DOI: 10.11817/j.ysxb.1004.0609.2021-37898
钕铁硼永磁废料中稀土回收循环利用现状及展望
宋 强1,2,3,童 雄1,2,谢 贤1,2,张文杰1,2,曹 阳1,2,杜云鹏1,2,程雅芝1,2
(1. 昆明理工大学 国土资源工程学院,昆明 650093;
2. 金属矿尾矿资源绿色综合利用国家地方联合工程研究中心,昆明 650093;
3. 云南缘矿科技开发有限公司,昆明 650093)
摘 要:钕铁硼(NdFeB)永磁材料在众多领域被广泛应用,在生产NdFeB永磁材料的过程中,会产生大量废料,直接丢弃不仅会污染环境,还是对二次资源的浪费。文章综述了NdFeB永磁废料中稀土循环利用的技术现状,目前处理废料中稀土的技术方法分为火法和湿法。其中,对比分析了氯化法、合金法、选择氧化法等火法在工业应用中的现状和存在的不足;并且详细阐述了全溶法、盐酸优溶法、草酸沉淀法、硫酸复盐法、溶剂萃取法等湿法的研究情况,深入剖析了不同方法存在的局限和优势;同时介绍了电沉积法、生物浸出法、氢化法、机械化学法等新技术方法的研究现状。在此基础上,展望了未来回收循环利用NdFeB永磁废料中稀土技术方法发展的方向。
关键词:
文章编号: 中图分类号:TD983 文献标志码:A
稀土作为国家战略资源,独特的4f层电子结构和纳米材料使稀土材料具有优异的电、光、催化、磁等性质,使其在陶瓷、国防、航空、电子信息、新能源等领域有着极其重要的应用[1-5]。其中,NdFeB永磁材料由于具有高剩磁、高矫顽力、高磁能积等特点而在风力发电、新能源汽车、电机、耳机等现代工业广泛应用[6-8]。具体消耗情况见如图1所示,且需求量逐年增长[9]。
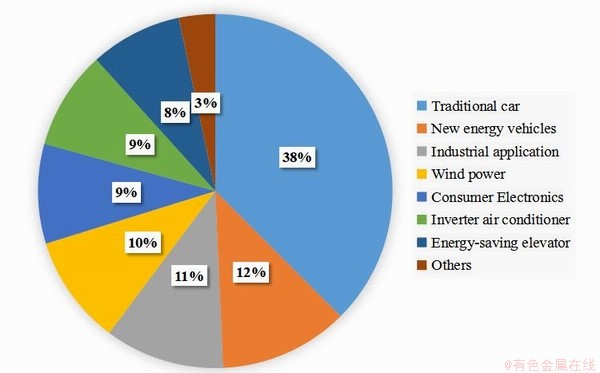
图1 高性能NdFeB永磁材料不同行业消费占比
Fig. 1 Consumption proportions of high-performance NdFeB permanent magnet materials in different industries
NdFeB永磁材料中含有30%左右的稀土元素(Nd、Pr、Dy、Tb等),在永磁材料需求日益增长的背景下,其产量以5%的增量增长,2020年稀土永磁材料产量接近20万吨[10-11]。巨大的产量使不可再生的稀土资源急剧消耗,虽然我国稀土储量位居世界第一,但粗犷的开采方式和急剧的消耗使我国稀土资源终将面临枯竭的困境[12]。随着绿色环保、循环经济的理念不断深入,越来越重视二次资源中稀土的绿色高效循环利用,以此缓解稀土资源供应的压力。在生产NdFeB永磁材料过程中,由于产品易氧化、开裂等因素从而会产生大量废料,直接丢弃不仅污染环境,还是资源的极大浪费[13-14]。相较于美国、日本和欧洲等发达国家,我国对NdFeB永磁废料的利用还远远不够。只有极少数企业具备永磁废料中稀土循环利用工艺,但也存在处理成本高、技术不成熟等弊端。在国务院颁布《国务院关于促进稀土行业持续健康发展的若干意见》中,明确指出要积极开展稀土资源回收工作[15-16]。本文综述了当前NdFeB永磁废料中稀土元素循环利用的技术现状及存在的弊端,并探讨其发展趋势,为我国NdFeB永磁废料中稀土元素绿色高效循环利用提供借鉴。
1 NdFeB永磁材料及废料来源
1.1 NdFeB永磁材料
进入20世纪后,永磁材料得到了飞速发展,图2所示不同时期永磁材料及其磁能积的关系[17-18]。其中,稀土永磁体材料经历了SmCo5为代表的第一代、Sm-Co为代表的第二代及Nd2Fe14B为代表的永磁合金。由于Co价格过于昂贵,严重制约第一和第二代永磁材料的应用和发展[19]。NdFeB永磁材料最早通过粉末冶金技术被制备,其单晶的饱和磁化强度Ms=1.6T,磁晶各向异性HA=73KOe,居里温度Tc=585K。在此基础上,实验室制备的NdFeB永磁材料的磁体能级超过55MGOe,因此也被称为“磁王”[20]。
在后续的研究中,以Cu、Al、Ga、Dy等元素对NdFeB系永磁材料进行改性研究,通过取代主相晶格的原子来改变材料的磁化强度Ms、磁晶各向异性HA、居里温度Tc等性质[21-22]。也存在“参杂型”元素,利用其它金属元素置于主相晶粒间,起到抑制软磁相生成,改善晶间相的作用,增强矫顽力和抗腐蚀性,提高NdFeB性能。
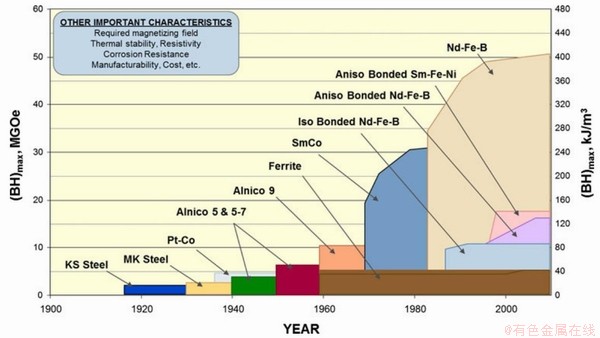
图2 不同种类永磁体的磁能积变化关系[18]
Fig. 2 The relationship between the magnetic energy product of different types of permanent magnets
1.2 NdFeB永磁废料的来源
优良的性质使NdFeB永磁材料拥有广阔的应用前景,目前永磁材料的生产制造流程如图3所示[23]。在生产过程中由于质量要求高、易氧化、开裂等因素往往导致产品报废率较高,原料利用率只有70%左右,会产生大量的废料,每年中国产生的废料约有10万吨,其中含有大量稀土资源[24]。
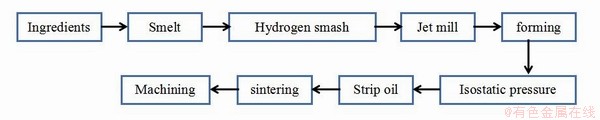
图3 NdFeB永磁材料生产流程图[23]
Fig. 3 Production flow chart of NdFeB permanent magnet material
在生产NdFeB永磁材料的各个阶段,均会伴随废料的产生,一般包括:原料预处理过程中的损耗、熔炼过程中被氧化的钕、氢碎和气流磨磨粉阶段中产生的超细粉、制粉阶段被空气氧化的粉末、烧结过程中被氧化的NdFeB块状料及机加工时边角料和表明处理产生的不合格产品等[25]。由此可以看出,NdFeB永磁废料在生产过程中很难避免且废料量大,对废料中稀土绿色高效循环利用,不仅能提高稀土利用率,更是生产迫切需要的,具有显著的经济效益。
2 火法回收
2.1 氯化法
