
Preparation and characterization of La-Co alloy nanowire arrays by electrodeposition in AAO template under nonaqueous system
GONG Xiao-zhong(龚晓钟)1, TANG Jiao-ning(汤皎宁)2, LI Jun-qing(李均钦)2, LIANG Yong-kang(梁永康)1
1. College of Chemistry and Chemical Engineering, Shenzhen University, Shenzhen 518060, China;
2. College of Material, Shenzhen Key Laboratory of Special Functional Materials, Shenzhen University,Shenzhen 518060, China
Received 10 July 2007; accepted 14 November 2007
Abstract:
La-Co alloy nanowires can be made in pulse reversal current(PRC) and direct current(DC) electrodepositions under nonaqueous system, with the porous anodic aluminum oxide(AAO) as template. This membrane is subject to the dual-oxidation (two-step) anodizing. Scanning electron microscope(SEM) examination shows that all of the nanowires have uniform diameter about 200 nm, and their diameters are determined by the pore diameter of applied AAO template. X-ray energy dispersion analysis indicates that the chemical composition of La and Co elements is very close to 1?2 in stoichiometry. X-ray diffraction pattern investigation demonstrates that La-Co nanowire is the face-centered cubic(FCC) LaCo13.
Key words:
La-Co alloy; nanowire arrays; electrodeposition; AAO template;
1 Introduction
Recently, nanosized materials, such as nanoparticle, nanowire and nanoarray have received much attention due to their extraordinary electronic[1], optical[2] and chemical[3] properties. In order to obtain nanostructure, several technologies, such as lithographic method and the bottom up approach, have been developed to promote the progress. The anodic aluminum oxide(AAO) template medium technique is currently employed to fabricate the regular and aligned nanoarrays[4-5]. The template synthesis for metal nanowires, pioneered by MARTIN et al[6], MOSKOVITS et al[7] and SEARSON et al[8], involves the deposition of metal into the cylindrical pores of an inert, nonconductive host material (such as porous Al2O3 films). In addition, the electrochemical deposition(ECD) is regarded as an inexpensive and easy method to synthesize material. The combination of AAO template and ECD is an efficient method to fabricate nanoarrays of metals[9]. Being conventionally functional materials, rare-earth(RE) compounds (hydroxides, oxides, rare earth alloy, etc.) have been widely used in various fields in the past decades, including high-performance luminescent devices, magnets and catalysts[10-11]. Lanthanum, one of the rare earth(RE) elements, has been applied successfully in many fields, such as metallurgy[12] and chemical engineering[13-14]. So far, many researches have been conducted or under way[15-19]. However, there is few published works about the template- electrodeposition preparation of La-Co alloy nanowire arrays in nonaqueous system. Metal compounds transferred from RE, such as LaCo5, SmCo5, Sm2Co17 and Nd2Fe14B, dominate the field of permanent magnets, which includes the high coercive fields iHc and/or maximum energy product (BH)max. Development of RE permanent magnet market will be described in this work.
Porous AAO template is a widely studied material, which is used for the fabrication of one-dimensional nanometer-scale structure as a medium template in recent years. The reason is that its morphology exhibits a lot of homogeneous parallel pores growing perpendicular to surface with a narrow distribution of diameter from 10 to 400 nm and its depth can be well controlled from several nanometers to several micrometers. It also exhibits good chemical inertia and fine physical stability. As a result, by combining its properties with the electrochemistry, vacuum, electroplating and chemical aggregation synthesized technology, the template can be widely applied in controllably ordered growth and assembling techniques of various nanowires and nanotubes[20].
In this work, the new method was adopted, i.e. the growth of La-Co alloy nanowire arrays in AAO template was introduced in means of template-electrodeposition under carbamide-NaBr-KBr-formamide of low- temperature molten salt system. The structure and morphology of the nanowires were characterized and discussed based on the initial research on the magnetic performance of La-Co alloy nanowires.
2 Experimental
2.1 Preparation of AAO template
The high purity aluminum (99.999%) plate was cut into pieces of 1 cm×2 cm. The aluminum pieces were subject to special treatment. A mirror surface was achieved by electropolishing in the solution with H2ClO4 to alcohol ethylic volume ratio in 1?3 at room temperature[21].
