DOI:10.19476/j.ysxb.1004.0609.2018.01.20
基于AOP协同氧化浸出碲渣中的碲和有价金属
郭学益,许志鹏,李 栋,田庆华
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中国有色金属工业清洁冶金工程研究中心,长沙 410083)
摘 要:
以粗铋碱性精炼产生的碲渣为原料,基于高级氧化技术(AOP),在硫酸体系中协同氧化浸出碲渣中的碲和有价金属,研究NaCl浓度、H2O2体积分数、H2O2滴加速度、H2SO4浓度、浸出温度、浸出时间、气体流速和液固比等工艺参数对碲、铜、铋、锑和铅等金属浸出行为的影响,确定最佳工艺参数。结果表明:在NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度 1.2 mL/min、H2SO4浓度2.76 mol/L、浸出温度60 ℃、浸出时间2.5 h、气体流速2.5 L/min和液固比10 mL/g的优化条件下,碲、铜和铋的浸出率分别达95.75%、91.88%和90.23%,而锑和铅的浸出率仅分别为4.84%和0.08%,实现碲渣中碲的高效浸出及有价金属的有效分离和富集。
关键词:
文章编号:1004-0609(2018)-01-0167-08 中图分类号:TF84 文献标志码:A
碲属于稀散元素,其丰度几乎是所有金属及非金属中最小的[1]。碲与其化合物具有许多优良性能,被广泛用于冶金、化工、医药卫生、电子信息、宇航、能源等工业领域,被誉为“现代工业、国防与尖端技术的维生素”[2]。工业纯碲广泛用作合金添加剂,以改良钢的力学性能[3];碲的铋或锑的化合物是良好的制冷材料,用于制作雷达、水底导弹等特殊冷却器[4];碲化铅和碲化铋用于制作感光材料[5];碲汞镉合金是红外发射体和探测器的最佳材料[6];碲锑锗是制作可擦写存储光盘的主要材料[7]。
碲虽然用途巨大,但是资源稀缺,大部分的碲伴生在铜、铅、金、银的矿物中,在四川石棉县境内的大水沟发现了世界唯一的一处独立碲矿[8]。碲渣是现今提取碲的主要原料,主要来自于铜、铅阳极泥火法处理过程及粗铋碱性精炼过程。从碲渣中提取碲的方法主要有碱法工艺[9-10]和酸法工艺[11-12]:碱法工艺是目前工业上回收碲所采用的方法,其主要步骤为碱浸→净化→中和沉碲→煅烧→造液→电解[13],该工艺原料适应强、环境污染小,但流程冗长复杂,且碲的回收率低;酸法工艺主要包括酸浸→还原→除杂→造液→电解等步骤,郑雅杰等[2]采用硫酸浸出—二氧化硫还原的方法提取中和渣中的碲,硫酸浸出中和渣时,碲浸出率达99.99%,二氧化硫还原时,碲回收率达99.84%,但二氧化硫还原时盐酸浓度达3.2 mol/L,设备腐蚀严重,且存在二氧化硫和酸雾污染,操作环境较差。
高级氧化技术(AOP)是在光、电、催化剂、氧化剂等协同作用下,在反应体系中产生活性极强的羟基自由基,使水体中具较高化学稳定性化合物被氧化分解的技术[14]。AOP具有氧化能力强、反应彻底、可连续操作及占地面积小等优点,尤其对难处理复杂物料具有较大的应用价值[15-16]。为高效分离碲渣中碲,避免碲的分散,本文作者基于AOP(O3/H2O2组合),在硫酸体系中氧化浸出碲渣中的碲和有价金属,对浸出过程的金属行为及分离工艺条件进行系统研究,以期为碲渣清洁高效回收提供理论和工艺依据。
1 实验
1.1 原料
实验原料为国内某有色金属冶炼厂的碲渣,其来源于粗铋碱性精炼过程。将碲渣于110 ℃下干燥24 h,然后破碎至粒径小于74 μm。碲渣的化学组成如表1所列。根据表1可知,碲渣中铋、铅、铜、锑和碲的含量分别为35.40%、29.10%、13.70%、8.29%和6.38%(质量分数),具有较高的回收价值。碲渣的XRD谱如图1所示,其结果表明碲渣中晶相主要为Bi、PbSb2O4、PbTe和CuBi2O4。
表1 碲渣的化学组成
Table 1 Chemical composition of tellurium slag

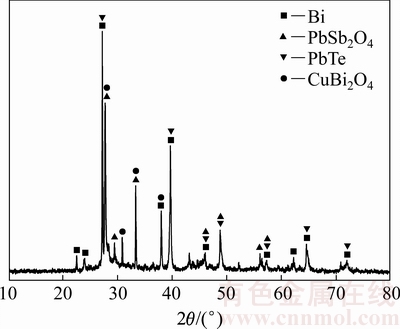
图1 碲渣的XRD谱
Fig. 1 XRD pattern of tellurium slag
1.2 实验原理
表2所列为常见氧化剂的氧化还原电位,由表2可见,·OH(羟基自由基)氧化还原电位为2.80 V,仅次于F2(2.87V),是水中氧化能力最强的氧化剂[17]。AOP能在反应体系中产生大量活性极强的·OH,而·OH的强氧化性能够打开碲渣的稳定结构[18-19],使Te、Cu、Bi等被释放而进入浸出液中,而锑和铅则生成相应的沉淀富集于浸出渣中。该过程发生的主要化学反应 如下:
2O3+H2O2→2·OH+3O2 (1)