钕铁硼废料中稀土元素相较于其他金属物质更活泼,能和氯离子表现出更强亲和力,生成稀土氯化物而与其它物质如Fe分离。目前,在氯化法中用于分离回收稀土的氯化剂一般为:FeCl2、NH4Cl、MgCl2-KCl等[26-27]。其中,当FeCl2作氯化剂时由于钕铁硼废料中含有氧,容易氧化Fe2+,因此在反应过程中往往需要添加碳粉,脱除废料中所含的氧,促使稀土元素被氯化完全。Uda等以永磁废料为研究对象,采用氯化法回收其中稀土,研究表明当FeCl2为氯化剂时,添加碳粉在800℃条件下能有效促进氯化反应进行。反应过程中加入过量的FeCl2能促使稀土氧化物完全转化为稀土氯化物,再利用彼此间沸点的差异采用真空蒸馏的方式使其分离,最终永磁废料中稀土回收率接近96%,且所得稀土氯化物纯度超过99%[28]。NH4Cl作氯化剂时在较高温度下能发生分解,产生的干燥HCl气体对稀土元素进行氯化,且能适用于超细粒永磁废料。Itoh等将NH4Cl和钕铁硼永磁材料置于反应器中,在300℃的条件下反应3h,将所得固体产物用去离子水浸出溶解,最终稀土回收率可达87%[29]。Tom Lorenz等以NH4Cl为氯化剂回收风力电机废旧永磁材料中的稀土元素,在225-325℃的反应温度下NH4Cl发生分解,产生的干燥HCl使稀土转变成稀土氯化物,再将其溶解与醋酸缓冲溶液中,所发生的化学反应如式(1-6)所示。该工艺能回收约85%的稀土,相较于最优条件下盐酸优溶法化学试剂消耗减少45%,并且得到额外具有经济价值产品(质量分数为2.9%的氨水溶液),且在德国弗莱堡工厂已经开始应用[30]。
氯化反应:
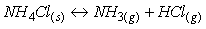 (1)
(1)
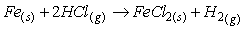 (2)
(2)
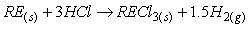 (3)
(3)
醋酸缓冲溶剂溶解反应:
 (4)
(4)
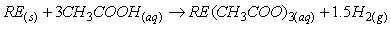 (5)
(5)
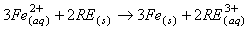 (6)
(6)
MgCl2-KCl作氯化剂时需在较高温度下使其处于熔融态,与稀土元素反应生存稀土氯化物,与其他金属分离。Hua等以MgCl2-KCl为氯化剂回收钕铁硼中稀土元素,在800-1200℃反应温度下使氯化剂为熔融态,再与破碎后的永磁材料进行反应,Nd能将熔融态的MgCl2还原成Mg,同时转化为NdCl3,最终得到的产物为RECl3-MgCl2-KCl混合物,再经真空蒸馏分离回收稀土,回收率能达到90%[31]。
氯化法回收稀土对一般钕铁硼废料有较好效果,但对氧化严重的废料回收效果较差。该方法最终得到的产物一般均为稀土氯化物,由于这类物质以潮解,因此还需转化为稀土氧化物,间接增加了工业流程,提高了生产成本。
2.2 合金法
合金法在回收钕铁硼废料中稀土时,由于稀土元素Nd与一些金属物质能形成低熔点合金,从而达到分离稀土的目的[32]。常用的金属如Mg、Ag等,Mg能在较低温度下形成熔融态,稀土Nd与熔融态Mg结合形成MgNd、Mg2Nd、Mg3Nd、Mg41Nd5等合金[33-34]。同样,稀土Nd也能与熔融态Ag形成AgNd、Ag2Nd、Ag51Nd14等合金,从而将稀土元素提出来[35-36]。再利用高温蒸汽使合金中稀土分离,得到较高纯度稀土产品。
Takeda等在如图4所示反应装置中回收钕铁硼废料中稀土,位于装置底部的Ta坩埚中装入Mg,在高温条件下Mg变为蒸汽挥发,在装置顶部冷凝后滴入进装有钕铁硼废料的Fe坩埚中。此时处于熔融态的Mg与废料中稀土Nd结合形成Mg-Nd合金,再经Fe坩埚壁上的缝隙滴落进底部坩埚中。经过充分反应直至Nd被提取完成,再经高温蒸馏使Nd从合金中分离,在最佳反应条件下,废料中稀土Nd的回收率和最终产物纯度分别为99%和98%[37]。在此基础上,Takeda探究了熔融Ag直接提取钕铁硼永磁磁铁残渣中的稀土Nd,在1273K条件下残渣中90%的Nd能被提取回收,最终所得Ag-Nd合金在空气中氧化,以Nd2O3形式存在于合金中。虽然银液作为一种有效提取Nd的介质,但所得产物合金中Nd2O3的分离难度较大[38]。
该方法在回收永磁废料中稀土时能得到稀土金属单质,一定程度缩减后后续工艺流程。但该工艺在反应过程中所需温度较高,不仅对操作设备要求比较高,而且能耗高,同时所得合金产物还需高温蒸馏,使工艺较冗长繁琐。
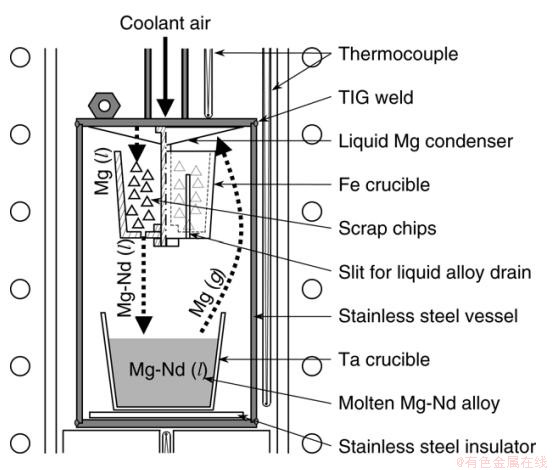
图4 镁合金法实验装置示意图[37]
Fig.4 Schematic illustration of the apparatus for neodymium extraction from scrap alloys using magnesium circulation.
2.3 选择氧化法
选择氧化法回收钕铁硼废料中稀土时主要利用不同温度下铁和稀土对氧亲和力的差异性,并依据Nd-Fe-O相图,再控制反应过程中氧分压,最终使稀土优先与氧结合形成稀土氧化物,而铁以金属态形式存在,从而达到分离回收的目的[39-40]。其中,Fe-Nd-O (1077℃)、Fe-Dy-O (977℃)、Fe-Pr-O (1027℃)的相图如图5所示,由图可知,在温度为1077℃时,当氧分压<10-15atm条件下NdFeO3会分解成Fe(s)和Nd2O3(s);当氧分压<10-17atm时,温度在977℃和1027℃条件下NdFeO3均会分解[41]。减小氧分压的本质是脱氧过程,在处理过程中一般加氢或加钙来达到脱氧的目的。Nakamoto等在Fe-Nd-O相图基础上,通过石墨坩埚控制系统氧势,加入钙来脱除氧,使系统中氧势控制在10-17-10-18atm之间,最终得到Fe单质和稀土氧化物,且稀土回收率超过99%。选择氧化法控制氧势分离稀土和铁,得到的稀土产品中往往还有较多氧化硼,降低稀土纯度[42]。为了降低稀土氧化物中硼的含量,而提出玻璃渣法(Glass Slag Method)。Saito等以采用玻璃渣法回收钕铁硼材料中稀土Nd,利用B2O3作氧化剂,使稀土Nd氧化成Nd2O3,而氧化硼被还原为硼单质进入铁中,有效提高了Nd2O3的纯度[43]。
该方法一定程度上能有效回收钕铁硼废料中稀土元素,并且基本无需消耗其它化学试剂,对环境友好。但是,所得稀土氧化物产物纯度不高,往往还含有铁和硼杂质,虽然可以延长反应时间或磁吸引来提高稀土产品纯度,由于反应温度整体较高,耗能大。增加生产成本。
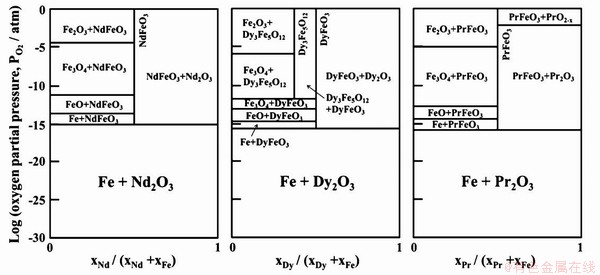
图5 Nd-Fe-O系热力学平衡图(1077℃、1027℃、977℃)[41]
