
Recovering of copper with metallic aluminum
Nizamettin DEMIRK?RAN, Asim K?NK?L
Chemical Engineering Department, Faculty of Engineering, Inonu University, Malatya 44280, Turkey
Received 27 January 2011; accepted 5 May 2011
Abstract:
The cementation of copper ions from aqueous copper sulfate solutions by using spherical aluminum metal particles was examined. The effects of the experimental parameters on copper cementation were investigated and evaluated. Reaction rate increases with increasing copper concentration, reaction temperature, stirring speed and decreasing pH. It was observed that the reaction follows the first-order kinetics, and progresses according to the diffusion controlling step.
Key words:
recovering metal; cementation; copper; aluminum; kinetics;
1 Introduction
With rapid industrial development and greater use of chemical products, high grade ores have depleted. As a result, the hydrometallurgical methods have gained a great importance in recovering metallic values from industrial wastes and low grade ores [1-2].
Industrial waste solutions obtained after the chemical and hydrometallurgical processing contain toxic and/or precious metal ions. These solutions are frequently discharged directly to the environment. When the levels of hazardous metal ions in industrial effluents exceed the permissible levels, it will cause environmental pollutions, and natural life is negatively affected. Therefore, from both environmental and economical perspectives, recovering valuable and/or toxic metal from waste solutions and purification of electrolyte solutions are important process [2-6].
Different methods are employed to recover the metal or remove the undesired impurities from the leach or industrial waste solutions, such as neutralization with acid or base solutions, chemical precipitation, crystallization, solvent extraction, flotation, ion exchange, adsorption onto different adsorbents, reverse osmosis, chelating ligands, electrodialysis and electrowinning [1, 3, 5-7].
These methods have some drawbacks. For example, chemical precipitation requires extremely long settling time; ion exchange and adsorption process are slow and expensive, and may require frequent regeneration for adequate performance; reverse osmosis, electrodialysis, electrowinning and solvent extraction methods are very expensive, and have high operating costs [3-6].
Cementation is an important chemical process used in industry to precipitate and recover valuable metals from leach or waste solutions. Cementation method has some advantage, such as recovery of metals in essentially pure metallic form, simple control requirements, low energy consumption and in general low cost process. The main disadvantages of the technique are excess sacrificial metal consumption and redox potential of sacrificing metal [3, 5, 6].
Cementation reactions, known as metal displacement reactions or contact reduction reactions, are processes where a metal ion presented in a solution or melt is reduced to the metallic state with a more active metal placed in the solution or melt [3, 6, 8-10].
A cementation reaction can be described as
nAa++mB0→nA0+mBb+ (1)
where n, m, A, B, a+, and b+ represent the stoichiometric coefficients, the noble and reductant metals, and valences of the noble and reductant metal, respectively [3, 11].
Copper is one of the most prevalent and valuable metals used in industry. Copper cementation has been performed to remove Cu2+ ions from electrowinning or electroplating solutions, or recover copper from leach solutions [3, 9, 12].
Copper cementation has been studied by various researchers. This subject is still widely investigated. Iron, nickel, zinc, and aluminum have been used as the reductant metal in copper cementation reactions [2, 4-6, 9, 12-19].
2 Experimental
The cementation reactions were conducted in a temperature controlled and mechanically stirred jacketed glass reactor of 500 mL in volume. The mechanical stirrer had a digital controller unit, and its agitator shaft and blade (45 mm in diameter) were made of Teflon. Temperature control unit had a constant-temperature- circulator. The solution pH was adjusted by adding H2SO4 and NaOH solutions. The cementation solutions containing Cu2+ ions were prepared by using CuSO4·5H2O. Spherical aluminum particles (1.5-2.0 mm of average diameter) used in the experiments were prepared by using aluminum foil.
After 300 mL of Cu2+ containing solution was put into the glass reactor and brought to the desired reaction temperature, 1.5 times of stoichiometrically required metallic aluminum was added into the reactor, and the reactor content was stirred at predetermined stirring speed. The progress of the cementation reaction was followed by measuring the amounts of unprecipitated copper ions in the solution. Aliquots of 5 mL solution were withdrawn at regular intervals during cementation, and were immediately filtered using filter paper. The filtered samples were analyzed for copper ion content by ethylene diamine tetraacetic acid sodium salt (EDTA) titration using murexide as the indicator [9, 20].
The amounts of recovered or deposited copper were calculated according to difference between the initial (C1) and final (C2) copper concentrations of the solution. The fraction of cemented copper is x(Cu)=C2/C1.