PbTe+H2SO4+8·OH→PbSO4+H2TeO4+4H2O (2)
2Bi+3H2SO4+6NaCl+6·OH→2BiCl3+3Na2SO4+6H2O (3)
CuBi2O4+4H2SO4+6NaCl→CuSO4+2BiCl3+3Na2SO4+4H2O (4)
1.3 实验方法
量取一定体积已配制好的H2SO4和NaCl混合溶液加入700 mL高型烧杯中,将高型烧杯置于恒温水浴锅中加热,当加热到目标温度时,向溶液中加入40 g碲渣和一定体积的双氧水(质量分数为30%),其中双氧水通过恒流泵精确控制流速缓慢加入,然后再利用曝气头向溶液中通入臭氧。臭氧气体采用臭氧发生器制备,臭氧气体的流量通过流量计控制。反应结束后趁热过滤,取滤液进行检测,通过测定溶液中金属离子浓度判断金属的浸出率(R),计算公式如式(5)所示。
表2 常见氧化剂氧化还原电位
Table 2 Oxidation reduction potential of common oxidant

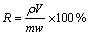 (5)
(5)
式中:R为金属的浸出率,%;ρ为金属离子浓度,g/L;V为溶液体积,L;m为碲渣质量,g;w为该金属在碲渣中的质量分数,%。
采用电感耦合等离子体(Optimal 5300DV,Perkin-Elmer Instruments)检测滤液中Te、Bi、Cu、Sb、Pb浓度。
2 结果与讨论
2.1 NaCl浓度的影响
图2所示为NaCl浓度对协同氧化浸出过程的影响。固定条件:H2O2体积分数15%、H2O2滴加速度 0.8 mL/min、H2SO4浓度1.84 mol/L、浸出温度30 ℃、浸出时间2 h、气体流速1 L/min、液固比10 mL/g。
由图2可见,NaCl浓度对碲、铜、铋的浸出率均有较大的影响。随着NaCl浓度的增大,碲和铜的浸出率均快速上升再趋于平缓。碲的浸出率增幅较铜更明显。铋的浸出率随着NaCl浓度的增大,先下降然后快速升高。随着NaCl浓度从0增加至0.75 mol/L,碲的浸出率由24.30%升高至68.34%,铜的浸出率由65.20%升高为88.88%,铋的浸出率由52.64%升高至74.50%。增加NaCl浓度能有效促进碲、铜、铋的浸出,其原因是Cl-具有较强的配位作用,在其作用下,碲、铜、铋与Cl-形成相应的配离子,而提高碲、铜、铋的浸出率[20-21]。另外,Cl-对碲的浸出具有较强催化作用[22-24]。锑、铅的浸出率则一直几乎为0,富集于浸出渣中。综上所述,确定NaCl浓度为0.75 mol/L。
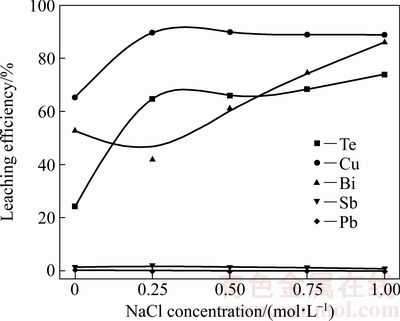
图2 NaCl浓度对各金属浸出率的影响
Fig. 2 Effect of NaCl concentration on leaching efficiency of metals
2.2 H2O2体积分数的影响
图3所示为H2O2体积分数对协同氧化浸出过程的影响。固定条件:NaCl浓度0.75 mol/L、H2O2滴加速度 0.8 mL/min、H2SO4浓度1.84 mol/L、浸出温度30 ℃、浸出时间2 h、气体流速1 L/min、液固比10 mL/g。

图3 H2O2体积分数对各金属浸出率的影响
Fig. 3 Effect of H2O2 volume fraction on leaching efficiency of metals
由图3可见,H2O2体积分数对碲、铜、铋的浸出率均有较大的影响。随着H2O2体积分数的增大,碲、铜和铋的浸出率均逐渐上升再趋于平缓。碲的浸出率增幅比铜和铋增幅更显著。随着H2O2体积分数从0增加至25%,碲的浸出率由44.35%升高至78.23%,铜的浸出率由84.86%升高至90.53%,铋的浸出率由68.69%升高至78.18%。H2O2体积分数的增大有利于在反应体系中产生更多活性高的·OH,更有效打开碲渣的稳定结构,促进碲、铜、铋等金属的浸出[25]。而锑、铅的浸出率则几乎为0。综上所述,确定H2O2体积分数为20%。
2.3 H2O2滴加速度的影响
图4所示为H2O2滴加速度对协同氧化浸出过程的影响。固定条件:NaCl浓度0.75 mol/L、H2O2体积分数20%、H2SO4浓度1.84 mol/L、浸出温度30 ℃、浸出时间2 h、气体流速1 L/min、液固比10 mL/g。
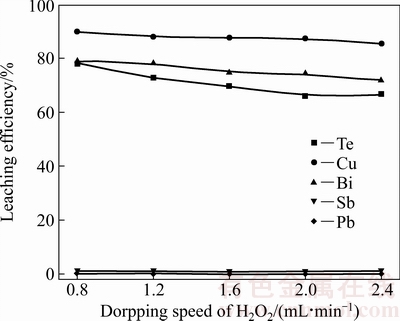
图4 H2O2滴加速度对各金属浸出率的影响
Fig. 4 Effect of dropping speed of H2O2 on leaching efficiency of metals
