
Removing cadmium from electroplating wastewater by waste saccharomyces cerevisiae
DAI Shu-juan(代淑娟)1, 2, WEI De-zhou(魏德洲)1, ZHOU Dong-qin(周东琴)1,
JIA Chun-yun(贾春云)1, WANG Yu-juan(王玉娟)1, LIU Wen-gang(刘文刚)1
1. School of Resources and Civil Engineering, Northeastern University, Shenyang 110004, China;
2. Shenyang Research Institute of Non-ferrous Metal, Shenyang 110141, China
Received 25 September 2007; accepted 7 January 2008
Abstract:
The appropriate condition and scheme of removing cadmium from electroplating wastewater were investigated by adsorption-precipitation method using waste saccharomyces cerevisiae(WSC) as sorbent. Effect factors on biosorption of cadmium in cadmium-containing electroplating wastewater by waste saccharomyces cerevisiae and precipitation process of waste saccharomyces cerevisiae after adsorbing cadmium were studied. The results show that removal rate of cadmium is over 88% after 30 min adsorbing under the condition of cadmium concentration 26 mg/L, the dosage of waste saccharomyces cerevisiae 16.25 g/L, temperature 18 ℃, pH 6.0 and precipitation time 4 h. Biosorption-precipitation method is effective to remove cadmium in cadmium-containing electroplating wastewater by waste saccharomyces cerevisiae. The SEM, infrared spectroscopy and Zeta-potential of the cells show that chemical chelating is the main adsorption form; electrostatic attraction, hydrogen bonding and van der Waals force all function in adsorption process; and ―NH2―,―C=O―,―C=O―NH―,―CH3, ―OH are the main adsorption groups.
Key words:
electroplating wastewater; waste saccharomyces cerevisiae; cadmium biosorption;
1 Introduction
Electroplating is one of the three main industries that discharge heavy metals in the world. And, with the development of electroplating, the quantity of the electroplating wastewater increases fast in these years. The cadmium concentration of the cadmium-containing electroplating wastewater after treatment cannot meet the domestic discharge standard[1-3], so removal of cadmium in electroplating wastewater is very important. The cadmium will first pollute the ground and water, then it will enter the grist, vegetable and poultry, even human beings through food chain, causing diseases such as renal damage and anemia due to excess cadmium. At present, many technologies, such as sulfuration method, electrolyse, membrane and ion-exchange process, can be used for the treatment of wastewater polluted by heavy metals. However, these methods are less effective and more expensive when heavy metal concentration in the wastewater is low, and some of them are easy to cause the second pollution[4].
Removal of heavy metals by biosorption has many advantages, such as fast adsorption speed, removing heavy metal ions selectively under low concentration, high adsorption efficiency, wide range of pH and temperature, less investment and running cost, in addition, some heavy metals can be recovered[5]. Recently, many experts have done a lot of work in biosorption of heavy metals[6-14]. And there are more and more research about the biosorption of heavy metals by waste saccharomyces cerevisiae in food industry. Because of abundant waste saccharomyces cerevisiae and low price, heavy metals can be recycled by burning. This has more obvious advantages compared with other biosorbents.
The appropriate condition and scheme of removing cadmium from electroplating wastewater were investigated by adsorption-precipitation method by waste saccharomyces cerevisiae. And a new approach is explored to utilize waste saccharomyces cerevisiae and remove cadmium in cadmium-containing wastewater.
2 Experimental
2.1 Experimental material and object of disposal
Waste saccharomyces cerevisiae used in experiment was obtained from a brewhouse. It was crisp, in piece shape and had aroma. It wasn’t active, and their morphology was round or ellipse with diameter of 4-8 μm. The microscopic image of WSC is shown in Fig.1.
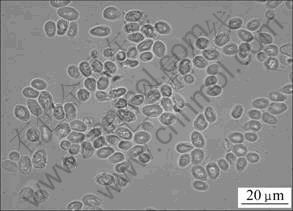
Fig.1 Microscopic image of waste saccharomyces cerevisiae
The concentration of cadmium in the electroplating wastewater is about 26 mg/L, and there are also a little lead, zinc and chrome in it. The waste water is non-color, non-odor and translucent, and the pH and density are about 8.0 and 1×103 kg/m, respectively.
