2017, 43(12): B299-B307. DOI: 10.1016/j.ceramint.2017.04.098.
[26] EL-HABAAK G, ASKALANY M, ABDEL-HAKEEM M. The effect of mineralogy of calcined shales on the alkali activation and geopolymerization reactions: A case study from Abu-Tartur plateau, Western Desert, Egypt [J]. Applied Clay Science, 2018, 162: 90-100. DOI: 10.1016/j.clay.2018. 05.025.
[27] ELIMBI A, TCHAKOUTE H K, NJOPWOUO D. Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements [J]. Construction & Building Materials, 2011, 25(6): 2805-2812. DOI: 10.1016/j.conbuildmat.2010. 12.055.
[28] ZHANG Yong, GUO Zhao-hui, HAN Zi-yu, XIAO Xi-yuan. Effects of AlN hydrolysis on fractal geometry characteristics of residue from secondary aluminium dross using response surface methodology [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(12): 2574-2581. DOI: 10.1016/S1003-6326(18)64904-0.
[29] GUNST R F. Response surface methodology: Process and product optimization using designed experiments [J]. Technometrics, 1996, 38(3): 284-286. DOI: 10.1080/ 00401706.1996.10484509.
[30] HE R, LI D, YANG K, YANG Z, LI T, REN B. Process optimization and modeling of recycling Mo (VI) from spent Mo-Fe2O3/Al2O3 catalyst by roasting with sodium carbonate using response surface methodology (RSM) [J]. International Journal of Refractory Metals and Hard Materials, 2019, 87: 105162. DOI: 10.1016/j.ijrmhm.2019.105162.
[31] SAHA P, WAGHMARE D. Parametric optimization for autogenous butt laser welding of sub-millimeter thick SS 316 sheets using central composite design [J]. Optics & Laser Technology, 2020, 122: 105833. DOI: 10.1016/j.optlastec. 2019.105833.
[32] AHMAD M A, ALROZI R. Optimization of preparation conditions for mangosteen peel-based activated carbons for the removal of Remazol Brilliant Blue R using response surface methodology [J]. Chemical Engineering Journal, 2010, 165(3): 883-890. DOI: 10.1016/j.cej.2010.10.049.
[33] GANJIAN E, PEYRAVI M, QOREYSHI A A, JAHANSHAHI M, RAD A S. Adsorption photobioreactor as a co-treatment system for ammonium and phosphate removal by the response surface method [J]. Waste Management & Research, 2017, 35(7): 766-775. DOI: 10.1177/0734242x 17708051.
[34] LOPEZ-DELGADO A, ROBLA J I, PADILLA I, LOPEZ- ANDRES S, ROMERO M. Zero-waste process for the transformation of a hazardous aluminum waste into a raw material to obtain zeolites [J]. Journal of Cleaner Production, 2020, 255: 120178. DOI: 10.1016/j.jclepro.2020.120178.
[35] ZHOU He-ping, QIAO Liang, FU Ren-li. Effect of the fluoride additives on the oxidation of AlN [J]. Materials Research Bulletin, 2002, 37(15): 2427-2435. DOI: 10.1016/S0025-5408(02)00942-X.
[36] YEN Chun-ting, TUAN Wei-hsing. Pre-oxidation of AlN substrates for subsequent metallization [J]. Journal of Materials Science Materials in Electronics, 2015, 26(8): 5910-5916. DOI: 10.1007/s10854-015-3160-7.
[37] LIN Chih-yuan, LU Fu-hsing. Oxidation behavior of AlN films at high temperature under controlled atmosphere [J]. Journal of the European Ceramic Society, 2008, 28(3): 691-698. DOI: 10.1016/j.jeurceramsoc.2007.07.015.
[38] DUCHESNE D J, HIPPS K W, GRASHER B A, NORTON M G. The formation of transition aluminas during oxidation of AlN [J]. Journal of Materials Science Letters, 1999, 18(11): 877-879. DOI: 10.1023/A:1006604528478.
[39] MOHAPATRA D, PARK K H. Selective recovery of Mo, Co and Al from spent Co/Mo/γ-Al2O3 catalyst: Effect of calcination temperature [J]. Journal of Environmental Science and Health, Part A, 2007, 42(4): 507-515. DOI: 10.1080/10934520601188409.
