- Abstract:
- 1 Introduction▲
- 2 Materials and methods▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Plots of adsorption vs pH for fluoride ion adsorption onto Zr-loaded garlic peel particles
- Fig.2 Plot of adsorption of fluoride anion onto Zr-loaded garlic peel powder in solutions with different co-existing anions (pH=3.0)
- Fig.3 Isotherm adsorption plots of fluoride onto Zr-loaded garlic peel sorbent
- Fig.4 Langmuir isotherm model for adsorption of fluoride onto Zr-loaded garlic peel sorbent
- Fig.5 Adsorption of fluoride anion onto Zr-GP sorbent at different contact time (pH=3.0)
- Fig.6 Pseudo-second-order plot of fluoride adsorption kinetics on Zr-loaded garlic peel sorbent (pH=3.0)
J. Cent. South Univ. Technol. (2011) 18: 1448-1453
DOI: 10.1007/s11771-011-0860-x![]()
Removal of fluoride from aqueous solution onto Zr-loaded garlic peel (Zr-GP) particles
HUANG Kai(黄凯)1, SHAO Jiu-gang(邵久刚)1, ZHU Hong-min(朱鸿民)1, Inoue Katsutoshi2
1. School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing,Beijing 100083, China;
2. Department of Applied Chemistry, Saga University, Saga 840-8502, Japan
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract:
Garlic peel, as the raw material, was modified by loading with zirconium(IV), exhibiting quite good uptaking behaviour for fluoride anion. The adsorption experiments were carried out in batch shaking vessels, and the process was strongly dependent on the pH value. The adsorption fits Langmuir model well, and the maximum adsorption capacities at equilibrium pH 2 and 6 are evaluated to be 1.10 and 0.89 mol(fluoride)/kg of Zr-loaded garlic peel gel, respectively. The evaluation of effects of coexisting anions such as nitrate, sulfate and phosphate shows that nitrate and sulfate have no negative effect on the adsorption of fluoride, while phosphate has a little effect. Adsorption kinetics of fluoride is well described by pseudo-second-order rate equation, and the corresponding adsorption rate constant is calculated to be 3.25×10-3 g/(mg·min).
Key words:
bio-adsorption; fluoride removal; zirconium-loaded garlic peel; aqueous solution;
1 Introduction
Water contamination by fluoride is a major concern in many places of the world. When its concentration in drinking water is more than 1.5 mg/L, which is the maximum allowable concentration of fluoride by the World Health Organization (WHO), it can become harmful to people’s health, for example, causing dental or skeletal fluorosis [1-2]. In most places of China, the problem of excessive fluoride in drinking water is particularly serious, where highly endemic diseases widespread [3]. So, it is quite urgent to develop more advanced and cost-effective techniques to decrease and remove fluoride from the water.
Several defluoridation technologies of drinking water, such as ion exchange, precipitation-coagulation, reverse osmosis, electrodialysis and nanofiltration, have been developed for fluoride removal from water [4]. Ion exchange is a reliable and simple way to remove fluoride from water while its selective uptaking for fluoride is usually not so satisfactory for water because the coexisting anions such as sulfate, carbonate, phosphate and OH- in the real drinking water may impede the effective stabilization of fluoride onto the resins [5]. Since the resins are synthesized by using the limited amount of fossil oil as the preliminary materials, which is expensive and not naturally degradable, it is not a good treatment choice in the present stage when the establishment of a sustainable society becomes the endeavor target of mankind. Precipitation-coagulation process is a good way to remove fluoride from the water [6], for its easy operation, low cost and extensive application, while one of its dominant disadvantages is its difficulty to decrease the equilibrium concentration of fluoride in water below the allowable limit level regulated by WHO, so it can only be employed as one of the primary unit operations in the practical water treatment. Moreover, the produced mud still has the potential to cause secondary pollution and hazard. As for other methods such as reverse osmosis [7], electrodialysis [8], and nanofiltration [9], they all exhibit excellent purification capacity for fluoride removal, but expensive operation cost also limits their extensive application, especially for the wide countryside areas.