Two-step anodizing method was used to produce an ordered porous aluminum oxide layer on the surface of the aluminum plate. The interval between anodic alumina porous, or called as the cell size, was controlled by the voltage supplied for anodization. In this experiment, a 35 V DC voltage was applied between the AAO template working electrode and graphite counter electrode in 0.3 mol/L H2C2O4 aqueous solution for 1 h at room temperature. The precursor template was then rinsed in distilled water and the oxide layer were dissolved in the mixed acid of 6% H3PO4 (mass fraction) and 1.5% CrO3 (mass fraction) for 1 h at room temperature.
The second anodizing step was performed in the same experimental condition as the first step (temperature, electrolyte concentration and anodizing voltage). The template membrane was separated from the Al substrate by immersing the treated sheet in saturated HgCl2 solution. The membrane was rinsed with distilled water and then immersed in 5% H3PO4 solution for about 30 min at 30 ℃ to dissolve the barrier-type part on the bottom of nanoholes. The hole in AAO template was about 200 nm in diameter and 10 mm in depth after this treatment. Finally, a platinum film was deposited by vacuum evaporation onto one surface of the AAO template so as to provide a conductive contact.
2.2 Preparation of La-Co alloy nanowire arrays
The La-Co alloy nanowire arrays were fabricated through electrodeposition using AAO template of PRC and DC[22]. The electrolyte and electrodeposition parameters are listed in Table 1. A graphite rod was used as the counter electrode and the Pt/AAO.
Table 1 Electrolyte and electrodeposition parameters

Template was used as working electrode. The electrodeposition was normally conducted for 30 min for the La-Co alloy nanowire growth, and then the deposited specimen was cleaned with deionized water to remove the remaining contaminants. Finally, the free-standing La-Co alloy nanowire arrays were obtained by removing the AAO template with NaOH solution.
2.3 Characterization of La-Co alloy nanowire arrays
The phases of La-Co alloy nanowire were identified by the X-ray diffractometry (XRD, X’PERT PRO) using Cu Kα radiation. The morphology of the AAO templates and La-Co alloy nanowire arrays was observed by scanning electron microscopy (SEM, JEOL JSM-5910LV). The chemical composition of the nanowires was determined with energy dispersive X-ray spectrometer (EDS, Oxford Instruments 7274).
3 Results and discussion
3.1 Mechanism of two-step anodizing method with AAO template
In the process of aluminum anodizing, the formation of the oxidized-alumina porous membrane on the metal aluminum is the interactive result by the chemical dissolution and the electrochemical dissolution under the function of electric field, and it is a complicated process involving physical, chemical, electrochemical and other disciplines. The said two-step refers to the oxidization for two times. Firstly, the aluminum plates are subject to oxidization under certain
conditions for a certain period and the alumina membrane is obtained, as shown in Fig.1(a). From the self- organized mode, if oxidation duration is longer, the nanoholes will be arrayed more regularly. This is because the growth of nanoholes is interacted by the vertical electrical-field force of outside electric field and the horizontal electric-field force repelling each other among the HC2O4- groups in the electrolyte, and consequently is in the disorder state. If oxidation time is long enough, together with the rise of temperature and increasing the thermal movement of HC2O4- and other factors, HC2O4- in the nanoholes is inclined for even distribution, which, actually, can weaken the orientation of horizontal electric field and enable the growth of nanoholes from disorder to order state. Near to the barrier layer (“c” in Fig.1), the nanoholes will be arrayed more regularly. In the aluminum anodizing process, if the vertical growth rate of nanoholes is converging to the dissolving rate of AAO membrane in electrolyte, the thickness of porous layer will become unvaried. If the oxidation is stopped, and the alumina membrane is eliminated by the dissolution in released liquid or by reverse-stripping method[23], the entire AAO membrane will make level shift to the aluminum base body. We can have the aluminum, as shown Fig.1(b). The orderly nano-traces at the plane of barrier layer “c” can be kept completely[24]. At Last, the oxidation for second time will be conducted under identical conditions. The nanoholes will continue growing alongside the original sequences and traces and the orderly nanohole arrays will be acquired. Obviously, if the duration for the fist-time oxidation is longer, the nanohole array obtained by means of dual-oxidation will be more orderly.

Fig.1 Process of two-step anodizing oxidation
Fig.2 shows the SEM images of the as-anodized and prolonged AAO templates. The diameter of the pores in the as-anodized template is about 50 nm, and is enlarged to about 200 nm for 30 min. As the time prolongs, the diameter of the pores increases, but the distance between the pore centers does not change, i.e. about 200 nm.