3 Results and discussion
3.1 Effect of parameters on copper cementation
The effect of initial copper ion concentration on copper cementation was studied at 0.010, 0.025, 0.050, 0.100 mol/L, respectively. The temperature, stirring speed, and pH were 343 K, 450 r/min, and 1, respectively. The results are given in Fig. 1. It is observed that the precipitation ratio of metallic copper increases as the concentration of copper ion is increased.
The effect of pH on copper cementation was investigated at pH value of 1.0, 1.5, 2.0, respectively. The concentration of solution, stirring speed, and temperature in these experiments were 0.025 mol/L, 450 r/min, and 343 K, respectively. Figure 2 shows the results of the effect of pH. It is clear that the maximum cementation yield is obtained at pH of 1. At higher pH values, a decrease in the yield is observed.
In order to determine the effect of stirring speed on cementation reactions, the experiments were carried out at the concentration of 0.025 mol/L, temperature of 343 K, and pH value of 1. The effect of stirring speed was tested at 250, 350, 450 and 550 r/min, respectively. The results are given in Fig. 3. The results indicate that the cemented fraction of copper increases as the stirring speed is increased.

Fig. 1 Effect of initial concentration of copper on copper cementation
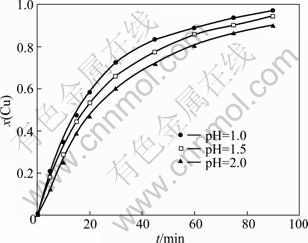
Fig. 2 Effect of pH value on copper cementation
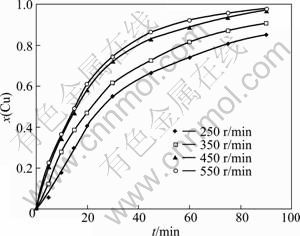
Fig. 3 Effect of stirring speed on copper cementation
The effect of temperature was examined at 313, 323, 333, 343, and 353 K, respectively. In these tests, the other parameters are chosen at the concentration of 0.025 mol/L, stirring speed of 450 r/min, and pH value of 1. The effect of temperature is given in Fig. 4. It shows that temperature has a significant effect on the acceleration of copper cementation.
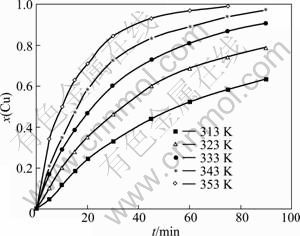
Fig. 4 Effect of temperature on copper cementation
3.2 Kinetic analysis
Cementation reactions are heterogeneous electrochemical reactions, and require a transfer of electrons between the dissolving and precipitating metals. Ions in the solution are reduced to zero valence on a solid metallic surface. Reaction steps are: diffusion of ions of the depositing metal to the deposit-solution interface from the bulk of the solution; conduction of electrons from the dissolving metal through the deposit; incorporation of the deposited metal atoms into a crystal lattice; release of the dissolving metal ions into solutions; transfer of the dissolving metal ions to the deposit-solution vicinity through the deposit layer; diffusion of the dissolving metal ions into the bulk of the solution [3].
The cementation reaction between copper ions containing solution and metallic aluminum particles occurs according to the following reaction:
3Cu2++ 2Al0→3Cu0+2Al3+ (2)
Due to the difference between the electrode potentials of the two metals, copper ions are easily reduced to its metallic state on aluminum metal surface. The standard reduction potentials of copper and aluminum are 0.34 and -1.67 V, respectively.
The half-cell reactions are
Cu2++2e![]() Cu0 (φ0=0.34 V) (3)
Cu0 (φ0=0.34 V) (3)
Al0![]() Al3++3e (φ0=1.67 V) (4)
Al3++3e (φ0=1.67 V) (4)
?φ0 of the reaction is positive (+2.01 V), and the standard Gibbs free energy ?G0 is to be negative (?G0=-nF?φ0). Therefore, a spontaneous heterogeneous reaction takes place through the galvanic cell.
Cementation occurs through a series of shorted electrochemical cells, in which electrons for reduction of Cu2+ are transferred from the cementation agent through the growing copper deposit. Aluminum, which supplies the electrons, is oxidized at anodic sites on their surface [12].
It is reported that cementation reactions follow the first-order kinetics [4, 9], and the rate-controlling step is the diffusion of the depositing metal ions to the reductant metal surface.
Kinetic analysis was performed according to the first-order kinetics in the present study. The model for the first-order reaction is
-ln[1-x(Cu)]=kt (5)
where x (Cu) is the cemented copper fraction at time t; k and t are the apparent rate constant and time, respectively. Cementation data obtained from the experiments were used to perform -ln[1-x(Cu)] versus time graphs. The results obtained from the graphs (apparent rate constant k and R2 values) are given in Table 1.