Fig. 5 Thermodynamic equilibrium diagram of Nd-Fe-O system (1077℃, 1027℃, 977℃)
3 湿法回收
3.1 全溶法
全溶法是通过盐酸将铁钕硼废料中能溶解的稀土和铁等元素全部溶解,也被称为盐酸全溶法。再以H2O2为氧化剂使铁以Fe3+形式存在溶液中,结合后续沉淀或萃取工艺分离回收溶液中稀土元素[44-45]。
Padhan等以盐酸为溶剂将NdFeB废料全部溶解,再以萃取剂NaCyanex302分离回收溶液中Dy和Nd,整个过程在pH=1.2的条件下Dy和Nd的分离系数达到53.65%,分离效果明显[46]。Lai等以4mol/L盐酸溶解处理NdFeB废料,待全部溶解后再分布沉淀,最终得到的稀土产品纯度高于99.4%,能直接用于新材料的生产[47]。全溶法在溶解NdFeB废料时,溶液中除稀土外还存在大量杂质离子,对后续沉淀法和萃取法都有明显干扰。如沉淀过程中杂质离子与稀土离子出现沉淀pH范围相近,降低所得稀土产品纯度;萃取分离过程中常见萃取剂易受到Fe3+等杂质离子影响,出现乳化现象,降低分离效果,增加分离成本[48]。因为,在工业生产中往往需要增加除杂工序,提高分离效率。环烷酸、N503等萃取剂与Fe3+结合效果明显,常被用于净化脱除溶液中Fe3+;Fe2+和稀土离子在草酸环境中出现沉淀的pH值范围相差明显,借助草酸调节溶液pH值能有效分离杂质Fe2+。陈云锦等在NdFeB废料全部溶解后,以H2O2氧化Fe2+,萃取剂N503萃取Fe3+净化溶液,再以膦酸萃取剂P507提取溶液中稀土元素,该处理工艺不仅提高了稀土回收率,还增加了萃取剂循环利用率[49]。Tian等以6mol/L盐酸溶解NdFeB废料,并添加酒石酸和六次甲基四胺(HMTA)螯合剂提高盐酸溶解浸出率,再通过草酸去除杂质Fe2+,最终回收的稀土产品纯度达到96%,且回收率超过90%[50]。Mehmet等以硫酸为溶剂将NdFeB废料全部溶解后,加入MnO2将超过99%的Fe2+氧化成Fe3+,再以Ca(OH)2为沉淀剂去除杂质Fe3+,最终得到合格稀土产品[51]。
全溶法处理NdFeB废料虽然原理和操作简单,但在溶解废料过程中盐酸消耗量大,对环境污染严重,生产环境差,且在溶解过程中会伴随大量杂质离子的溶出,不仅增加了后续分离除杂的难度,还降低稀土产品纯度。
3.2 盐酸优溶法
针对全溶法中酸消耗量大的弊端,盐酸优溶法被提出。首先经过氧化焙烧,使NdFeB废料中稀土、Fe和其它金属物质均转化为氧化物,再通过调节溶液pH值控制稀土氧化物优先与盐酸反应溶解,使稀土元素以离子形式进入溶液中,而残渣等停留在滤渣中[52-53]。溶液中的稀土离子再经萃取剂提取纯化,结合草酸使稀土沉淀,最终能得到纯度较高的稀土氧化物。
Liu等经过研究发现在800℃温度下对NdFeB废料进行焙烧,能有助于后续稀土分离,经过焙烧后的废料采用HCl对其溶解,涉及反应如式(7-10)所示,加入2g/L NaNO3能显著提高稀土和B从Fe中分离,经过EHD萃取后使用草酸进行沉淀,最终得到的稀土产品纯度超过99%,且硼的回收率超过99.5%,具体流程见图6所示[54]。
 (7)
(7)
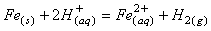 (8)
(8)
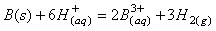 (9)
(9)
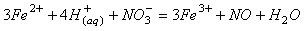 (10)
(10)
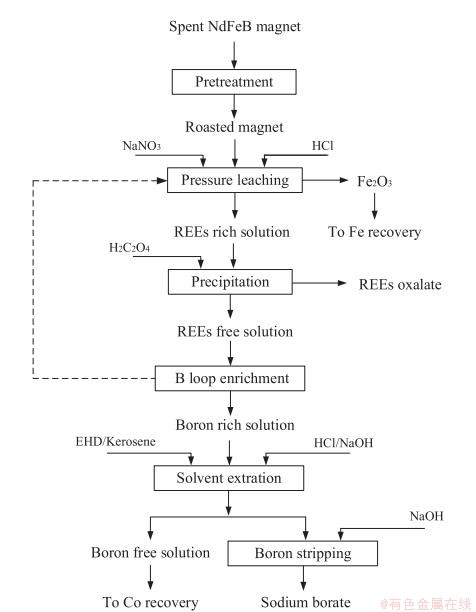
图6 盐酸优溶法回收NdFeB废料中稀土流程图
Fig.6 The process of recovering rare earth from NdFeB waste by hydrochloric acid excellent solution method
江泽佐等采用氧化焙烧-盐酸优溶-氧化除铁-萃取分离的工艺处理NdFeB回收料,研究表明90℃条件下以NaClO3为氧化剂能使Fe2+的氧化率达到99.99%,在pH=4的溶液中形成Fe(OH)3,最终得到质量合格的稀土产品[55]。
盐酸优溶法在处理NdFeB废料过程中,焙烧温度的控制至关重要,使稀土和铁能转化成Re2O3和Fe2O3,同时避免稀土铁酸盐形成,为后续盐酸控制pH,优先溶解稀土氧化物奠定基础。相较于盐酸全溶法,该方法对酸的消耗量有了明显降低,资源利用率有了提高[56]。但目前对焙烧的理论研究较为欠缺,在实际工业处理中很难避免稀土铁酸盐(NdFeO3)的产生,使Nd和Fe无法有效分离。并且,在调控pH选择沉淀过程中,在产生Fe(OH)3沉淀的同时,部分稀土也会形成沉淀,造成稀土损失。王毅军等在盐酸优容处理NdFeB废料后,采用晶型碳酸沉淀,稀土损失率接近10%[57]。
综上所述,盐酸优溶法在处理NdFeB废料时,工艺流程有了明显缩短,且化学试剂消耗降低,对环境更加友好,具有应用前景。但在废料溶解过程中效率较低,同时产生大量废水,对焙烧过程物相变化的理论研究较欠缺,缺乏科学指导,还需进一步深入研究。
3.3 草酸沉淀法
NdFeB废料经过溶解后,溶液中的Fe2+和稀土离子与草酸形成的盐类物质溶解度有显著差异,借助其溶解度差异使铁和稀土分离。通过控制草酸加入量、溶液pH值和反应温度等因素变量,在最佳条件下得到较高纯度的Re2(C2O4)3,再经灼烧而转变成稀土氧化物[58-59]。尹小文等以草酸沉淀法分离回收NdFeB废料浸出液中稀土,在溶液pH=1.5-2、草酸用量比为1.5、温度80℃的条件下对稀土沉淀效果最佳,再将烘干的稀土草酸盐在800℃温度下焙烧,最终产品中稀土氧化物含量达到99.3%,具体流程见图7所示[60]。
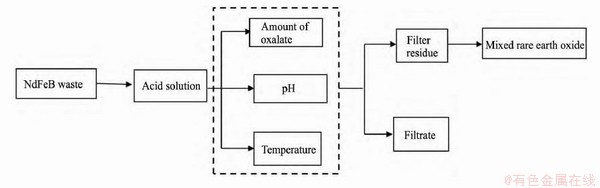
图7 草酸盐沉淀稀土流程图
Fig. 7 Flow chart of oxalate precipitation of rare earth
草酸沉淀法处理浸出液中稀土和Fe时,还存在大量杂质离子,会严重影响最终产品纯度。为此也有相关研究尽量减少浸出液中杂质离子含量,Innocenzi等以柠檬酸为抑制剂,加入至废料浸出液中,有效抑制废料中Mn的浸出,不仅减少了溶解过程酸的消耗量,还大幅提高了最终产品中稀土氧化物的纯度[61]。