In comparison, adsorption is one of the most popular purification methods due to its high selectivity, low cost, high efficiency and easy post-treatment after adsorption. Recently, a series of low-cost adsorbents derived from agricultural waste or natural materials [10-11] have been investigated intensively for toxic substances removal from aqueous solutions, such as apple and orange juicing residue, waste tea, peanut hull, rice straw, and coconut hull. Several adsorbents special for the removal of fluoride anion from the aqueous solution have been developed, namely activated alumina, bone char, calcite, clay, zeolite, rare metal-loaded resins, etc [4,12-13]. Activated alumina is quite effective for the removal of fluoride, while the leaked Al3+ in the drinking water may bring about senile dementia for people. Bone char is usually applied to the purification of fluoride and arsenic, but the source of this material is not so abundant. As for calcite, clay and zeolite, their cost-effectiveness still needs to be improved. In contrast to these above-mentioned adsorbents, the rare metal- loaded adsorbents exhibit fairly good adsorption properties for their selective affinity to fluoride anion, high adsorption capacity, easy operation and harmless characteristic, and draw the interests of more and more researchers. For this kind of adsorbent, it is significant to choose the suitable matrix materials used for loading of rare metals and consequently affect the adsorption behavior for fluoride anion directly. The biomass produced in the agricultural industry is one of the most promising candidates for this application for its effectiveness, availability and abundance. And in this research aspect, orange juicing residue has been used as the matrix material to load rare metal ions, showing quite excellent adsorption properties for anions including fluoride, arsenic and phosphate [14-15]. While for the wide application of this technique, more biomass should be sieved to develop highly cost-effective adsorbents in fluoride purification if taking into account of the serious reality of fluoride pollution.
In this study, a bioadsorpbent was developed for fluoride removal with a kind of easily available agricultural waste, garlic peel (GP), which can be an alternative to the conventional costly defluoridation products such as ion exchange resins and electrodialysis. In China, huge amount of garlic is consumed every year, and lots of peel is disposed, causing a severe problem in the community. So, for the environmental interest, this biowaste could be low-cost adsorbent and remove fluoride from water. The aim of the present study is to evaluate the feasibility of using garlic waste for fluoride removal from aqueous solution and the effect of common experimental parameters, such as the initial pH value of the solution, equilibrium concentration of fluoride, contact time, and coexisting anions.
2 Materials and methods
2.1 Chemicals and solutions
All the chemical reagents used in the present study were supplied by the National Chemical Reagents Company, Beijing, China. Fluoride working solutions were prepared from sodium fluoride by dissolving it in deionized water. Aqueous solutions of zirconium(IV) were prepared by dissolving analytical grade zirconium oxychloride octahydrate (ZrOCl2·8H2O) in 0.1 mol/L hydrochloric acid. Analytical grade sodium chloride, sodium nitrate, sodium sulfate and sodium phosphate were used to prepare 1.0 mol/L stock solutions, and mixed at an arbitrary volume ratio to adjust the concentration of the coexisting anions in the solution. Analytical grade sodium citrate and sodium nitrate were employed to prepare the total ion strength adjusting buffer (TISAB) solution for the pretreatment of all sampling solutions before testing by using ion selective electrode of fluoride.
2.2 Preparation of saponified GP gel
The garlic peel used in this study was obtained from the local market of Jianxiangqiao open fair in Beijing. It was pretreated and activated according to the similar process of saponified orange waste as reported [10,14]: About 50 g of garlic peel was taken along with 4 g of NaOH in a juice mixture and crushed into fine particles, and then was transferred into a beaker. A certain amount of deionized water was added, and the suspension was stirred for 24 h under intensive magnetic stirring at room temperature in order to facilitate the saponification. The pH value of the suspension was maintained at around 12.0 by adding NaOH solution. After stirring, the suspension was washed repeatedly by deionized water until neutral pH by filtration, and the obtained wet gel was dried in an air convection oven for 12 h at 60 °C. After above treatment, the smell of garlic peel became very weak, and then this dried gel was further used for zirconium loading treatment.
2.3 Preparation of Zr(IV)-loaded GP gel
In order to load zirconium, the preparation experiment was carried out according to the prescription reported [14-15], i.e., 5 g of above saponified GP gel was mixed with 500 mL of 0.1 mol/L zirconium solution at pH 1.8 for 24 h. The gel was then filtered and washed with deionized water until neutral pH, and dried in an air convection oven for 12 h at 60 °C. The dried gel was crushed again and sieved to obtain a particle size of below 300 μm for the adsorption tests.