2.2 Experimental method
The biosorption experiments were carried out in batches as follows. Waste saccharomyces cerevisiae and the cadmium-containing electroplating wastewater were put into beaker, according to different ratio. And then, the solution in the beaker was mixed by magnetic force (THZ-92B desk constant temperature vibration vessel). The samples were centrifuged (desk centrifugal machine, TGL-16G) at 15 000 r/min for 5 min and the liquid was used to determine metal ion concentration. The absorption rate is expressed as
Q=(1-ρ/ρ0)×100% (1)
where ρ0 and ρ are the concentrations of cadmium in the liquid before and after biosorption, respectively, mg/L.
Electroplating wastewater after adsorption by waste saccharomyces cerevisiae was put into the vessel for certain time. Suspension and deposit were centrifuged respectively at 15 000 r/min for 5 min. And the solid was dried in low temperature and weighted. The precipitation efficiency R is calculated as
R=m2/(m1+m2)×100% (2)
where m1 and m2 are masses of the waste saccharomyces cerevisiae in the suspension and deposit, respectively, mg.
The equation about the removing rate of cadmium after biosorption and precipitation can be expressed:
M=Q×R×100% (3)
The concentrations of heavy metals were analyzed by an atomic absorption spectrophotometer (AA-6300, Japan).
The waste saccharomyces cerevisiae with and without cadmium were washed by distilled water for three times, fixed after airing in natural condition, dehydrated by ethanol, and was sprayed by gold. The surface characteristics were observed by Scanning Electrical Microscope(SSX-550).
Infrared absorption spectra were recorded on a Perkin Elmer Spectrum One FT-IR Spectrophotometer using different dried biomass before and after biosorption. The cells were washed using distilled water for three times and dried at 50-60 ℃. The sample was mixed with KBr at ratio of 1?100 and compacted to pellet under high pressure.
3 Results and analysis
3.1 Biosorption experiment results
The biosorption of heavy metals is a complicated process, which is affected by adsorbent, kind and concentration of heavy metals and other environmental factors. Effects of pH, dosage of adsorbent, contact time, temperature on adsorption were studied under the condition of concentration of cadmium in wastewater 26 mg/L, and stirring speed 800 r/min.
3.1.1 Effect of pH
pH value can affect the chemical characteristic, activity of functional groups and competition of metal ions in solution[15]. pH was measured by acidity meter ( PHS-25) and was adjusted by HCl and NaOH, under the condition of biomass of waste saccharomyces cerevisiae 15 g/L, at room temperature about 18 ℃ and contacting time 10 min. The effects of pH value on cadmium adsorption are shown in Fig.2.
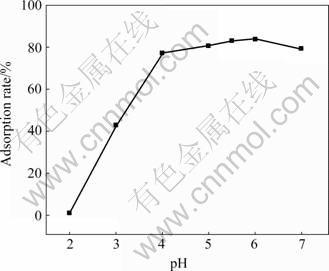
Fig.2 Effect of pH on adsorption
From Fig.2, the adsorption rate of cadmium is low when the pH is lower than 4.0. The better adsorption result is obtained in the pH range of 4.0-7.0, and the adsorption rate reaches 83.71% when pH value is 6.0. The pH value of the solution becomes about 7 after adsorption, which shows that waste saccharomyces cerevisiae adsorbs cadmium and H+ so that pH value of the solution rises. It is obvious that the waste saccharomyces cerevisiae can adsorb cadmium in a wide range of pH, and it has the best adsorption effect when the solution is nearly neutral. The characteristic of this technology is beneficial to industrialized practice because the cost is lower, operation is more convenient,equipment eroding can be avoided or decreased and pH can be adjusted according to discharge standard of water.
3.1.2 Effect of dosage of adsorbent
The effects of dosage of adsorbent were observed under the condition of pH 6.0, room temperature 18 ℃, contact time 10 min, as shown in Fig.3.
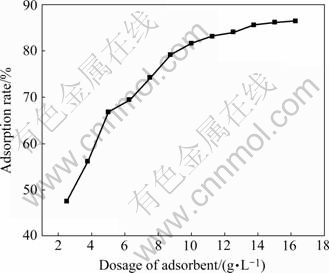
Fig.3 Effect of dosage of adsorbent on adsorption
From Fig.3, it can be seen that adsorption rate of cadmium increases as the dosage of adsorbent increases. The adsorption rate increases slowly, when the dosage of adsorbent increases from 15 to 16.25 g/L. In practical operation, dosage of adsorbent, ratio of capability and price should be considered in order to approach to certain adsorption rate. when the dosage of adsorbent is just at 16.25 g/L, the adsorption rate gets to 86.44%.