草酸沉淀法在一定程度上能有效分离稀土与铁,生产工艺简单,生产流程短。但由于NdFeB废料中Fe的含量高,直接草酸沉淀会消耗大量草酸,并且草酸价格较高,制约了该方法在工业生产中的应用。
3.4 硫酸复盐法
由于稀土硫酸盐在溶液中能与Na2SO4反应,生产复盐沉淀,从而与溶液中Fe2+分离,具体反应见式(11)所示。
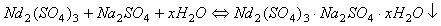 (11)
(11)
该方法处理NdFeB废料时,首先经过H2SO4溶解浸出,使稀土和铁分别以稀土硫酸盐和FeSO4形式存在溶液中,再控制温度加入NaSO4,使稀土形成复盐沉淀,而Fe停留在溶液中,经过滤后,稀土硫酸复盐沉淀与NaOH结合形成稀土氢氧化物沉淀,再经高温灼烧后得到纯度较高稀土氧化物产品,具体流程见图8所示[62-63]。
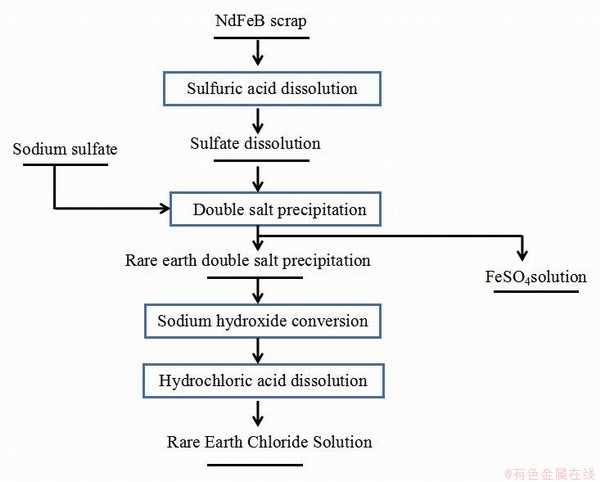
图8 钕铁硼废料回收的硫酸复盐沉淀法流程
Fig. 8 Process of sulfuric acid double salt precipitation method for NdFeB waste recovery
唐杰等对比了草酸沉淀法和硫酸复盐法回收NdFeB废料中稀土的效果,研究表明,草酸沉淀法不仅对稀土难以沉淀完全,并且非稀土杂质含量较高,而硫酸复盐法得到的Nd2O3纯度较高,且对Nd的回收率超过82%[64]。
硫酸复盐法在工业回收NdFeB废料过程中,对稀土的回收率能达到80%以上,整体操作流程简短,反应条件温和,成本相对较低,具有明显优势,尤其是对含Nd含量高的稀土废料效果显著,化学试剂消耗较少,更利于环境环保。但在生产中,对温度和Na2SO4用量(<理论量50%)需要严格控制,避免副反应发生;在草酸沉淀稀土过程中,一旦草酸加入量过多,会使稀土浸出率下降。整个操作过程忽视了溶液中硫酸亚铁的回收,不仅使资源被浪费,同时直接排放还会造成环境污染。
3.5 溶剂萃取法
NdFeB废料中稀土等物质被溶解后,大量稀土与非稀土离子共同存在于溶液中,草酸沉淀、硫酸复盐等方法虽然一定程度能有效分离出稀土元素,但整体效率较低。溶剂萃取法通过有机萃取剂与溶液中稀土离子结合,而达到分离稀土的目的[65-67]。该方法具有处理量大、分离系数和效率高、针对性强等优势。溶剂萃取法至关重要的是有机萃取剂的选择,目前对溶液中稀土有较好萃取性能的传统萃取剂有:D2EHPA、PC88A、Cyanex272、Cyanex923、Cyanex272、TBP、CA12等[68-75]。Yoon等对比分析了萃取剂D2EHPA和PC88A对Dy和Nd的回收效果,D2EHPA整体萃取分离Dy的性能更优于PC88A,当D2EHPA浓度为0.1mol/L、相比O:A=1:1、经过2级萃取后,能有效分离Dy,分离系数βDy/Nd= 410.68[76]。Mohammadi等采用1L三己基(十烷基四)膦酸酯萃取硝酸浸出液中稀土,以EDTA选择性络合分离Nd、Dy,在最优参数条件下,最终制备的Nd2O3和Dy2O3纯度分别为99.6%和99.8%[77]。溶剂萃取剂法虽然能生产单一稀土元素的产品,但在萃取过程中,萃取剂自身具有一定毒性,且易受到杂质离子影响而产生乳化现象,阻碍连续生产等弊端。
针对传统萃取剂的问题,离子液体萃取剂逐渐被开发。一般由有机阳离子与无机、有机阴离子组合组合而成,以液态盐类形式存在,依靠离子液体与溶液中金属离子结合能力的差异而达到分离富集的目的。相较于传统萃取剂,离子液体萃取剂具有分离系数高、循环利用效果好、不挥发、萃取体系稳定等优势,在回收NdFeB废料中有价金属的领域越来越受到关注。。Zhou等设计并合成新型离子液体[P66614]2[IOPAA]用于回收NdFeB废料浸出液中的稀土元素,以[P66614]Cl萃取剂脱除杂质铁,且稀土回收率达到99.85%,并揭示了离子液体萃取剂与稀土结合的机理,如式(12)所示[78]。
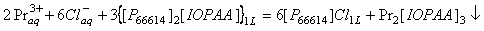 (12)
(12)
Matsumiya等以[P2225][TFSA]离子液体萃取剂分离回收NdFeB废料中的稀土,在最优条件下对Pr、Nd和Dy的萃取率分别为96.7%、97.6%和96.3%[79]。Kikuchi等采用硝酸溶解铁钕硼废料后,通过调节溶液pH去除杂质铁离子,在以合成的离子液体萃取剂[A336][NO3]提取分离溶液中的Nd、Pr和Dy,离子液体与稀土的结合能力为Dy
3能提高离子液体萃取性能[80]。还存在[C12mim][TFSA]、 [C4mim][TFSA]+TBP等离子液体萃取剂,均能有效分离溶液中稀土元素[81-82]。虽然离子液体萃取剂对稀土分离回收效果显著,但也存在合成成本较高,且能溶于溶液而造成损失等弊端,目前,该方法还停留在实验室阶段,离工业生产应用还存在一定距离。
4 其他方法
4.1 电沉积法
在永磁材料NdFeB生产的过程中也会伴随超细钕铁硼废料的产生,相较于传统NdFeB废料,超细钕铁硼废料中杂质较少,主要以铁和稀土(>98wt%)为主,同时废料表面污染物少。传统火法和湿法处理这类废料时存在较多弊端,其中火法消耗能量高,生产成本高;湿法需需要大量盐酸、草酸等溶解和沉淀剂,生产工艺繁琐且伴随大量废水。在此基础上,借助电化学选择性氧化将Fe(Ⅱ)转变成Fe(Ⅲ),再使稀土形成沉淀被回收,即电沉积法[83-84]。Prakash等采用图9所示的装置进行原位电化学氧化反应,研究发现溶液中Fe(Ⅱ)被氧化的程度与阳极所通的电流强度关系如图10所示,当电流强度为1.2A时,经过4h的氧化溶液中99%的Fe(Ⅱ)被氧化,阳极所涉及的反应(13-15)如式。再经过草酸处理,最终能得到99.2%的稀土氧化物,且稀土回收率超过98%,溶液中残留的Fe(Ⅲ)也能被循环利用,提高了超细钕铁硼废料的利用率[85]。
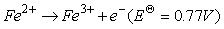 (13)
(13)
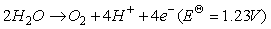 (14)
(14)
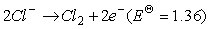 (15)
(15)
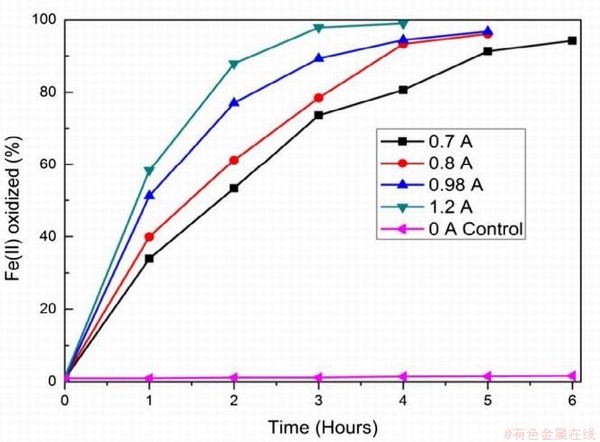
图9 电解装置的示意图
Fig.9 Schematic illustration of the electrolysis set-up.