由图4可见,H2O2滴加速度对碲、铜、铋的浸出率影响较大。随着H2O2滴加速度的增大,碲、铜、铋的浸出率缓慢降低,而锑、铅的浸出率几乎为0。随着H2O2滴加速度由0.8 mL/min增加至2.4 mL/min时,碲的浸出率由78.23%降为66.53%,铜的浸出率由89.81%降为85.39%,铋的浸出率由79.00%降为71.78%。碲、铜、铋的浸出率缓慢降低,其原因是由于H2O2滴加速度增大,体系中在短时间内将产生大量的·OH,而·OH 寿命短,在体系中存在时间有限[26-28],导致部分·OH来不及与碲渣反应就消失,使碲、铜、铋等浸出率降低。综上所述,确定H2O2滴加速度为1.2 mL/min。
2.4 H2SO4浓度的影响
图5所示为H2SO4浓度对协同氧化浸出过程的影响。固定条件:NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度 1.2 mL/min、浸出温度30 ℃、浸出时间2 h、气体流速1 L/min、液固比10 mL/g。
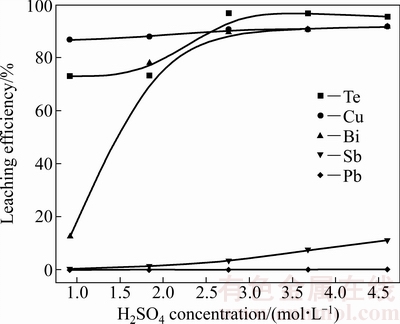
图5 H2SO4浓度对各金属浸出率的影响
Fig. 5 Effect of H2SO4 concentration on leaching efficiency of metals
由图5可见,H2SO4浓度对碲、铜、铋和锑的浸出率均有较大的影响。随着H2SO4浓度的增大,碲、铜、铋和锑的浸出率均快速上升再趋于平缓。其中铋的浸出率增幅较碲、铜、锑明显,而铅的浸出率几乎为0。随着H2SO4浓度由0.92 mol/L增加至2.76 mol/L,碲的浸出率由73.00%升高至96.80%,铜的浸出率由86.77%升高至90.62%,铋的浸出率由12.50%升高至89.70%,锑的浸出率由0.11%增加至3.20%。这是由于,根据勒沙特列原理,增加H2SO4浓度,有利于反应(1)~(4)的正向进行,有效促进碲、铜和铋的浸出。综上所述,确定H2SO4浓度为2.76 mol/L。
2.5 浸出温度的影响
图6所示为浸出温度对协同氧化浸出过程的影响。固定条件:NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度 1.2 mL/min、H2SO4浓度2.76 mol/L、浸出时间2 h、气体流速1 L/min、液固比10 mL/g。
由图6可见,浸出温度对铜和铋的浸出率均有较大的影响。随着浸出温度的升高,铜和铋的浸出率均逐渐下降再趋于平缓。而碲、锑和铅的浸出率几乎没有变化。随着浸出温度由30 ℃增加至90 ℃,铜的浸出率由93.71%降为90.49%,铋的浸出率由89.67%降为82.08%。而碲的浸出率一直维持在96%左右。浸出温度增大,铜、铋的浸出率反而降低,其原因是O3在水溶液中的溶解度随着温度的升高而降低][[29-30],以及H2O2的分解速度随着温度升高而加快[31]。锑的浸出率维持4%左右,铅的浸出率几乎为0。综上所述,,确定浸出温度为60 ℃。

图6 浸出温度对各金属浸出率的影响
Fig. 6 Effect of leaching temperature on leaching efficiency of metals
2.6 浸出时间的影响
图7所示为浸出时间对协同氧化浸出过程的影响。固定条件:NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度1.2 mL/min、H2SO4浓度2.76 mol/L、浸出温度60 ℃、气体流速1 L/min、液固比10 mL/g。

图7 浸出时间对各金属浸出率的影响
Fig. 7 Effect of leaching time on leaching efficiency of metals
由图7可见,随着浸出时间的延长,碲、铜和铋的浸出率逐渐升高然后趋于平缓,而锑和铅的浸出率几乎没有变化。随着浸出时间由1.5 h延长至2.5 h,碲的浸出率由95.00%升高为97.53%,铜的浸出率由90.45%升高为91.18%,铋的浸出率由79.83%升高为89.70%。延长反应时间有利于协同氧化过程进行更加彻底,但反应时间太长不利于生产,综上所述,确定反应时间为2.5 h。
2.7 气体流速的影响
图8所示为气体流速对协同氧化浸出过程的影响。固定条件:NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度 1.2 mL/min、H2SO4浓度2.76 mol/L、浸出温度60 ℃、浸出时间2.5 h、液固比10 mL/g。
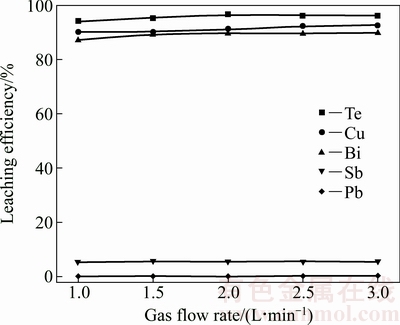
图8 气体流速对各金属浸出率的影响