2.4 Adsorption experiments
Batch-wise adsorption tests for fluoride were performed to evaluate the effect of operating parameters like contact time, pH, equilibrium concentration of fluoride, and coexisting anions on the adsorption behavior of fluoride on the Zr(IV)-loaded GP gel. All batch adsorption experiments were carried out in 50 mL polyethylene (PE) plastic vessels by taking 25 mg dried gel together with 15 mL of the fluoride solution. The vessels were stoppered and then shaken in a horizontal thermostated shaker (thermostatic shaking incubator) maintained at 25 °C and 150 r/min for about 24 h to attain equilibrium. The concentrations of fluoride before and after adsorption were measured by using an ion-selective electrode method, outlined in the Methods of Examination of Water and Wastewater [16] with a pHJC-3 pH meter as the indicator of ion potential. The amount of adsorption at equilibrium Qe (mol/kg) of fluoride is calculated from the following equation:
Qe=[(Ci-Ce)/m]?V (1)
where Ci (mol/L) and Ce (mol/L) are the fluoride concentrations before and after adsorption, respectively, m (kg) is the mass of dried gel, and V (L) is the volume of the aqueous solution.
The procedure of kinetic tests was basically identical to that of equilibrium tests. The aqueous samples were taken at preset time intervals and the concentrations of fluoride were similarly measured. The amount of adsorption at time t, Qt (mol/kg), can be calculated by
Qt=[(Ci-Ct)/m]?V (2)
where Ct (mol/L) is the fluoride concentration at contact time of t.
The adsorption (A), calculated according to Eq.(3), is defined as the ratio of difference of fluoride concentration before and after adsorption (Ci-Ce) to its initial concentration (Ci):
A=(Ci-Ce)/Ci (3)
3 Results and discussion
3.1 Description of Zr-GP gel
The prepared Zr-GP gel exists in the form of fine particles with white color, and it is found to be very tough, light and hydrophobic, and easy to float on the surface of the water. By dipping and shaking in the aqueous solution for several minutes, it will settle down to the bottom when being saturated with water.
3.2 Effect of pH value
Usually, the ion exchange capacity is strongly affected by the pH value of the solution and by the surface chemistry of the solids, degree of ionization and the adsorbate species. The adsorption of fluoride at various equilibrium pH values was tested and a quantitative removal was observed from pH 1 to 7, as shown in Fig.1. The maximum adsorption of fluoride was obtained in the pH range of 2-4. For an initial concentration of 10 mg/L, the final concentration of fluoride after adsorption at the pH range of 2-4 was found to be much lower than the permissible discharge limit (1.5 mg/L). When the pH value of the test fluoride solutions increased from 1 to 7, adsorption percentage of fluoride varied, i.e. firstly increased to 97.2%, after pH 4 reduced to 46.7% at pH 7. Depending on the pH value of the testing solution, fluoride exists in two species, i.e. HF and F-. At pH lower than 4, the dominant species in the aqueous solution is the neutral species (HF) while F- is the main species at pH larger than 4. These ions are supposed to be adsorbed onto Zr(IV)-loaded GP gel with the substitution of hydroxyl ions and/or water molecules, which are coordinated with Zr(IV) ion under its hydrated condition. This has been confirmed by the fact that the pH increases a little after the adsorption of fluoride at various pH values. The decrease of fluoride adsorption ratio after pH 4 can be attributed to the competition between hydroxyl ions and fluoride ions for adsorption sites, and in the case of strongly acidic solution (pH≤2), the dominant species of fluoride is neutral HF whose protonated species are weakly favored for adsorption onto the active sites, eventually leading to low removal of fluoride from the solution at low pH. Above depicted adsorption behavior is quite popular and typical in the similar studies [14-15].