Irina等以硫酸为溶剂处理NdFeB废料,经过电化学氧化处理后,采用H2CO4使稀土沉淀,从而与Fe分离。对NdFeB进行电解前往往需无机酸(HCl、HNO3、H2SO4)将其溶解,经过电解后还需草酸沉淀稀土,但稀土氟化物不溶于氢氟酸,能避免草酸沉淀直接分离得到稀土沉淀[86]。Yang等以HF为回收溶剂处理超细钕铁硼废料,经过一步沉淀最终能得到可以电解的稀土氟化物,极大降低了化学试剂的消耗,是一种更加简便高效的方法[87]。
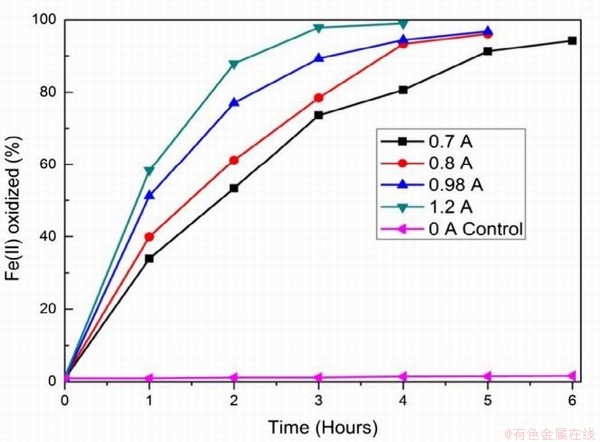
图10 Fe2+氧化速率与电流密度的关系
Fig.10 Rate of Fe2+ oxidation as a function of current density
4.2 生物浸出法
在环保、绿色、高效的生产理念下,传统火法和湿法处理NdFeB废料存在能耗过大、化学试剂消耗过多易造成环境污染等问题。生物浸出是在微生物的帮助下以水溶性形式对不溶性金属化合物进行的生物转化,它基于生物氧化和络合作用对金属离子的迁移,所涉及的微生物在此过程中具有多种功能[88-89]。首先,它们产生Fe(III)离子和/或质子,再将它们浓缩在样品材料和细菌细胞之间的界面中,从而提高浸出率。借助生物浸出处理二次资源已经收到广泛关注,在电子设备、催化剂、荧光粉、电池及金属加工行业的炉渣和煤灰等领域有较好研究和应用[90-91]。对NdFeB废料的处理相对较少,但生物浸出法处理二次资源的优势也促使该方法在处理NdFeB废料中的研究。Romy等最先开展了生物浸出法处理NdFeB废料,对比分析了不同细菌对稀土的浸出效率,经过研究发现酸性氧化铁硫杆菌(Acidithiobacillus ferrooxidans)和氧化亚铁螺旋体(Leptospirillum ferrooxidans)对NdFeB废料中稀土的浸出率最高,其中Dy、Nd和Pr的浸出率分别为86%、91%和100%。该研究为常规工艺提供了一种可管理,经济高效且环保的替代方法[92]。
生物浸出法处理NdFeB废料能极大降低化学试剂消耗,更加绿色环保,更加符合当前社会绿色发展的理念。相较于传统湿法和火法工艺,具有处理量大、成本低、环保等显著优势,是未来发展极具潜力的方法。但是,该技术目前还停留在实验室阶段,离工业应用还有一定差距,还需筛选浸出效率更高的微生物细菌,降低细菌培养所需的环境要求,深入了解微生物代谢机理,为其更广泛应用奠定基础。
4.3 氢化法
由于NdFeB接触H2后容易发生晶界断裂,以此推演出氢化-歧化-脱氢工艺处理NdFeB废料。在处理过程中NdFeB吸收H2,生成氢爆粉,再经歧化脱氢使稀土Nd分离,所涉及的反应如式(16-17)所示[93]。
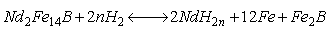 (16)
(16)
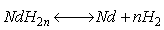 (17)
(17)
整个反应处于高温环境中,且温度越高,反应式中n越趋于1。氢化法在处理NdFeB废料过程中,Nd在回收阶段易发生氧化挥发,使其再被用致永磁材料中时,往往会使材料性能下降[94-95]。因此,在循环利用中还需与新稀土Nd搭配使用。该方法当前还处于实验室阶段,但其高效和环保的优势使其成为研究热点。虽然氢化法对废料中Nd的回收效果好,但对Fe的循环利用较差[96]。Koji等结合氢化法的优势,提出氢化-碳基铁法回收NdFeB废料中稀土和Fe,经过氢化歧化处理后的废料,在高温高压条件下以CO为催化剂与Fe反应,最终使Fe以Fe(CO)5的形式被回收,回收率超过90%[97]。结合碳基铁法能使废料中Fe的循环利用率大幅提高,但CO的添加给生产操作带来难度,且反应所需较长时长,生产效率较低。
4.4 机械化学法
传统处理NdFeB废料的方法一般需要高温高压条件下,并且在效率、成本等方面任然存在壁垒,需要开发回收条件更温和的工艺。机械化学法最早由日本岛跟大学提出,在球磨动能的配合下,常温常压下能被硫酸和草酸组成的溶剂高效回收,对得到纯度超过80%的Nd[98]。该技术的开发使回收工艺条件温和,成本大大降低,是稀土循环利用技术的重大突破,极具应用前景。Sasai等将消磁的废烧结钕铁硼磁体用行星式铣削的方式处理,用直径为10mm的ZrO2球在300rpm速度下降其粉碎成粗颗粒,再用盐酸和草酸组成的溶剂回收其中稀土,最终稀土回收率和纯度均超过95%[99]。经过近几年的发展,机械化学法回收处理NdFeB废料的研究不断深入,但离工业应用还有一定距离。
5 结论和展望
NdFeB废料随着其产量的逐年增加而日益增多,对废料中稀土元素绿色高效循环利用的意识也在逐渐增强。目前,对NdFeB废料中稀土回收的技术方法进行了大量研究,并取得了丰富的研究成果。其中,不同技术方法的优势和不足见表1所示。
表1 不同技术方法回收NdFeB废料中稀土的优势和劣势
Table 1 Advantages and disadvantages of different technical methods for recycling rare earths in NdFeB waste
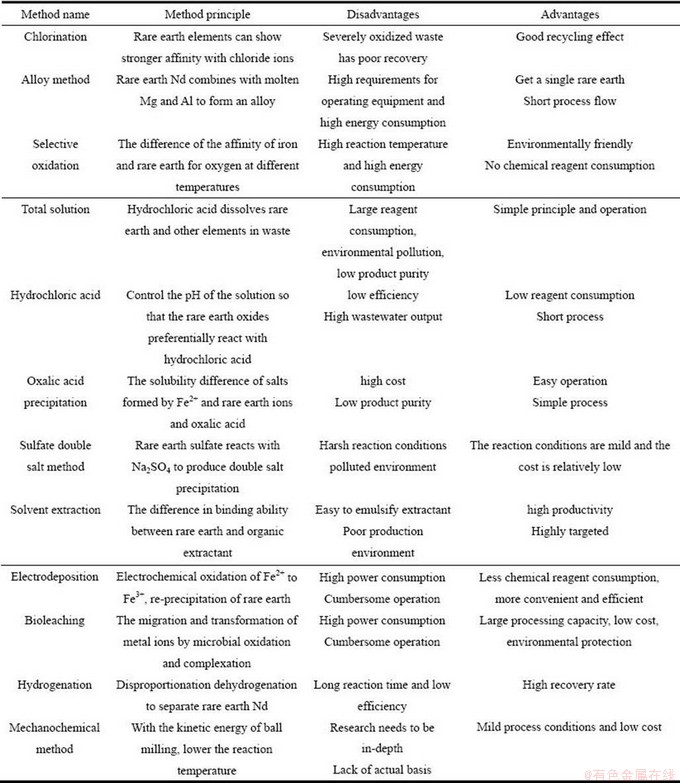
虽然对NdFeB废料中稀土回收的工艺方法在不断完善,更加绿色高效,但也存在较多弊端。为了更好拖动NdFeB废料中稀土的综合利用,在今后的研究主要从以下几个方面入手:①开发先的回收工艺,缩短生产流程,降低生产成本;②开发并筛选性能更优萃取剂,尤其是离子萃取剂,更高效率回收浸出液中稀土元素,降低离子液体萃取剂溶解度,争取早日工业化应用;③对微生物浸出研究进一步深入,培育性能更优、针对性更强、稳定性更高的菌种,使其能在更温和的条件回收废料中稀土元素;④深入研究机械化学法处理NdFeB废料技术,探究其最佳工艺条件,在最低能耗条件下回收稀土;⑤对新工艺技术的开发创新,NdFeB废料中回收稀土工艺成本整体较高,急需成本更低的工艺技术来推断产业链的发展。
6 结语
我国虽然作为稀土大国,但肆意的开采使不可再生的稀土资源消耗急剧增强。急需加大力度对二次资源中稀土的循环利用,不仅能极大缓解稀土的供应压力,还能提高企业的经济效益。相较于欧美等发达国家,我国在稀土循环领域还有巨大差距,处理技术和意识也较落后。随着相关政策的出台,对二次资源的利用意识逐渐增加,已经积极开展相关研究,共同促进我国对FeNdB废料中稀土循环利用技术的提升。
REFERENCES
[1] LI W Z, LIU M Y, GONG L, et al. Emerging various electronic and magnetic properties of silicene by light rare-earth metal substituted doping[J]. Superlattices and Microstructures, 2020, 148: 106712.
[2] ZHAO X, TIAN X, YAO Z Q, et al. The origin of large magnetostrictive properties of rare earth doped Fe-Ga as-cast alloys[J]. Journal of Magnetism and Magnetic Materials, 2020, 524(15): 167289.
[3] KHUILI M, FAZOUAN N, MAKARIM H A, et al. First-principles calculations of rare earth (RE=Tm, Yb, Ce) doped ZnO: Structural, optoelectronic, magnetic, and electrical properties[J]. Vacuum, 2020, 181: 109603.
[4] LI S, LU Y D, QU Y, et al. Dielectric and thermal properties of aluminoborosilicate glasses doped with mixed rare-earth oxides[J]. Journal of Non-Crystalline Solids, 2020, 11(19): 120550.
[5] LINDA O, SATU P, ESA-MATTI T, et al. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications - A review[J].Journal of Cleaner Production, 2019, 236(1): 117573.
[6] JACIM J, THOMAS C, Eric D. Self-organized giant magnetic structures via additive manufacturing in NdFeB permanent magnets[J]. Additive Manufacturing, 2020, 34: 101288.
[7] CHEN H, YANG X, SUN L, et al. Effects of Ag on the magnetic and mechanical properties of sintered NdFeB permanent magnets[J]. Journal of Magnetism and Magnetic Materials, 2019, 485(1): 49-53.
[8] XU J J, XIAO Q F, MEI D D, et al. Fabrication and Properties of Micro-arc Oxidation Coatings on Sintered NdFeB Permanent Magnets[J]. Rare Metal Materials and Engineering, 2018, 47(4): 1059-1063.
[9] 华经情报网. 2018年高性能钕铁硼磁材行业现状及其发展趋势[DB/OL]. http://www.huaon.com/story/423220.
Huajing Information Network. The current status and development trend of high-performance NdFeB magnetic materials industry in 2018 [DB/OL]. http://www.huaon.com/story/423220.