Fig. 8 Effect of gas flow rate on leaching efficiency of metals
由图8可见,气体流速对碲、铜、铋、锑和铅等金属的浸出率影响较小。随着气体流速的增大,碲、铜和铋有少许的升高,但不明显,而锑和铅浸出率几乎不改变,维持在较小浸出率的水平而富集在浸出渣中。气体流速增大,不但能有效增加溶液中O3浓度,而且能对溶液进行搅拌,增加传质和传热,有利于各金属的浸出,但气体流速增大,导致O3逸出的速率加快,O3在溶液中停留时间变短,不利于·OH的生成。综合考虑,确定气体流速为2.5 L/min。
2.8 液固比的影响
图9所示为液固比对协同氧化浸出过程的影响。固定条件:NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度 1.2 mL/min、H2SO4浓度2.76 mol/L、浸出温度60 ℃、浸出时间2.5 h、气体流速2.5 L/min。
由图9可见,随着液固比的增加,铋的浸出率快速升高,当液固比由5 mL/g增大为10 mL/g时,铋的浸出率由44.70%升高为89.43%;随着液固比的增加,体系中液固两相的传质得到强化[32-33],有利于铋的浸出,而碲、铜、锑和铅的浸出率几乎没有变化。综上所述,确定液固比为10 mL/g。
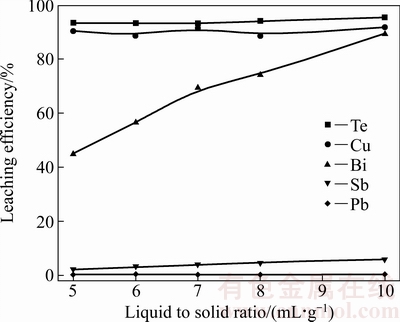
图9 液固比对各金属浸出率的影响
Fig. 9 Effect of liquid to solid ratio on leaching efficiency of metals
2.9 优化条件实验
通过以上的系列实验研究,可得出基于AOP协同氧化浸出碲渣中的碲和有价金属的优化工艺条件:NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度 1.2 mL/min、H2SO4浓度2.76 mol/L、浸出温度60 ℃、浸出时间2.5 h、气体流速2.5 L/min、液固比10 mL/g。在此优化条件下进行验证实验,实验结果如表3所列。
表3 优化条件实验结果
Table 3 Results of optimum experiment
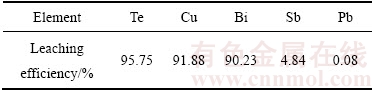
由表3可知,此优化条件下,碲、铜、铋等金属大部分进入浸出液中,碲、铜和铋的浸出率分别为95.75%、91.8%和90.23%,该浸出液可利用草酸沉铜→亚硫酸钠还原→水解沉铋分别回收其中的铜、碲和铋,而锑和铅浸出率较低富集于浸出渣中,浸出渣XRD谱如图10所示。
由图10可见,该浸出渣的主要物相为PbSO4,锑由于其含量较低,XRD谱中未能显示其物相。该浸出渣可利用硫化钠浸出分离锑,分锑渣再利用还原熔炼炼铅,实现锑和铅的回收。
据浸出液和浸出渣的特性,提出了碲渣的处理流程,如图11所示。
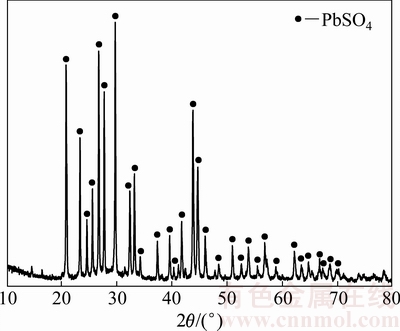
图10 浸出渣XRD谱
Fig. 10 XRD pattern of leaching residue
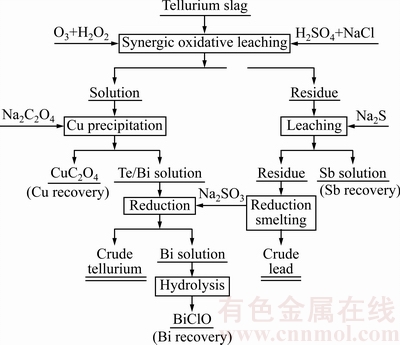
图11 碲渣回收的实验流程图
Fig. 11 Experimental flow sheet of tellurium slag recovery
3 结论
1) 基于AOP,在硫酸体系中氧化浸出碲渣中的碲和有价金属。AOP能在反应体系中产生大量活性极强的·OH,而·OH的强氧化性能够打开碲渣的稳定结构,使Te、Cu、Bi等被释放而进入到浸出溶中,而锑和铅则富集于浸出渣中,实现了碲渣中碲的高效浸出及有价金属的有效分离和富集。
2) 基于AOP协同氧化浸出过程的适宜条件为:NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度 1.2 mL/min、H2SO4浓度2.76 mol/L、浸出温度60℃、浸出时间2.5 h、气体流速2.5 L/min、液固比10 mL/g。在此条件下,碲、铜和铋的浸出率分别达95.75%、91.88%和90.23%,而锑和铅的浸出率仅分别为4.84%和0.08%。
3) 基于AOP协同氧化浸出过程中,NaCl浓度、H2SO4浓度、H2O2体积分数和液固比等因素对各金属浸出率影响显著,而H2O2滴加速度、浸出温度、浸出时间和气体流速等因数影响较小。
REFERENCES
[1] WANG Shi-jie. Tellurium, its resourcefulness and recovery[J]. JOM, 2011, 63(8): 90-93.