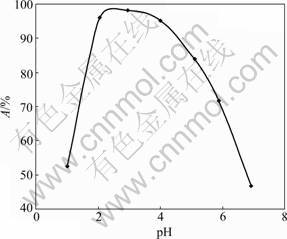
Fig.1 Plots of adsorption vs pH for fluoride ion adsorption onto Zr-loaded garlic peel particles
3.3 Effect of coexisting anions
Coexisting anions such as nitrate, sulfate and phosphate may compete with fluoride ions during the adsorption process for the active sites on the Zr(IV)-GP gel. Hence, the effect of these competitive anions on the uptaking of fluoride ions should be evaluated. The anion solutions of nitrate, sulfate and phosphate were prepared by dissolving appropriate amount of their sodium salts. Adsorption experiments were carried out by adding a certain volume of these anion solutions, respectively, in the fluoride solution to make the testing solutions containing 10 mg/L and 0.1 mol/L individual coexisting anion. The effect of various coexisting anions on fluoride adsorption by gel is shown in Fig.2, which indicates that nitrate and sulfate have no significant inverse effect on the adsorption of fluoride, while phosphate will suppress the uptaking of fluoride onto gel to some degree, decreasing the adsorption of fluoride from 97.2% to 77.6% at pH 3. This adsorption behavior may be explained by comparing the insolubility of the corresponding anions with zirconium. Obviously, nitrate and sulfate are difficult to form insoluble compounds with Zr(IV), while it is quite easy for the case of phosphate, meaning that the phosphate will show much better competitive capability than nitrate and sulfate. But even that, its affinity to Zr(IV) is still much inferior to fluoride. The excellent selective adsorption of fluoride onto the Zr(IV)-loaded GP gel will make it effective in the treatment process for the practical water, in which the anions of nitrate, sulfate and chloride usually exist.
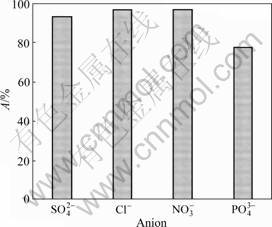
Fig.2 Plot of adsorption of fluoride anion onto Zr-loaded garlic peel powder in solutions with different co-existing anions (pH=3.0)
3.4 Biosorption isotherms
The adsorption isotherms of fluoride on the Zr-GP gel are shown in Fig.3, from which it can be evaluated that the maximum loading capacity for this gel is 0.89 mmol/g and 1.10 mmol/g at equilibrium pH 6.0 and 2.0, respectively. The larger adsorption capacity at pH 2.0 suggests that OH- has significant effect on the adsorption of fluoride, which is quite consistent with the results shown in Section 3.2. And further study shows that the adsorption process fits the Langmuir model, as shown in Fig.4, which suggests that the uptaking of fluoride on this gel follows a kind of monolayer adsorption style. By collecting some data about the adsorbents for fluoride removal reported in the literatures and listing their uptaking capacity in Table 1, it can be found that the Zr-loaded garlic peel exhibits quite excellent adsorption properties. Considering the low cost of garlic peel as the raw materials, the gel developed in present study will show much better commercial potential in application.
3.5 Biosorption kinetics
Figure 5 indicates the effect of contact time on the adsorption of fluoride on the Zr-GP gel. It can be seen that the adsorption equilibrium will be attained after at least 4 h, which is quite long and not good for fast removal of fluoride from the water if being applied in the practical life. This kind of phenomena is quite popular and the reason for such slow adsorption of this Zr-GP gel may be attributed to the tight and hydrophobic surface, and the radius of fluoride anion. Compared with other faster biosorbents for fluoride removal reported [17], more improvement needs to be done in the next work.