[10] HESAMODDIN G, AHAD G, ALIREZA H, et al. Studies on effective interaction parameters in extraction of Pr and Nd using Aliquat 336 from NdFeB magnet-leaching solution: Multiple response optimizations by desirability function[J]. Journal of Molecular Liquids, 2021, 324(15): 115123.
[11] KOMAL H, A product classification approach to optimize circularity of critical resources-the case of NdFeB magnets[J]. Journal of Cleaner Production, 2019, 230(1): 90-97.
[12] HOLGERr P, ERIKA M. The global rare earth element exploration boom: An analysis of resources outside of China and discussion of development perspectivesJ[J]. Resources Policy, 2017, 52: 134-153.
[13] KARTIKEY K Y, MALLAVARPU A, SINGH D K, et al. NdFeB magnet recycling: Dysprosium recovery by non-dispersive solvent extraction employing hollow fibre membrane contactor[J]. Separation and Purification Technology, 2018, 194(3): 265-271.
[14] RITA S, MATTHIAS B. Estimates of global REE recycling potentials from NdFeB magnet material[J]. Resources, Conservation and Recycling, 2016, 113: 12-27.
[15] YANG Q, SANGWON S. Economic feasibility of recycling rare earth oxides from end-of-life lighting technologies[J]. Resources, Conservation and Recycling, 2019, 15: 104432.
[16] 陈丽杰, 李子良, 龚傲, 等. 从稀土废料中回收稀土的研究进展[J]. 中国稀土学报, 2019, 37(3): 259-272.
CHEN Li-jie, LI Zi-liang, GONG Ao, et al. Research Progress of Rare Earth Recovery from Rare Earth Waste[J]. Journal of the Chinese Society of Rare Earths, 2019, 37(3): 2590-272.
[17] COEY J M D. Perspective and Prospects for Rare Earth Permanent Magnets[J]. Engineering, 2020, 6(2): 119-131.
[18] KRAMER M J, MCCALLUM R W, ANDERSON I A, et al. Prospects for Non-Rare Earth Permanent Magnets for Traction Motors and Generators[J]. J O M, 2012, 64(7): 752-763.
[19] SAGAWA M, FUJIMURA S, TOGAWAN, et al. New material for permanent magnets on a base of Nd and Fe (invited)[J]. Journal of Applied Physics, 1984, 55(6): 2083-2087.
[20] HERBST J F. R2Fe14B materials: Intrinsic properties and technological aspects[J]. Review of Modern Physics, 1991, 63(63): 819-898.
[21] KIM A S, CAMP F E. Effect of minor grain boundary additives on the magnetic properties of NdFeB magnets[J]. IEEE Transactions on Magnetics, 1995, 31(6): 3620-3622.
[22] TOKUNAGA M, HARADA H, TROUT S R. Effect of Nb additions on the irreversible losses of Nd-Fe-B type magnets[J]. IEEE Transactions on Magnetics, 1987, 23(5): 2284-2286.
[23] 石富. 稀土永磁材料制备技术[M]. 冶金工业出版社, 2007: 35-37.
SHI Fu. Preparation technology of rare earth permanent magnet materials[M]. Metallurgical Industry Press, 2007: 35-37.
[24] 王龙君. 钕铁硼废料中稀土的选择性分离与回收研究[D]. 赣州: 江西理工大学, 2020: 11-12.
WANG Long-jun. Research on selective separation and recovery of rare earths in NdFeB waste[D]. Ganzhou: Jiangxi University of Science and Technology, 2020: 11-12.
[25] LAMICHHAN T N, SETHURAMAN L, DALAGANT A, et al. Additive manufacturing of soft magnets for electrical machines-a review[J]. Materials Today Physics, 2020, 15: 100255.
[26] MURASE K, MACHIDA K, ADACHI G. Recovery of rare metals from scrap of rare earth intermetallic material by chemical vapour transport[J]. Journal of alloys and compounds,1995, 217(2): 218-225.
[27] MOCHIZUKIo Y, TSUBOUCHI N, SUGAWARA K. Selective Recovery of Rare Earth Elements from Dy containing NdFeB Magnets by Chlorination[J]. Acs Sustainable Chemistry and Engineering, 2013, 1(6): 655-662.
[28] UDA T. Recovery of Rare Earths from Magnet Sludge by FeCl2[J]. Materials Transactions, 2002, 43(1): 55-62.
[29] ITOH M, MIURA K, MACHIDA K I. Novel rare earth recovery process on Nd-Fe-B magnet scrap by selective chlorination using NH4Cl[J]. Journal of Alloys and Compounds, 2009, 477(1-2): 484-487.
[30] TOM L, MARTIN B. Recycling of rare earth elements from FeNdB-Magnets via solid-state Chlorination[J]. Journal of Cleaner Production[J]. 2019, 215: 131-143.
[31] HUA Z S, WANG J, WANG L, et al. Selective Extraction of Rare Earth Elements from Nd Fe B Scrap by Molten Chlorides[J]. ACS Sustainable Chemistry and Engineering, 2014, 2 (11): 2536-2543.
[32] STERLING E A, SINCLAI C W. The effect of precipitation on recrystallization in a Mg-Nd alloy[J]. Materialia, 2020, 10: 100643.
[33] KANG Y, JIN L, Chartrand P, et al. Thermodynamic evaluations and optimizations of binary Mg-light rare earth (La, Ce, Pr, Nd, Sm) systems[J]. Calphad, 2012, 38: 100-116.
[34] LIU J N, BIAN D, ZHENG Y F, et al. Comparative in vitro study on binary Mg-RE (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu) alloy systems[J]. Acta Biomaterialia, 2020, 102(15): 508-528.
[35] HUANG G X, ZHANG L G, JIA B, et al. Thermodynamic assessment of the Ag-Nd system[J]. Journal of Alloys and Compounds, 2009, 471(1-2): 176-179.
[36] WANG S L, WANG C P, LIU X J, et al. Thermodynamic assessments of the Ag-Gd and Ag-Nd systems[J]. Journal of Alloys and Compounds, 2009, 476(1-2): 245-252.
[37] TAKEDA O, OKABE T H, UMETSU Y. Recovery of neodymium from a mixture of magnet scrap and other scrap[J]. Journal of Alloys and Compounds, 2006, 408-412(3): 387-390.
[38] TAKEDA O, OKABE T H, UMETSU Y. Phase equilibrium of the system Ag-Fe-Nd, and Nd extraction from magnet scraps using molten silver[J]. Journal of Alloys and Compounds, 2004, 379(1-2): 305-313.
[39] BIAN Y, GUO S, JIANG L, et al. Extraction of Rare Earth Elements from Permanent Magnet Scraps by FeO-B2O3, Flux Treatment[J]. Journal of Sustainable Metallurgy, 2015, 1(2): 151-160.
[40] TAKEDA O, NAKANO K, SATO Y. Recycling of rare earth magnet waste by removing rare earth oxide with molten fluoride[J]. Materials Transactions, 2014, 55(2): 334-341.
[41] PARIDA S C, DASH S, SINGH Z, et al. Thermodynamic Studies on NdFeO3(s) [J]. Journal of Solid State Chemistry, 2002, 164(1): 34-41.
[42] NAKAMOTO M, KUBO K, KATAYAMA Y, et al. Extraction of rare earth elements as oxides from a neodymium magnetic sludge[J]. Metallurgical and Materials Transactions B, 2001, 43(3): 468-476.
[43] SAITO T, SATO H, OZAWA S, et al. The extraction of Nd from waste Nd-Fe-B alloys by the glass slag method [J]. Journal of Alloys and Compounds, 2003, 353(1): 189-193.
[44] YANG Y X, WALTON A, SHERIDAN R, et al. REE recovery from end-of-life NdFeB permanent magnet scrap: a critical review[J]. Journal of Sustainable Metallurgy, 2017, 3(1): 122-149.
[45] 卞玉洋. 从钕铁硼废料中回收稀土元素的新工艺研究[D]. 上海: 上海大学, 2016:57-61.
BIAN Yu-yang. Research on the new technology of recovering rare earth elements from NdFeB waste[D]. Shanghai: Shanghai University, 2016: 57-61.
[46] PADHANA E, NAYAK A K, SARANGI K. Recovery of neodymium and dysprosium from NdFeB magnet swarf[J]. Hydrometallurgy, 2017 (174): 210-215.
[47] LAI W H, LIU M, L C Y, et al. Recovery of a composite powder from NdFeB slurry by co-precipitation[J]. Hydrometallurgy, 2014, 150: 27-33.
[48] JUDGEA W D, AZIMI G. Recent progress in impurity removal during rare earth element processing: A review[J]. Hydrometallurgy, 2020, 196: 105435.
[49] 陈云锦. 全萃取法回收钕铁硼废渣中的稀土与钴[J]. 中国资源综合用, 2004(06): 10-12.
CHEN Yun-jin. Recovery of Rare Earth and Cobalt in NdFeB Waste Residue by Full Extraction Method[J]. China Resources Comprehensive Utilization, 2004(06): 10-12.