[2] 郑雅杰, 乐红春, 孙召明. 铜阳极泥处理过程中中和渣中碲的提取与制备[J]. 中国有色金属学报, 2012, 22(8): 2360-2365.
ZHENG Ya-jie, LE Hong-chun, SUN Zhao-ming. Extraction and preparation of tellurium from neutralization sludge of process for treatment of anode slime[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(8): 2360-2365.
[3] 袁武华, 王 峰. 国内外易切削钢的研究现状和前景[J]. 钢铁研究, 2008, 36(5): 56-57.
YUAN Wu-hua, WANG Feng. Present research status and prospects on free cutting steel at home and abroad[J]. Research on Iron & Steel, 2008, 36(5): 56-57.
[4] SICILIANO T, DI GIULIO M, TEPORE M, FILIPPO E, MICOCCI G, TEPORE A. Ammonia sensitivity of RF sputtered tellurium oxide thin films[J]. Sensors and Actuators B, 2009, 138(2): 550-555.
[5] TSIULYANU D, TSIULYANU A, LIESS H D, EISELE I. Characterization of tellurium-based films for NO2 detection[J]. Thin Solid Films, 2005, 485(1): 252-256.
[6] SHENAI-KHATKHATE D V, WEBB P, COLE-HAMILTON D J, BLACKMORE G W, MULLION J B. Ultra-pure organotellurium precursors for the low temperature MOVPE growth of II/VI compound semiconductors[J]. Journal of Crystal Growth, 1988, 93(1): 744-749.
[7] EL-MALLAWANY R A H. Tellurite glasses handbook: Physical properties and data[M]. New York: CRC press, 2011.
[8] 毛景文, 陈毓川, 周剑雄, 杨白川. 四川省石棉县大水沟碲矿床地质, 矿物学和地球化学[J]. 地球学报, 1995, 16(3): 276-290.
MAO Jing-wen, CHEN Yu-chuan, ZHOU Jian-xiong, YANG Bai-chuan. Geology, mineralogy, and geochemistry of the Dashuigou tellurium deposit in Shimian County, Sichuan[J]. Acta Geoscientia Sinica, 1995, 16(3): 276-290.
[9] 王少锋, 汪 琼, 杨静静, 张存磊, 席珍强. 碱浸提取碲的工艺研究[J]. 浙江理工大学学报, 2013, 30(2): 254-256.
WANG Shao-feng, WANG Qiong, YANG Jing-jing, ZHANG Cun-lei, XI Zhen-qiang. Study on process of tellurium extraction through alkaline leaching[J]. Journal of Zhejiang Institute of Science and Technology, 2013, 30(2): 254-256.
[10] FAN You-qi, YANG Yong-xiang, XIAO Yan-ping, ZHAO Zhuo, LEI Ying. Recovery of tellurium from high tellurium-bearing materials by alkaline pressure leaching process: thermodynamic evaluation and experimental study[J]. Hydrometallurgy, 2013, 139: 95-99.