[40] KATNANI A D, PAPATHOMAS K I. Kinetics and initial stages of oxidation of aluminum nitride: Thermogravimetric analysis and X-ray photoelectron spectroscopy study [J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 1987, 5(4): 1335-1340. DOI: 10.1116/ 1.574765.
[41] LI Qi, YANG Qun, ZHANG Guo-fan, SHI Qing. Investigations on the hydrolysis behavior of AlN in the leaching process of secondary aluminum dross [J]. Hydrometallurgy, 2018, 182: 121-127. DOI: 10.1016/ j.hydromet.2018.10.015.
[42] MEEK T T, HOLCOMBE C E, DYKES N. Microwave sintering of some oxide materials using sintering aids [J]. Journal of Materials Science Letters, 1987, 6(9): 1060-1062. DOI: 10.1007/BF01729132.
(Edited by ZHENG Yu-tong)
中文导读
采用火法工艺去除二次铝灰中的氮化铝
摘要:为了促进二次铝灰的再生利用,采用添加冰晶石并高温焙烧二次铝灰的火法工艺以去除其中的氮化铝。采用响应面法进行实验设计和参数优化。结果表明,添加适量冰晶石可以有效促进二次铝灰中氮化铝的氧化,但是过量的冰晶石会降低这种促进作用。相比于保温时间,焙烧温度和冰晶石质量分数对脱氮率的影响较为显著(p<0.0001)。脱氮率的预测值与实验值吻合较好(R2=0.9894, =0.9775),验证了模型的有效性。根据二次模型,确定了最佳工艺参数为焙烧温度750 °C,保温时间194 min,冰晶石质量分数17.7%。在此条件下,最大实际脱氮率达到94.71%,二次铝灰中氮化铝质量分数仅为0.55%。以处理过的二次铝灰为主要原料,制备的免烧砖抗压强度达到18.62 MPa (GB/T 2542-2012)。
=0.9775),验证了模型的有效性。根据二次模型,确定了最佳工艺参数为焙烧温度750 °C,保温时间194 min,冰晶石质量分数17.7%。在此条件下,最大实际脱氮率达到94.71%,二次铝灰中氮化铝质量分数仅为0.55%。以处理过的二次铝灰为主要原料,制备的免烧砖抗压强度达到18.62 MPa (GB/T 2542-2012)。
关键词:二次铝灰;氮化铝;火法工艺;脱氮率;响应面法
Foundation item: Project(2017YFB0306001) supported by National Key R&D Program of China
Received date: 2020-07-10; Accepted date: 2020-10-26
Corresponding author: LIN Gao-yong, PhD, Professor; Tel: +86-13507422779; E-mail: mater218@163.com; ORCID: https://orcid.org/ 0000-0001-8004-4123
Abstract: A new process of AlN removal from secondary aluminum dross (SAD) by pyrometallurgical treatment with added cryolite was applied for solving the problem of recycling the secondary aluminum dross. The response surface methodology (RSM) was used to design experiments and optimize parameters. The results show that adding the appropriate amount of cryolite can effectively promote the oxidation of AlN in the SAD, and too much cryolite will reduce the promotion effect. The effects of roasting temperature and cryolite on the denitrification rate are the most significant (p<0.0001) followed by holding time. Predicted values of the denitrification rate are found to be in good agreement with experimental values (R2=0.9894 and  =0.9775), which confirms the validity of the model employed. The optimum conditions of roasting temperature of 750 °C, holding time of 194 min, mass fraction of cryolite of 17.7% are obtained according to the quadratic model. Under these conditions, the maximum actual denitrification rate reaches 94.71% and the AlN content in the SAD is only 0.55 wt%. The unfired brick with compressive strength of 18.62 MPa (GB/T 2542-2012) was prepared based on the roasted SAD.
=0.9775), which confirms the validity of the model employed. The optimum conditions of roasting temperature of 750 °C, holding time of 194 min, mass fraction of cryolite of 17.7% are obtained according to the quadratic model. Under these conditions, the maximum actual denitrification rate reaches 94.71% and the AlN content in the SAD is only 0.55 wt%. The unfired brick with compressive strength of 18.62 MPa (GB/T 2542-2012) was prepared based on the roasted SAD.