[50] TIAN Y L, LIU Z W, ZHANG G Q. Recovering REEs from NdFeB wastes with high purity and efficiency by leaching and selective precipitation process with modified agents[J]. Journal of Rare Earths, 2019, 37: 205-210.
[51] MEHMET A, CHENNA R, MUXING G, et al. Hydrometallurgical recycling of NdFeB magnets: Complete leaching,iron removal and electrolysis[J]. Jouranl of rare earths, 2017, 35(5): 574-584.
[52] 吴继平, 邓庚凤, 邓亮亮, 等. 从钕铁硼废料中提取稀土工艺研究[J]. 有色金属科学与工程, 2016, 7(01): 119-124.
WU Ji-ping, DENG Gen-feng, DENG Liang-liang, et al. Study on the Extraction Process of Rare Earth from NdFeB Waste[J]. Nonferrous Metal Science and Engineering, 2016, 7(01): 119-124.
[53] VACLAV G, MILAN C. New technology for lanthanide recovery from spent Nd-Fe-B magnets[J]. South African Journal of Chemical Engineering, 2020, 33: 35-38.
[54] LIU F P , ANTTI P, WANG J L, et al. Recovery and separation of rare earths and boron from spent Nd-Fe-B magnets[J]. Minerals Engineering, 2020(145): 106097.
[55] 江泽佐, 钟春兰, 卢阶主, 等. 钕铁硼回收料盐酸优溶液氯酸钠氧化法除铁[J]. 化工技术与开发, 2018, 47(08): 55-57+68.
JIANG Ze-zuo, ZHONG Chun-lan, LU Jie-zhu, et al. Removal of Iron in Hydrochloric Acid Preferential Solution of Nd-Fe-B Recovery Material by Sodium Chlorate Oxidation[J]. Technology & Development of Chemical Industry, 2018, 47(08): 55-57+68.
[56] KIKUCHI Y, MATSUMIYA M, KAWAMI S. Extraction of rare earth ions from NdFeB magnet wastes with TBP in tri-capryl-methyl-ammoniumnitrate[J]. Solvent Extraction Research and Development Japan, 2014, 21: 137-145.
[57] 王毅军, 刘宇辉, 郭军勋, 等. 用盐酸优溶法从NdFeB废料中回收稀土[J]. 湿法冶金, 2006, 25(4): 195-197.
WANG Yi-jun, LIU Yu-hui, GUO Jun-xun, et al. Recovery of rare earths from NdFeB waste by hydrochloric acid optimization[J]. Hydrometallurgy of China, 2006, 25(4): 195-197.
[58] ELWERTL T, GOLDMANN D, SCHMIDT F, et al. Hydrometallurgical recycling of sintered NdFeB magnets[J]. World Metall, 2013, 66(4): 209-219.
[59] HOOGERSTAETE V, BLANPAIN B, GERVEN T V, et al. From NdFeB magnets towards the rare-earth oxides: a recycling process consuming only oxalic acid[J]. Royal Society of Chemistry, 2014, 4: 64099-64111.
[60] 尹小文, 刘 敏, 赖伟鸿, 等. 草酸盐沉淀法回收钕铁硼废料中稀土元素的研究[J]. 稀有金属, 2014, 38(6): 1093-1098.
YIN Xiao-wen, LIU Min, LAIi Wei-hong, et al. Study on the Recovery of Rare Earth Elements from NdFeB Waste by Oxalate Precipitation[J]. Rare Metals, 2014, 38(6): 1093-1098.
[61] INNOCENZI V, VEGLIO F. Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations [J]. Journal of Power Sources, 2012, 211: 184-191.
[62] ABRAHAMIS T, XIAO Y, YANG Y. Rare-earth elements recovery from post-consumer hard-disc drives[J]. Mineral Processing and Extractive Metallurgy, 2015, 124(2): 106-115.
[63] 林河成. 从钕铁硼废料制取氧化钕的研究[J]. 中国有色冶金, 1997(3): 31-33.
LIN He-cheng. Study on the preparation of neodymium oxide from NdFeB waste materials[J]. China Nonferrous Metallurgy, 1997(3): 31-33.
[64] 唐杰, 魏成富, 赵导文, 等. 烧结钕铁硼废料中Nd2O3的回收[J]. 稀有金属与硬质合金, 2009, 37(1): 9-18.
TANG Jie, WEI Cheng-fu, ZHAO Dao-wen, et al. Recovery of Nd2O3 from Sintered NdFeB Waste[J]. Rare Metals and Cemented Carbides, 2009, 37(1): 9-18.
[65] PADHANA E, NAYAK A K, SARANGI K. Recovery of neodymium and dysprosium from NdFeB magnet swarf[J]. Hydrometallurgy, 2017, 174: 210-215.
[66] MIURA K, ITOH M, MACHIDA K. Extraction and recovery characteristics of Fe element from Nd-Fe-B sintered magnet powder scrap by carbonylation[J]. Journal of Alloys and Compounds, 2008, 466: 228-232.
[67] LI M R, CHEN Z Q, ZHANG Fe M. Solvent effect on the synergic extraction of praseodymium(III) and neodymium(III) with l-phenyl 3-methyl-4-benzoyl-5pyrazolone and 1,10-phenanthroline[J]. Polyhedron, 1987, 6: 957-962.
[68] RADHIKA S, KUMAR B N, LAKSHMI K M, et al. Solvent extraction and separation of rare-earths from phosphoric acid solutions with TOPS 99[J]. Hydrometallurgy, 2011, 110: 50-55.
[69] HSIANG C K, PEI S Y, RUEY S J. Solvent extraction of La(III) and Nd(III)from nitrate solutions with 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester[J]. Chemical Engineering Journal, 2006, 119: 167-174.
[70] PANDA N, DEVI N, MISHRA S. Solvent extraction of neodymium(III) from acidic nitrate medium using Cyanex 921 in kerosene[J]. Journal of Rare Earths, 2012, 30 (8): 794-797.
[71] EIHEFNY N E. Kinetics and mechanism of extraction and stripping of neodymium using a Lewis cell[J]. Chemical Engineering and Processing, 2007, 46: 623-629.
[72] BANDA R, JEON H, Lee M. Solvent extraction separation of Pr and Nd from chloride solution containing La using Cyanex 272 and its mixture with other extractants[J]. Separation and Purification Technology, 2012, 98: 481-487.
[73] PRESTON J. The recovery of rare earth oxides from a phosphoric acid by product Part 4. The preparation of magnet-grade neodymium oxide from the light rare earth fraction[J]. Hydrometallurgy, 1996, 42: 151-167.
[74] ESMAEIL J, MALEK S. The production of rare earth elements group via tributyl phosphate extraction and precipitation stripping using oxalic acid[J]. Arabian Journal of Chemistry, 2012, 4: 367-372.
[75] TIAN M M, JIANG Q, LIAO W P. Studies on synergistic solvent extraction of rare earth elements from nitrate medium by mixtures of 8-hydroxyquinoline with Cyanex301 or Cyanex302[J]. Journal of Rare Earths, 2013, 31(6): 604-608.
[76] YOON H S, KIM C J, CHUNG K W, et al. Solvent extraction, separation and recovery of dysprosium (Dy) and neodymium (Nd) from aqueous solutions: Waste recycling strategies for permanent magnet processing[J]. Hydrometallurgy, 2016, 165: 27-43.
[77] MOHAMMADI M, FORSBERG K, KLOO L, et al. Rasmuson A.Separation of Nd(III), Dy(III) and Y(III) by solvent extraction using D2EHPA and EHEHPA[J]. Hydrometallurgy, 2015, 156: 215-224.
[78] ZHOU H Y, WANG Y L, GUO X G, et al. The recovery of rare earth by a novel extraction and precipitation strategy using functional ionic liquids[J]. Journal of Molecular Liquids, 2018, 254: 414-420.
[79] MATSUMIYA M, KIKUCHIY, YAMADA T, et al. Extraction of rare earth ions by tri-n-butylphosphate/phosphonium ionic liquids and the feasibility of recovery by direct electrodeposition[J]. Separation and Purification Technology, 2014, 130: 91-101.
[80] LIN X, QU Z, CHEN Y, et al. A novel application of hematite precipitation for high effective separation of Fe from Nd-Fe-B scrap[J]. Scientific Reports, 2019, 9: 18362.
[81] SUN X Q, LUO H, DAI S. Solvent extraction of rare-earth ions based on functionalized ionic liquids[J], Talanta, 2012, 90: 132-137.
[82] GIRIDHAR P, VENKATENSAN K A, SUBRAMANIAM S, et al. Extraction of uranium (VI) by 1.1M tri-n-butylphosphate/ionic liquid and the feasibility of recovery by direct electrodeposition from organic phase[J]. Journal of Alloys and Compounds. 2008, 448: 104-108.
[83] 兰超群. 利用湿法与电沉积技术回收超细钕铁硼废料中稀土与铁的研究[D]. 包头: 内蒙古科技大学, 2019: 22-29.