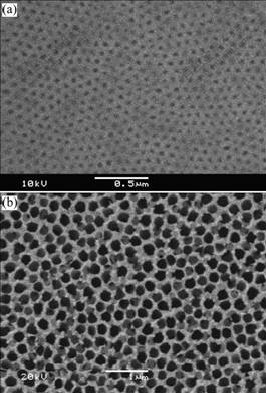
Fig.2 SEM images of AAO templates with interpore distance of 200 nm: (a) As-anodized; (b) Anodized for 30 min
3.2 Surface morphology and microstructure
Fig.3 shows the surface and cross-section morphologies of the DC and PRC La-Co alloy nanowire. It is found that, compared with the DC La-Co alloy nanowire (Fig.3(a)), the PRC La-Co alloy nanowire (Fig.3(b)) appears finer and compacter.

Fig.3 Surface and cross-section morphologies of La-Co alloy nanowires (SEM): (a) DC La-Co alloy nanowires; (b) PRC La-Co alloy nanowires
Fig.3 shows the typical SEM image of La-Co alloy nanowires prepared by electrodeposition in AAO template with pore diameter of about 200 nm. It can be seen that the average diameter of the nanowires is about 200 nm, which closely corresponds to the diameter of the pores in the used AAO. It can also be seen from Fig.3 that the length of the uniform nanowires is about 10 mm, and the quantity of the nanowires is very huge.
The results of the present experiment show that the means of PRC could give fine and long nanowires. The results may be attributed to increased electrochemical polarization of cathode during the on-time of pulse current period, which decreases the nucleation energy of the metal deposition on the electrode surface and increases nucleation rate. As a consequence of the high nucleation, it leads to the increase of the number of nucleation centre. In addition, in the process of PRC, the alternative of positive current and reversal current can make the surface state of electrodes change, such as adsorption phenomena, surface diffusion layer structure and electrical double layer structure, which results in the clearing and activation of the metallic surface in turn and is favorable to improve the purity of the composite[25-26]. Consequently, the texture of PRC nanowires possesses fine length and compact microstructure.
The principle that RE raises the electrodeposition rate of metal[27] can well explain the fact that the La-Co alloy is more inclined to form and more compacted once the RE La is added. To those RE elements (La, Ce, etc.) that lie at the Third Deputy Communities in Periodic Table, as their 4f electron does not tightly enclose the atomic nucleus and their screening factor is less than that of the other electrons with same principal quantum number, the effective nuclear charge number is large and strong adsorption capacity is demonstrated. When an appropriate amount of them are added into the plating liquid, firstly, they can make preferential adsorption defects on the surface of base body, thus lowering the substrate surface energy, improving the nucleation rate and accelerating the deposition; secondly, the RE, if it appears in the form of positive ions, can act as a catalyst to accelerate the decomposition of main salt CoCl2 and the reduction of Co2+; thirdly, the RE and some transition metal ions can reduce mutual activity and increase solubility each other, thus, it can carry with the deductive deposition of fundamental metal ions on the surface of base body; fourthly, the RE ions can also form complex with other organic or inorganic coordinating substance, consume some of the complex bases of complexant, and enlarge the concentration of main salt’s metal irons dissociative in the plating liquid, hence increase the electric potential difference of interface, promote the transition of active particles to the surface of base body and speed up the sedimentation rate.
3.3 XRD analysis
The X-ray diffraction pattern for the sample is shown in Fig.4. The diffraction peaks at 2θ=27.334?, 38.957?, 47.568?, 50.915?, 61.164?, 70.417? and 76.515? correspond to the (222), (422), (531), (620), (642), (660) and (753) diffraction peaks of face centered lattice- LaCo13 crystalline, respectively.
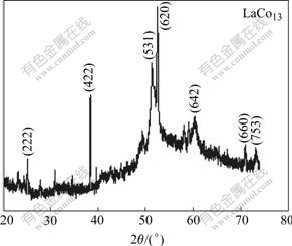
Fig.4 XRD pattern for sample consisting of La-Co alloy nanowires and AAO template
3.4 Energy disperse spectroscopy(EDS) analysis
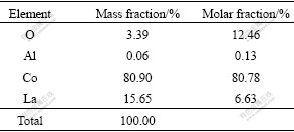
The chemical composition of the La-Co alloy nanowires is determined by energy dispersive X-ray spectrometer(EDS). The EDS spectrum, shown in Fig.5, reveals that the nanowires contain Co and La. Quantitative analysis results, given in Table 2, indicate that the composition of the nanowires is 21.63% La and 50.78% Co, which is very close to 1?2 of molar ratio. As the samples used in the EDS analysis can not be applied with vinyl alcohol protection film and the installation of samples must be done in the air, the surface of samples is oxidized partially (below 3.5%), while a few of aluminum (0.06%) residual is caused by the AAO template.