[11] 陈昆昆, 郑雅杰. 采用H2SO4-H2O2溶液从含贵金属的富碲渣中选择性提取碲[J]. 稀有金属, 2013, 37(6): 946-951.
CHEN Kun-kun, ZHENG Ya-jie. Selective extracting Te from tellurium richen residue containing precious metals by H2SO4-H2O2 solutions[J]. Chinese Journal of Rare Metals, 2013, 37(6): 946-951.
[12] 张博亚, 王吉坤. 加压酸浸预处理铜阳极泥的工艺研究[J]. 矿冶工程, 2007, 27(5): 41-43.
ZHANG Bo-ya, WANG Ji-kun. The technological research on pre-treating copper anode slime with pressure acid leaching method[J]. Mining and Metallurgical Engineering, 2007, 27(5): 41-43.
[13] 方 锦, 王少龙, 付世继. 从碲渣中回收碲的工艺研究[J]. 材料研究与应用, 2009, 3(3): 204-206.
FANG Jin, WANG Shao-long, FU Shi-ji. Study on process of recovering tellurium from the tellurium slag[J]. Materials Research and Application, 2009, 3(3): 204-206.
[14] 万金泉, 朱应良, 马邕文, 王 艳, 黄明智, 韩东晖. SR-AOPs 深度处理制浆造纸废水的研究[J]. 中国造纸, 2015, 34(1): 1-5.
WAN Jin-quan, ZHU Ying-liang, MA Yong-wen, WANG Yan, HUANG Ming-zhi, HAN Dong-hui. Pilot study on advanced treatment of papermaking wastewater by SR-AOPs[J]. China Pulp & Paper, 2015, 34(1): 1-5.
[15] 湛雪辉, 李朝辉, 湛含辉, 李 飞, 曹 芬, 李 侠. 臭氧-过氧化氢联合浸出方铅矿[J]. 中南大学学报(自然科学版), 2012, 43(5): 1651-1655.
ZHAN Xue-hui, LI Zhao-hui, ZHAN Han-hui, LI Fei, CAO Fen, LI Xia. Combined leaching of galena by ozone-hydrogen peroxide solution[J]. Journal of Central South University (Science and Technology), 2012, 43(5): 1651-1655.
[16] 李 飞. 由方铅矿直接制备活性PbO粉体的新技术及基础研究[D]. 长沙: 长沙理工大学, 2011.
LI Fei. New technologies and basic research on active PbO powder prepared by the galena directly[D]. Changsha: Changsha University of Science & Technology, 2011.
[17] GUEDES A M F M, MADEIRA L M P, BOAVENTURA R A R, COSTA C A. Fenton oxidation of cork cooking wastewater—overall kinetic analysis[J]. Water Research, 2003, 37(13): 3061-3069.
[18] 李 进. 臭氧氧化过程中羟基自由基的氧化性能的研究[D]. 北京: 北京化工大学, 2007.
LI Jin. Oxidation susceptibility of hydroxyl radical during ozonation[D]. Beijing: Beijing University of Technology, 2007.
[19] 解明媛, 金鹏康, 王晓昌, 葛碧洲. 催化臭氧化反应体系中羟基自由基的产生及影响因素[J]. 水处理技术, 2008, 34(6): 30-32.
XIE Ming-yuan, JIN Peng-kang, WANG Xiao-chang, GE Bi-zhou. Formation and influence factors of hydroxyl radical in catalytic ozonation system[J]. Technology of Water Treatment, 2008, 34(6): 30-32.
[20] 丁风华. 氯盐体系下含铋溶液净化及氧化铋制备的工艺研究[D]. 长沙: 中南大学, 2014.
DING Feng-hua. Study of purifying bismuth containing solution and preparing Bi2O3 in the chloride system[D]. Changsha: Central South University, 2014.
[21] WEN Jun-jie, ZHANG Qi-xiu, ZHANG Gui-qing, CAO Zuo-ying. Deep removal of copper from cobalt sulfate electrolyte by ion-exchange[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1534-1540.
[22] LI Dong, GUO Xue-yi, XU Zhi-peng, FENG Qi-ming. Metal values separation from residue generated in alkali fusion-leaching of copper anode slime[J]. Hydrometallurgy, 2016, 165: 290-294.
[23] 郑雅杰, 陈昆昆, 孙召明. SO2还原沉金后液回收硒碲及捕集铂钯[J]. 中国有色金属学报, 2011, 21(9): 2258-2264.
ZHENG Ya-jie, CHEN Kun-kun, SUN Zhao-ming. Recycling Se and Te and capturing Pt and Pd from solution after precipitating gold by SO2 reduction[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2258-2264.
[24] 马玉天, 龚竹青, 陈文汨, 李宏煦, 阳征会, 黄 坚. 从硫酸溶液中还原制取金属碲粉[J]. 中国有色金属学报, 2006, 16(1): 189-194.
MA Yu-tian, GONG Zhu-qing, CHEN Wen-mi, LI Hong-xu, YANG Zheng-hui, HUANG Jian. Preparation of powdered-tellurium by reduction from sulfuric acid solution[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(1): 189-194.