LAN Chao-qun. Research on the recovery of rare earth and iron in ultrafine neodymium iron boron waste using wet method and electrodeposition technology[D]. Baotou: Inner Mongolia University of Science and Technology, 2019: 22-29.
[84] DTUUA T, KIM K H, UCHIMIYA M, et al. Global demand for rare earth resources and strategies for green mining[J]. Environmental Research, 2016, 150: 182-190.
[85] PRAKASH V, SUNB Z, SIETSMA J, et al. An environmentally friendly electro-oxidative approach to recover valuable elements from NdFeB magnet waste[J]. Separation and Purification Technology, 2018, 191: 384-391.
[86] IRINA M, EKATERINA S, MARIA O, et al. Electrochemical leaching of rare-earth elements from spent NdFeB magnets[J]. Hydrometallurgy, 2020, 192: 105264.
[87] YANG Y S, LAN C Q, WANG Y C, et al. Recycling of ultrafine NdFeB waste by the selective precipitation of rare earth and the electrodeposition of iron in hydrofluoric acid[J]. Separation and Purification Technology, 2020, 230: 115870.
[88] SAND W, GEHRKE T, JOZS P G, et al. (Bio) chemistry of bacterial leaching-direct vs indirect bioleaching[J]. Hydrometallurgy, 2001, 59: 159-175.
[89] ASGHARI I, MOUSAVI S M, AMIRI F, et al. Bioleaching of spent refinery catalysts: a review[J]. J Ind Eng Chem. 2013, 4: 1069-1081.
[90] LEE J, PANDEY B D. Bio-processing of solid wastes and secondary resources for metal extraction-a review[J]. Waste Manage. 2012, 32: 3-18.
[91] DEPLANCHE K, MURRAY A, MENNAN C, et al. Biorecycling of precious metals and rare earth elements[J]. Nanomaterials, 2011, 14: 279-314
[92] ROMY A, KATRIN B, RUDOLF S, Oliver Gutfleisch, Sylvia Schnell, Stefan Ratering. Critical raw materials-Advanced recycling technologies and processes: Recycling of rare earth metals out of end of life magnets by bioleaching with various bacteria as an example of an intelligent recycling strategy[J]. Minerals Engineering, 2019, 134: 104-117.
[93] SHERIDAN R S, SILLITON R, ZAKOTINK M. Anisotropic powder from sintered NdFeB magnets by the HDDR processing route[J]. Journal of Magnetism and Magnetic Materials, 2012, 324(1): 63-67.
[94] ZAKOTNIK M, HARRIS I R, WILLAMS A J. Possible methods of recycling NdFeB-type sintered magnets using the HD/degassing process[J]. Journal of Alloys and Compounds, 2008, 450(450): 525-531.
[95] 李现涛, 岳明, 李萌, 等. 块状烧结钕铁硼废料的氢处理回收技术研究进展[J]. 稀土, 2016, 37(03): 123-128.
LI Xian-tao, YUE Ming, LI Meng,et al. Research progress in hydrogen treatment and recovery technology of massive sintered NdFeB waste[J]. Chinese Rare Earths, 2016, 37(03): 123-128.
[96] 吴继平. 从钕铁硼废料中提取稀土工艺及机理研究[D]. 赣州: 江西理工大学, 2015:67-68.
WU Ji-ping. Study on the technology and mechanism of rare earth extraction from NdFeB waste materials[D]. Ganzhou: Jiangxi University of Science and Technology, 2015: 67-68.
[97] MIURA K, ITOH M, MACHIDA K I. Extraction and Recovery Characteristics of Fe Element from Nd-Fe-B Sintered Magnet Powder Scrap by Carbonylation [J]. Journal of Alloys and Compounds, 2020, 466(1-2): 228-232.
[98] 中华人民共和国科学技术部. 日本开发出从废磁铁中高效回收稀土钕的新技术[J]. 稀土, 2014, 35(04): 34.
Ministry of Science and Technology of the People’s Republic of China. Japan has developed a new technology for the efficient recovery of rare earth neodymium from waste magnets[J]. Chinese Rare Earths, 2014, 35(04): 34.
[99] SASAI R, SHIMAMURAN. Technique for recovering rare-earth metals from spent sintered Nd-Fe-B magnets without external heating[J]. Journal of Asian Ceramic Societies, 2016, 4(2): 155-158.
Current Status and Prospect of Recycling and Utilization of Rare Earth in NdFeB Permanent Magnet Waste
SONG Qiang 1,2,3, TONG Xiong1,2, XIE Xian1,2, ZHANG Wen-jie1,2, CAO Yang1,2, DU Yun-peng1,2, CHENG Ya-zhi1,2
(1. Faculty of Land and Resources Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China;
2. National and Local Joint Engineering Research Center for green Comprehensive Utilization of Gangue Resources from Metal Tailings Resources, Kunming 650093, China;
3.Yunnan Yuankuang Technology Development Co. LTD, Kunming 650093, Yunnan, China)
Abstract:Neodymium iron boron (NdFeB) permanent magnet materials are widely used in many fields. In the process of producing NdFeB permanent magnet materials, a large amount of waste will be generated. Direct discarding will not only pollute the environment, but also waste secondary resources. This article summarizes the technical status of the recycling of rare earths in NdFeB permanent magnet waste. The current technical methods for treating rare earths in waste are divided into fire method and wet method. Among them, comparative analysis of the current situation and shortcomings of fire methods in industrial applications such as chlorination method, alloy method, and selective oxidation method; The research status of wet methods such as solvent extraction method has deeply analyzed the limitations and advantages of different methods; at the same time, the research status of new technology methods such as electrodeposition method, biological leaching method, hydrogenation method and mechanochemical method are introduced. On this basis, the future development direction of recycling and recycling of rare earth technology in NdFeB permanent magnet waste is prospected.
Keywords:NdFeB permanent magnet material; Waste; Rare earth;Recycling; Fire method; Wet method
Foundation item:Project (U1802252) supported by the National Natural Science Joint Fund
Received date: ;Accepted date:
Corresponding author:XIE Xian; Tel: 15987188290;E-mail:kgxianxie@126.com。
基金项目:国家自然科学联合基金资助项目(U1802252)
收稿日期: ;修订日期:
通信作者:谢贤,副教授,博士。电话:15987188290;E-mail:kgxianxie@126.com。
[9] 华经情报网. 2018年高性能钕铁硼磁材行业现状及其发展趋势[DB/OL]. http://www.huaon.com/story/423220.
[16] 陈丽杰, 李子良, 龚傲, 等. 从稀土废料中回收稀土的研究进展[J]. 中国稀土学报, 2019, 37(3): 259-272.
[23] 石富. 稀土永磁材料制备技术[M]. 冶金工业出版社, 2007: 35-37.
[24] 王龙君. 钕铁硼废料中稀土的选择性分离与回收研究[D]. 赣州: 江西理工大学, 2020: 11-12.
[45] 卞玉洋. 从钕铁硼废料中回收稀土元素的新工艺研究[D]. 上海: 上海大学, 2016:57-61.
[49] 陈云锦. 全萃取法回收钕铁硼废渣中的稀土与钴[J]. 中国资源综合用, 2004(06): 10-12.
[52] 吴继平, 邓庚凤, 邓亮亮, 等. 从钕铁硼废料中提取稀土工艺研究[J]. 有色金属科学与工程, 2016, 7(01): 119-124.
[55] 江泽佐, 钟春兰, 卢阶主, 等. 钕铁硼回收料盐酸优溶液氯酸钠氧化法除铁[J]. 化工技术与开发, 2018, 47(08): 55-57+68.
[57] 王毅军, 刘宇辉, 郭军勋, 等. 用盐酸优溶法从NdFeB废料中回收稀土[J]. 湿法冶金, 2006, 25(4): 195-197.
[60] 尹小文, 刘 敏, 赖伟鸿, 等. 草酸盐沉淀法回收钕铁硼废料中稀土元素的研究[J]. 稀有金属, 2014, 38(6): 1093-1098.
[63] 林河成. 从钕铁硼废料制取氧化钕的研究[J]. 中国有色冶金, 1997(3): 31-33.
[64] 唐杰, 魏成富, 赵导文, 等. 烧结钕铁硼废料中Nd2O3的回收[J]. 稀有金属与硬质合金, 2009, 37(1): 9-18.
[83] 兰超群. 利用湿法与电沉积技术回收超细钕铁硼废料中稀土与铁的研究[D]. 包头: 内蒙古科技大学, 2019: 22-29.
[95] 李现涛, 岳明, 李萌, 等. 块状烧结钕铁硼废料的氢处理回收技术研究进展[J]. 稀土, 2016, 37(03): 123-128.
[96] 吴继平. 从钕铁硼废料中提取稀土工艺及机理研究[D]. 赣州: 江西理工大学, 2015:67-68.
[98] 中华人民共和国科学技术部. 日本开发出从废磁铁中高效回收稀土钕的新技术[J]. 稀土, 2014, 35(04): 34.