Fig.5 EDS spectrum of La-Co alloy nanowires prepared by template electrodeposition in AAO
Table 2 Component of AAO template deposited La-Co alloy
3.5 Magnetic property of La-Co alloy nanowires
The magnetism of La-Co alloy nanowires are obtained from hysteresis loops measured with the magnetic field parallel to the wire axis. The testing conditions are as follows: sample sensitivity 2 mV, corresponding voltage 5 410 mV; sample sensitivity 500 mV, corresponding voltage 101.796 mV; at room temperature (as shown in Fig.6). The perfect symmetrical loop is displayed. The saturated magnetization intensity (Ms), coercive force(Hc) and the maximum energy product (BH)max are 129.63 Am2/kg, 0.010 8 T and 865 J/m3, respectively. This kind of rare earth alloy nanowire prepared from low temperature melting salt bath could be expected in same application as magnetic materials.
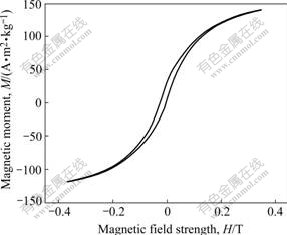
Fig.6 La-Co alloy settled layer hysteresis loop
4 Conclusions
1) We have successfully prepared the ordered and free-standing La-Co alloy nanowire arrays by electrodeposition method in the solution of urea-NaBr-KBr-formamide system using AAO templates.
2) The composition and crystal structure of nanowires can be controlled by pulse reversal electro- deposition(PRC) and direct current electrodeposition (DC). SEM and XRD results indicate that the diameter and length of the electrodeposited La-Co alloy nanowires are closed to those of the pores in used AAO template, around 200 nm. The obtained La-Co alloy nanowires are of face-centered lattice LaCo13 crystalline.
3) Both qualitative and quantitative EDS analysis have been made on the samples and the findings tell us that the sample contains 21.63% La and 50.78% Co (molar fraction), and the mole ratio between two elements is nearly 1?2.
4) The hysteresis loop of La-Co alloy can tell us that the saturation reaches when the field strength is 0.35 T, the saturated magnetic moment is 129.63 Am2/kg, the maximum energy product (BH)max is 865 J/m3 and the coercive force is 0.010 8 T.
References
[1] COBDEN D H. Nanowires begin to shine [J]. Nature, 2001, 409: 32-33.
[2] GOODSON T, VARNAVSKI O, WANG Y. Optical properties and applications of dendrimer-metal nanocomposites [J]. International Reviews in Physical Chemistry, 2004, 23(1): 109-150.
[3] KAMAT P V. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles [J] J Phys Chem B, 2002(106): 7729-7744.
[4] MARTIN C R. Nanomaterials: A membrane-based synthetic approach [J]. Science, 1994, 266(5193): 1961-1966.
[5] MASUDA H, FUKADA K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina [J]. Science, 1995, 268(5216): 1466-1468.
[6] SAPP S A, MITCHELL D T, MARTIN C R. Using template-synthesized micro- and nanowires as building blocks for self-assembly of supramolecular architectures [J]. Chem Mater, 1999, 11(5): 1183-1185.
[7] ROUTKEVITCH D, BIGIONI T, MOSKOVITS M, XU J M. Electrochemical fabrication of CdS nanowire arrays in porous anodic aluminum oxide templates [J]. J Phys Chem, 1996, 100(33): 14037-14047.
[8] SUN L, SEARSON P C, CHIEN C L. Finite-size effects in nickel nanowire arrays [J]. Phys Rev, 2000, B61: R6463-R6466.
[9] LEE Y H, LEU I C, WU Min-tao, YEN J H, FUNG K Z. Fabrication of Cu/Cu2O composite nanowire arrays on Si via AAO template-mediated electrodeposition [J]. Journal of Alloys and Compounds, 2007, 427(1/2): 213-218.
[10] TOMOTA M, MURAKAMI M. High-temperature superconductor bulk magnets that can trap magnetic fields of over 17 Tesla at 29K [J]. Nature, 2003, 421: 517-520.