Fig.3 Isotherm adsorption plots of fluoride onto Zr-loaded garlic peel sorbent
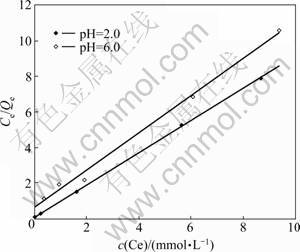
Fig.4 Langmuir isotherm model for adsorption of fluoride onto Zr-loaded garlic peel sorbent
Table 1 Adsorption capacity of various defluorination adsorbents


Fig.5 Adsorption of fluoride anion onto Zr-GP sorbent at different contact time (pH=3.0)
The kinetics of adsorption is an important route to understand the adsorption mechanism and rate- controlling steps. For adsorption phenomena, the most commonly adopted models are pseudo-first-order, pseudo-second-order and intraparticle diffusion models [1], which are employed to predict the adsorption kinetics in the present study. It is found that both the pseudo-first-order and pseudo-second-order kinetic models can be used to describe the adsorption process well, while the calculation value of the equilibrium loading capacity from the former model is only 0.12 mmol/g, which is quite different from the tested data of the experiment (0.20 mmol/g). So, the pseudo- first-order kinetic model cannot be used here. The calculation value obtained from pseudo-second-order model is 0.21 mmol/g, quite close to the experimental result, meaning that the adsorption of fluoride on the Zr-GP gel fits the pseudo-second-order kinetic step, as shown in Fig.6, and the corresponding adsorption rate constant in the case of initial concentration of 10 mg/L for fluoride is evaluated to be 3.25×10-3 g/(mg·min).
3.6 Adsorption mechanism
In the present study, garlic peel is used as the raw materials to prepare adsorbents, for it contains abundant functional components such as —NH2 and —COOH as reported in Ref.[18]. These active groups have strong affinity to the metal ions, e.g., Cu2+, Cd2+, Pb2+, Fe3+ and Zn2+, which makes garlic peel the excellent candidate for the fixation of above cations [19]. And these adsorption capabilities are quite popular among many biomass, such as apple or orange juicing residue which typically contains large amount of pectin with the functional group of —COOH [10, 14-15]. It can also adsorb ZrO2+ cation easily and become the Zr(IV)-loaded gel, which will further exhibit good affinity to fluoride. As to the uptaking for fluoride, the adsorption mechanism is usually related to the adsorbate species and the surface chemistry characteristics of the adsorbents. Above adsorption behavior of fluoride on the Zr-GP gel suggests that the ion-exchange is the possible adsorption mechanism, i.e., the gel possibly exists in the form of (H2O)n-Zr-GP-Zr-(OH)-m (with n and m denoting the number of molecules and ions) in the aqueous liquid, so fluoride species can replace the coordinated H2O molecules or hydroxyl anions of OH- on the gel with the species of HF molecules or F- anions. As mentioned above in Section 3.2, fluoride will exist in the forms of HF or F- depending on the solution pH, i.e. protonated HF appears at low pH (≤2) dominantly, which will replace the coordinated H2O molecules from the Zr-GP gel, and F- anion will substitute the coordinated hydroxyl anions of OH- on the gel. This kind of ion-exchange mechanism is also popular in other similar biosorption systems reported [1-2, 12-15, 17].
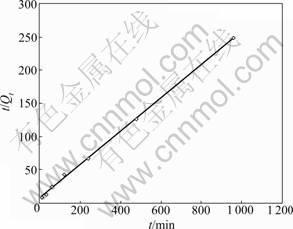
Fig.6 Pseudo-second-order plot of fluoride adsorption kinetics on Zr-loaded garlic peel sorbent (pH=3.0)
4 Conclusions
1) Biosorbent having high adsorption capacity for fluoride anion can be easily prepared by using cheap garlic peel as the raw materials via simple saponification and Zr(IV)-loading treatment. This biosorbent may be applied to the purification of drinking groundwater in the countryside areas where fluoride pollution is severe.
2) The pH value has strong effect on its adsorption behavior, and pH 2.0-4.0 is suitable for adsorption of fluoride. The adsorption capacity of the Zr-loaded garlic peel is evaluated to be 0.89 mmol/g and 1.10 mmol/g at equilibrium pH 6.0 and 2.0, respectively, which is much better than the current similar defluorination adsorbents.
3) The adsorption process fits the pseudo-second- order kinetic model, and the corresponding adsorption rate constant is evaluated to be 3.25×10-3 g/(mg·min).
References
[1] FAN X, PARKER D J, SMITH M D. Adsorption kinetics of fluoride on low cost materials [J]. Water Research, 2003, 37(20): 4929-4937.
[2] SRIMURALI M, ORAGATHI A, KATHIKEYAN J. A study on removal of fluorides from drinking water by adsorption onto low-cost materials [J]. Environmental Pollution, 1998, 99(2): 285-289.