[25] OH B T, SEO Y S, SUDHAKAR D, CHOE J H, LEE S M, PARK Y J, CHO M. Oxidative degradation of endotoxin by advanced oxidation process(O3/H2O2 & UV/H2O2)[J]. Journal of Hazardous Materials, 2014, 279: 105-110.
[26] 谢 刚. 基于H2O2的高级氧化技术降解不同水基质中痕量农药的研究[D]. 兰州: 兰州大学, 2015.
XIE Gang. Effectiveness of H2O2 based advanced oxidation processes for the degradation of trace pesticides in different water matrices[D]. Lanzhou: Lanzhou University, 2015.
[27] VINALS J, JUAN E, RUIZ M, FERRANDO E, CRUELLS M, ROCA A, CASADO J. Leaching of gold and palladium with aqueous ozone in dilute chloride media[J]. Hydrometallurgy, 2006, 81(2): 142-151.
[28] SOLIS MARCIAL O J, LAPIDUS G T. Chalcopyrite leaching in alcoholic acid media[J]. Hydrometallurgy, 2014, 147/148: 54-58.
[29] TIAN Qing-hua, WANG Heng-li, XIN Yun-tao, LI Dong, GUO Xue-yi. Ozonation leaching of a complex sulfidic antimony ore in hydrochloric acid solution[J]. Hydrometallurgy, 2016, 159: 126-131.
[30] 王华然, 王 尚, 李昀桥, 王福玉, 李迎凯, 尹 静. 臭氧在水中的溶解特性及其影响因素研究[J]. 中国消毒学杂志, 2009 (5): 481-483.
WANG Hua-ran, WANG Shang, LI Yun-qiao, WANG Fu-yu, LI Ying-kai, YIN Jing. Study on dissolving characteristics and influential factors of ozone in water[J]. Chinese Journal of Disinfection, 2009(5): 481-483.
[31] 张 清, 应超燕, 余可娜, 顾东虎, 肖海湖, 吴建一. 双氧水分解速率和稳定性研究[J]. 嘉兴学院学报, 2010, 22(3): 51-53.
ZHANG Qing, YING Chao-yan, YU Ke-na, GU Dong-hu, XIAO Hai-hu, WU Jian-yi. Study on deccomposition rate and stability of hydrogen peroxide[J]. Journal of Jinxing University, 2010, 22(3): 51-53.
[32] AYDOGAN S, ARAS A, CANBAZOGLU M. Dissolution kinetics of sphalerite in acidic ferric chloride leaching[J]. Chemical Engineering Journal, 2005, 114(1): 67-72.
[33] 张淑华, 李 涛, 朱炳辰, 朱子彬. 三相机械搅拌反应器气液传质[J]. 化工学报, 2005, 56(2): 220-226.
ZHANG Shu-hua, LI Tao, ZHU Bing-chen, ZHU Zi-bin. Gas-liquid mass transfer in three-phase mechanical agitated reactor [J]. Journal of Chemical Industry and Engineering (China), 2005, 56(2): 220-226.
Coordination oxidative leaching of tellurium and valuable metals from tellurium slag based on AOP
GUO Xue-yi, XU Zhi-peng, LI Dong, TIAN Qing-hua
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Cleaner Metallurgical Engineering Research Center, Nonferrous Metal Industry of China, Changsha 410083, China)
Abstract: Based on advanced oxidation process (AOP), tellurium slag, which came from basic refining of bismuth crude, was processed by coordination oxidative leaching in sulfuric acid. The effects of various parameters, such as concentration of NaCl and H2SO4, volume fraction of H2O2, dropping speed of H2O2, leaching temperature and time, gas flow rate and liquid to solid ratio on leaching behavior of Te, Cu, Bi, Sb and Pb were investigated. The optimal conditions were determined as follows: NaCl concentration of 0.75 mol/L, H2SO4 concentration of 2.76 mol/L, volume fraction of H2O2 of 20%, dropping speed of H2O2 of 1.2 mL/min, leaching temperature of 60 ℃ and leaching time of 2.5 h, liquid to solid ratio of 10. Under the optimal conditions, the leaching efficiencies of Te, Cu and Bi reach 95.75%、91.88% and 90.23%, while the leaching efficiencies of Sb and Pb are 4.84% and 0.08%, respectively, achieving efficient leaching of tellurium and separation and enrichment of valuable metals from tellurium slag relatively.