Table 1 Rate constants (k) and correlation coefficients (R2) values
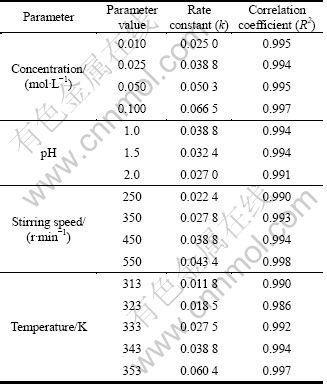
To determine the activation energy of cementation reaction, using the apparent rate constants obtained from the plot of -ln[1-x(Cu)] versus time for different temperatures (Table 1), an Arrhenius plot was drawn, and the activation energy of this process was calculated as 36.8 kJ/mol. This value of the activation energy indicates that the rate-controlling step of the reaction examined is the diffusion. In diffusion-controlled processes, the activation energy is mostly below 40 kJ/mol. The fact that the cementation rate is dependent on the stirring speed supports that the diffusion is the rate-controlling step.
3.3 Discussion on effects of parameters
One of the most important events in cementation reactions is whether deposit formed on the reductant metal surface is coherent. A coherent deposit formed on metal surface affects the reaction rate and controlling- step of reaction. The values of the parameters such as the concentration, temperature, stirring speed and pH affect the deposit formation and progression of reaction.
The removal of copper from solution involves two main processes, including adsorption of copper ions on the metallic aluminum surface and cementation of copper ions onto the metal. The presence of a passive oxide layer on aluminum is known, and it inhibits the reaction rate by creating a resistance to diffusion of copper ions on the metal surface. This oxide layer can be destroyed by acidity of medium. In this way, it is ensured a good contact between copper ions and metal surface, and the cementation rate enhances. Also, pH value of the process is an important economical issue for any cementation plant: corrosion damage of reactors, excess dissolution of the reducing metal and hydroxide precipitation [10]. When the pH value of solution is above a certain limit, hydroxides of copper ions and dissolved aluminum ions can form, and these species may cause a decreasing yield and grade of the precipitated copper. Strong acidic solutions prevent precipitation of hydroxides. Because of above reasons, we have used strong acidic solution in our work. The following side reaction, which represents the dissolution of aluminum, occurs in acidic reaction medium. Therefore, we have used excess aluminum in the experiments.
2Al0(s)+6H+(aq)![]() 2Al3+(aq)+3H2(g) (6)
2Al3+(aq)+3H2(g) (6)
In practice, the amount of aluminum consumed is always higher than the theoretical amount.
The rate of cementation reactions is proportional to the ion concentration in solution and the exposed metal area. The amount of metallic aluminum used in the experiments has been proportionally increased with increasing initial copper ion concentration, and it was observed that the cementation rate increased. When the amount of metal is increased, the effective surface area will be higher; and as a result, the amount of cemented copper will increase. Since the cemented copper accumulates on the surface of aluminum particles, a product layer occurs on the metal surface. This metallic layer depicts a resistance to the diffusion of copper ions through it. If the metallic layer formed is a coherent deposit, then the reaction rate can excessively decrease with increasing deposit mass. If the deposit is a porous layer, then the copper ions can diffuse towards the reducing metal surface through the porous deposit layer when the concentration of ions is high. In this situation, the thickness of the deposit layer increases, and the reaction rate can decrease. Since the cementation reactions are mostly diffusion controlled, the reaction rate can be accelerated by stirring. If the agitation of the solution is strong enough, the deposit formed can peel off from the metal surface. Also, agitation can facilitate the diffusion of ions through the deposit layer, and thus the cementation rate increases. It was determined that the reaction rate did not change at stirring speeds higher than 450 r/min. Examining the temperature effect on the cementation rate, it was observed that the copper deposit was not coherent on the reducing metal surface at temperatures higher than 333 K while was coherent at lower temperatures.
4 Conclusions
Recovering copper with aluminum particles by cementation reaction was investigated in this work. The effects of the parameters on cementation rate were examined and evaluated. It is determined that the activation energy is 36.8 kJ/mol, and the reaction rate is controlled by diffusion.
References
[1] JHA M K, KUMAR V, SINGH R J. Review of hydrometallurgical recovery of zinc from industrial wastes [J]. Resour Conserv Recy, 2001, 33: 1-22.
[2] CHANG F C, LO S L, KO C H. Recovery of copper and chelating agents from sludge extracting solutions [J]. Sep Purif Technol, 2007, 53: 49-56.
[3] NOSIER S A, SALLAM S A. Removal of lead ions from wastewater by cementation on a gas-sparged zinc cylinder [J]. Sep Purif Technol, 2000, 18: 93-101.
[4] BATOUTI M E. Cementation reactions in the presence of nitrogen compounds [J]. J Colloid Interf Sci, 2003, 263: 548-553.