[3] ZHENG Bao-shan. Research on the endemic fluoride toxication and pollution from industry in China [M]. Beijing: Press of China Environment Science, 1992. (in Chinese)
[4] MOHAPATA M, ANNAD S, MISSHRA B K. Review of fluoride removal from drinking water [J]. Journal of Environmental Management, 2009, 91(1): 67-77.
[5] MEENAKSHI S, VISWANATHAN N. Identification of selective ion-exchange resin for fluoride sorption [J]. Journal of Colloid and Interface Science, 2007, 308(2): 438-450.
[6] SUJANA M G, THAKUR R S, DAS S N, RAO S B. Defluorination of waste waters [J]. Asian Journal of Chemistry, 1997, 9(4): 561-570.
[7] GREENLEE L F, DESMOND F L, FREEMAN B D, MARROT B, MOULIN P. Reverse osmosis desalination: Water sources, technology, and today’s challenges [J]. Water Research, 2009, 43(9): 2317-2348.
[8] KABAY N, ARAR O, SAMATYA S, YUKSEL U, YUKSEL M. Separation of fluoride from aqueous solution by electrodialysis: Effect of process parameters and other ionic species [J]. Journal of Hazardous Materials, 2008, 153(1/2): 107-113.
[9] SIMONS R. Trace element removal from ash dam waters by nanofiltration and diffusion dialysis [J]. Desalination, 1993, 89(3): 325-341.
[10] DHAKAL R P, GHIMIRE K N, INOUE K. Adsorptive separation of heavy metals from an aquatic environment using orange waste [J]. Hydrometallurgy, 2005, 79(3/4): 182-190.
[11] DEMIRBAS A. Heavy metal adsorption onto agro-based waste materials: A review [J]. Journal of Hazardous Materials, 2008, 157(2/3): 220-229.
[12] ALAGUMUTHU G, RAJAN M. Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon [J]. Chemical Engineering Journal, 2010, 158(3): 451-457.
[13] Leyva-Ramos R, Rivera-Utrilla J, Medellin- Castillo N A, Sanchez-Polo M. Kinetic modeling of fluoride adsorption from aqueous solution onto bone char [J]. Chemical Engineering Journal, 2010, 158(3): 458-467.
[14] BISWAS B K, INOUE K, GHIMIRE K N, OHTA S, HARADA H, OHTO K, KAWAKITA H. The adsorption of phosphate from an aquatic environment using metal-loaded orange waste [J]. Journal of Colloid and Interface Science, 2007, 312(2): 214-223.
[15] BISWAS B K, INOUE K, GHIMIRE K N. Removal and recovery of phosphorus from water by means of adsorption onto orange waste gel loaded with zirconium [J]. Bioresource Technology, 2008, 99: 8685-8690.
[16] State Environmental Protection Administration of China. Methods of examination of water and wastewater [M]. Beijing: Press of China Environment Science, 2002. (in Chinese)
[17] ZHOU Y, YU C, SHAN Y. Adsorption of fluoride from aqueous solution on La3+-impregnated cross-linked gelatin [J]. Separation and Purification Technology, 2004, 36(2): 89-94.
[18] LIU Wei-lin, XU Liang-ping, YUAN Qiao-ping, HONG Yin-bo, ZHANG Yi-qin. Study on extraction condition of mixed amino acids from garlic [J]. Modern Food Science and Technology, 2006, 22(2): 1818-182. (in Chinese)
[19] HUANG Kai, JIAO Shu-qiang, ZHU Hong-min. An alternative of ion exchange resins by using biowastes for water purification [C]// NI Wei-dou, XU Jin-liang. Proceedings of Conference on China Technological Development of Renewable Energy Source. USA: Scientific Research Publishing, 2010: 105-109.
(Edited by YANG Bing)
Foundation item: Project(11140065) supported by Research Program for Returned Oversea Scholars of China
Received date: 2010-12-31; Accepted date: 2011-04-10
Corresponding author: HUANG Kai, PhD; Tel: +86-10-62334204, +86-13552537538; E-mail: khuang@metall.ustb.edu.cn
- Removal of fluoride from aqueous solution onto Zr-loaded garlic peel (Zr-GP) particles