Key words: tellurium slag; advanced oxidation process; coordination oxidation; separation; enrichment
Foundation item: Project(51234009) supported by the National Natural Science Foundation of China; Project (2014DFA90520) supported by the International Cooperation Program of Ministry of Science of China; Project (2013A100003) supported by the Production, Teaching and Research program of Guangdong Province, China
Received date: 2016-12-08; Accepted date: 2017-05-12
Corresponding author: LI Dong; Tel: +86-731-88876255; E-mail: yejin013_18@163.com
(编辑 王 超)
基金项目:国家自然科学基金重点项目(51234009);科技部国际合作专项(2014DFA90520);广东省产学研项目(2013A100003)
收稿日期:2016-12-08;修订日期:2017-05-12
通信作者:李 栋,讲师,博士;电话:0731-88876255;E-mail:yejin013_18@163.com
摘 要:以粗铋碱性精炼产生的碲渣为原料,基于高级氧化技术(AOP),在硫酸体系中协同氧化浸出碲渣中的碲和有价金属,研究NaCl浓度、H2O2体积分数、H2O2滴加速度、H2SO4浓度、浸出温度、浸出时间、气体流速和液固比等工艺参数对碲、铜、铋、锑和铅等金属浸出行为的影响,确定最佳工艺参数。结果表明:在NaCl浓度0.75 mol/L、H2O2体积分数20%、H2O2滴加速度 1.2 mL/min、H2SO4浓度2.76 mol/L、浸出温度60 ℃、浸出时间2.5 h、气体流速2.5 L/min和液固比10 mL/g的优化条件下,碲、铜和铋的浸出率分别达95.75%、91.88%和90.23%,而锑和铅的浸出率仅分别为4.84%和0.08%,实现碲渣中碲的高效浸出及有价金属的有效分离和富集。
[1] WANG Shi-jie. Tellurium, its resourcefulness and recovery[J]. JOM, 2011, 63(8): 90-93.
[2] 郑雅杰, 乐红春, 孙召明. 铜阳极泥处理过程中中和渣中碲的提取与制备[J]. 中国有色金属学报, 2012, 22(8): 2360-2365.
[3] 袁武华, 王 峰. 国内外易切削钢的研究现状和前景[J]. 钢铁研究, 2008, 36(5): 56-57.
[8] 毛景文, 陈毓川, 周剑雄, 杨白川. 四川省石棉县大水沟碲矿床地质, 矿物学和地球化学[J]. 地球学报, 1995, 16(3): 276-290.
[9] 王少锋, 汪 琼, 杨静静, 张存磊, 席珍强. 碱浸提取碲的工艺研究[J]. 浙江理工大学学报, 2013, 30(2): 254-256.
[11] 陈昆昆, 郑雅杰. 采用H2SO4-H2O2溶液从含贵金属的富碲渣中选择性提取碲[J]. 稀有金属, 2013, 37(6): 946-951.
[12] 张博亚, 王吉坤. 加压酸浸预处理铜阳极泥的工艺研究[J]. 矿冶工程, 2007, 27(5): 41-43.
[13] 方 锦, 王少龙, 付世继. 从碲渣中回收碲的工艺研究[J]. 材料研究与应用, 2009, 3(3): 204-206.
[14] 万金泉, 朱应良, 马邕文, 王 艳, 黄明智, 韩东晖. SR-AOPs 深度处理制浆造纸废水的研究[J]. 中国造纸, 2015, 34(1): 1-5.
[15] 湛雪辉, 李朝辉, 湛含辉, 李 飞, 曹 芬, 李 侠. 臭氧-过氧化氢联合浸出方铅矿[J]. 中南大学学报(自然科学版), 2012, 43(5): 1651-1655.
[16] 李 飞. 由方铅矿直接制备活性PbO粉体的新技术及基础研究[D]. 长沙: 长沙理工大学, 2011.
[18] 李 进. 臭氧氧化过程中羟基自由基的氧化性能的研究[D]. 北京: 北京化工大学, 2007.
[19] 解明媛, 金鹏康, 王晓昌, 葛碧洲. 催化臭氧化反应体系中羟基自由基的产生及影响因素[J]. 水处理技术, 2008, 34(6): 30-32.
[20] 丁风华. 氯盐体系下含铋溶液净化及氧化铋制备的工艺研究[D]. 长沙: 中南大学, 2014.
[23] 郑雅杰, 陈昆昆, 孙召明. SO2还原沉金后液回收硒碲及捕集铂钯[J]. 中国有色金属学报, 2011, 21(9): 2258-2264.
[24] 马玉天, 龚竹青, 陈文汨, 李宏煦, 阳征会, 黄 坚. 从硫酸溶液中还原制取金属碲粉[J]. 中国有色金属学报, 2006, 16(1): 189-194.
[26] 谢 刚. 基于H2O2的高级氧化技术降解不同水基质中痕量农药的研究[D]. 兰州: 兰州大学, 2015.
[30] 王华然, 王 尚, 李昀桥, 王福玉, 李迎凯, 尹 静. 臭氧在水中的溶解特性及其影响因素研究[J]. 中国消毒学杂志, 2009 (5): 481-483.
[31] 张 清, 应超燕, 余可娜, 顾东虎, 肖海湖, 吴建一. 双氧水分解速率和稳定性研究[J]. 嘉兴学院学报, 2010, 22(3): 51-53.
[33] 张淑华, 李 涛, 朱炳辰, 朱子彬. 三相机械搅拌反应器气液传质[J]. 化工学报, 2005, 56(2): 220-226.