[11] SCHAPPACHER M, FABRE T, MINGOTAUD A F, SOUM A. Preparation of a new nerve guide through controlled random copolymerization using rare earth catalysts [J]. Biomaterials, 2001, 22(21): 2849-2855.
[12] VAN DEUN R, BINNEMANS K. Lanthanide containing Schiff's base complexes with chloride counter-ions: Mesomorphic properties [J]. Materials Science and Engineering C, 2001, 18(1/2): 211-215.
[13] ZHANG S Y, GAO F M, WU C X. Chemical bond properties of rare earth ions in crystals [J]. Journal of Alloys and Compounds, 1998, 275/277: 835-837.
[14] HUANG Yun-hui, XU Zhi-gang, YAN Chun-hua, WANG Zhe-ming, ZHU Tao, LIAO Chun-sheng, GAO Song, XU Guang-xian. Soft chemical synthesis and transport properties of La0.7Sr0.3MnO3 granular perovskites [J]. Solid State Commun, 2000, 114: 43-47.
[15] ARENAS M A, DE DAMBORENEA J J, MEDRANO A, GARC?AB J A, RODR?GUEZ R. Corrosion behaviour of rare earth ion-implanted hot-dip galvanised steel [J]. Surface and Coatings Technology, 2002, 158/159: 615-619.
[16] HAUGSRUD R. On the effects of surface coatings on the high-temperature oxidation of nickel [J]. Corrosion Science, 2003, 45(6): 1289-1311.
[17] WANG K L, ZHANG Q B, SUN M L, WEI X G, ZHU Y M. Rare earth elements modification of laser-clad nickel-based alloy coatings [J]. Applied Surface Science, 2001, 174(3/4): 191-200.
[18] YI W, ZHENG C, FAN P, CHENG S, LI W, YING G. Effect of rare earth on oxidation resistance of iron base fluxing alloy spray-welding coating [J]. Journal of Alloys and Compounds, 2000, 311(1): 65-68.
[19] ZHANG Li-min, SUN Dong-bai, YU Hong-ying. Characteristics of plasma cladding Fe-based alloy coatings with rare earth metal elements [J]. Materials Science and Engineering A, 2007, 452/453: 619-624.
[20] LI Meng-ke, LU Mei, WANG Cheng-wei, LI Hu-lin. Preparation of well-aligned carbon nanotubes/silicon nanowires core-sheath composite structure arrays in porous anodic aluminum oxide templates [J]. Science in China (Series B), 2002, 45(4): 435-444.
[21] SAEDI A, GHORBANI M. Electrodeposition of Ni-Fe-Co alloy nanowire in modified AAO template [J]. Materials Chemistry and Physics, 2005, 91(2/3): 417-423.
[22] CHANG L M, ANA M Z, SHI S Y. Microstructure and characterization of Ni-Co/Al2O3 composite coatings by pulse reversal electrodeposit [J]. Materials Chemistry and Physics, 2006, 100: 395-399.
[23] YAKOVLEVA N M, YAKOVLEV A N, CHUPAKHINA E A. Structural analysis of alumina films produced by two-step electrochemical oxidation [J]. Thin Solid Films, 2000, 366(1/2): 37-42.
[24] MASUDA H, YAMADA H, SATOH M, ASOH H. Highly ordered nanochannel-array architecture in anodic alumina [J]. Appl PhysLett, 1997, 71(19): 2770-2772.
[25] MARLOT A, KERN P, LANDOLT D. Pulse plating of Ni-Mo alloys from Ni-rich electrolytes [J]. Electrochimica Acta, 2002, 48(1): 29-36.
[26] YIN K M, JAN S L. Current pulse plating of nickel-iron alloys on rotating disk electrodes [J]. Surface and Coatings Technology, 1996, 79(1/3): 252-262.
[27] GUO Zhong-cheng, GUO Shu-xian. Current status of electro- deposited multi-element composite coatings [J]. Electroplating & Pollution Control, 2001, 21(2): 4-12.
Foundation item: Project(04011311) supported by the Natural Science Foundation of Guangdong Province, China; Project(2006B14001001) supported by the Science and Technology Plan of Guangdong Province, China; Project(50471108) supported by the National Natural Science Foundation of China
Corresponding author: GONG Xiao-zhong; Tel: +86-755-26536141; E-mail: hxgxz@szu.edu.cn; cici_gxz@163.com
(Edited by LI Xiang-qun)