[5] DIB A, MAKHLOUFI L. Cementation treatment of copper in wastewater: Mass transfer in a fixed bed of iron spheres [J]. Chem Eng Process, 2004, 43: 265-1273.
[6] FOUAD O A, ABDEL BASIR S M. Cementation-induced recovery of self-assembled ultrafine copper powders from spent etching solutions of printed circuit board [J]. Powder Technol, 2005, 159: 127-134.
[7] SIROLA K, LAATIKAINEN M, LAHTINEN M, PAATERO E. Removal of copper and nickel from concentrated ZnSO4 solutions with silica-supported chelating adsorbents [J]. Sep Purif Technol, 2008, 64: 88-100.
[8] POWER G P, RITCHIE I M. A contribution to the theory of cementation (metal displacement) reactions [J]. Aust J Chem, 1976, 29: 699-709.
[9] DEM?RKIRAN N, EKMEKYAPAR A, K?NK?L A, BAYSAR A. A kinetic study of copper cementation with zinc in aqueous solutions [J]. Int J Miner Process, 2007, 82: 80-85.
[10] NOUBACTEP C. Elemental metals for environmental remediation: Learning from cementation process [J]. J Hazard Mater, 2010, 181: 1170-1174.
[11] FARAHMAND F, MORADKHANI D, SAFARZADEH M S, RASHCHI F. Optimization and kinetics of the cementation of lead with aluminum powder [J]. Hydrometallurgy, 2009, 98: 81-85.
[12] KARAVASTEVA M. Kinetics and deposit morphology of copper cementation onto zinc, iron and aluminum [J]. Hydrometallurgy, 2005, 76: 149-152.
[13] NADKARNI, R M, JELDEN, C E, BOWLES, K C, FLANDERS, H E, WADSWORTH M E. A kinetic study of copper precipitation on iron-part1 [J]. Trans Metall Soc AIME, 1967, 239: 581-585.
[14] MACKINNON D J, INGRAHAM, T R. Kinetics of Cu(II) cementation on a pure aluminum disc in acidic sulphate solutions [J]. Can Metall Quart, 1970, 9: 443-448.
[15] MACKINNON D J, INGRAHAM T R, KERBY, R. Copper cementation on nickel disc [J]. Can Metall Quart, 1971, 10: 165-169.
[16] MACKINNON D J, INGRAHAM, T R. Copper cementation on aluminum canning sheet [J]. Can Metall Quart, 1971, 10: 197-201.
[17] MASSE N, PIRON, D L. Effects of temperature and powder morphologies on the cementation rate of copper in alkaline zinc solution [J]. J Electrochem Soc, 1994, 141: 664-669.
[18] KANUNGO M, MISHRA K G, DAS S C. Study on morphology of copper deposited onto aluminium by immersion plating from an oxalate bath containing perchloric acid [J]. Miner Eng, 2003, 16: 1383-1386.
[19] HUNG Y P, MOHAMED N, DARUS H. Recovery of copper from strong chloride-based solution [J]. J Appl Sci, 2005, 5: 1328-1333.
[20] G?LENSOY H. Kompleksometrinin esaslar? ve kompleksometrik titrasyonlar [M]. Fatih Yay?nevi, ?stanbul, 1984. (in Turkish).
采用金属铝置换回收铜
Nizamettin DEMIRK?RAN, As?m K?NK?L
Chemical Engineering Department, Faculty of Engineering, Inonu University, Malatya 44280, Turkey
摘 要:研究了采用球形铝金属粒子从硫酸铜水溶液中置换铜离子。考察了各实验参数初始铜离子浓度、反应温度、搅拌速度、溶液pH值等对铜置换反应的影响。结果表明,反应速率随着铜离子浓度、反应温度和搅拌速度的增加及pH的降低而提高。置换反应遵循一级动力学方程,其控制步骤为扩散控制。
关键词:金属回收;置换;铜;铝;动力学
(Edited by LI Xiang-qun)
Foundation item: Project (2008/55) supported by Inonu University Research Fund, Turkey
Corresponding author: Nizamettin DEMIRK?RAN; Tel: +90-422-3774760; E-mail: nizamettin.demirkiran@inonu.edu.tr
DOI: 10.1016/S1003-6326(11)61123-0
Abstract: The cementation of copper ions from aqueous copper sulfate solutions by using spherical aluminum metal particles was examined. The effects of the experimental parameters on copper cementation were investigated and evaluated. Reaction rate increases with increasing copper concentration, reaction temperature, stirring speed and decreasing pH. It was observed that the reaction follows the first-order kinetics, and progresses according to the diffusion controlling step.