3.1.3 Effect of temperature
HCl was used to adjust pH to 6.0. The dosage of the waste saccharomyces cerevisiae was 16.25 g/L, and contact time was 10 min. According to the characteristic of climate, the experiments were performed between 16 ℃ and 34 ℃, and the effect of temperature was studied. The results are shown in Fig.4.
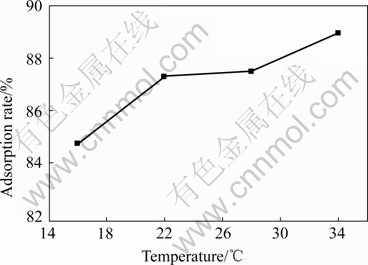
Fig.4 Effects of temperature on adsorption
Fig.4 shows that, the adsorption rate goes up with the increase of temperature from 16 ℃ to 34 ℃, but the increase is not obvious. And when temperature changes between 22 ℃ and 28 ℃, the adsorption rate can get over 87%. In practical operation, the running condition and the cost of further treatment should be considered. The techniques can be worked in higher temperature season without heating equipment.
3.1.4 Effect of contact time
By adjusting pH to 6.0, using the concentration of the waste saccharomyces cerevisiae 16.25 g/L, at room temperature (about 18 ℃), the effect of contact time was observed. The result is shown in Fig.5.
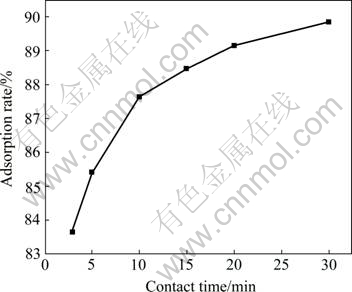
Fig.5 Effect of contact time on adsorption
Fig.5 shows that, the adsorption speed is large, and the adsorption rate is over 83% after adsorption for 3 min. The adsorption rate ascends with the increase of contact time, which approaches 89.85% after adsorption for 30 min. The fast adsorption is propitious to the practical application, which can decrease operation time, improve efficiency and increase the treatment quantity of wastewater.
3.2 Precipitation experiment result
The solid-liquid separation is the important process in the biosorption method. Because of small pellet diameter, little density, and low intensity, solid-liquid separation is difficult in the application of water treatment, which limits the application of the industrialization of biosorption method in waste water treatment. The familiar methods of solid-liquid separation are sedimentation, filtration and centrifugation. In this experiment, the precipitation rate of waste saccharomyces cerevisiae is fast, in addition, it has some advantages, such as no driving setting, simple operation, low cost and no impact adsorption process, so the precipitation experiment is carried out.
40 mL electroplating wastewater with pH 6.0 and 0.65 g waste saccharomyces cerevisiae were put into beaker of 100 mL, magnetically stirred for 30 min under the stirring speed of 800 r/min. And then the system was stayed statically. So the precipitation rate was investigated and the removal rate of cadmium was calculated. The results are shown in Fig.6.
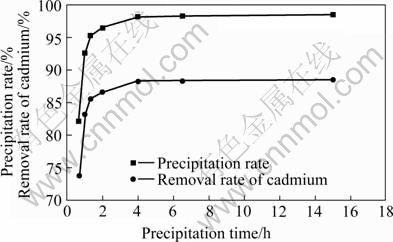
Fig.6 Effect of precipitation time on precipitation rate and removal rate of cadmium
As illustrated in Fig.6, the precipitation characteristic of waste saccharomyces cerevisiae is better. When it is precipitated for 40 min, the precipitation rate reaches over 82%, and the precipitation rate of waste saccharomyces cerevisiae increases with the increase of the precipitation time. Removal rate of cadmium gets over 88% when it is precipitated for 4 h. After the third treatment, cadmium concentration in the wastewater meets domestic discharge standard. After precipitation for more than 4 h, the removal rate rises up slowly. Therefore, if precipitation time is short, the effect is not good; while the time is long, it is no practice meaning, so the appropriate precipitation time is 1-4 h. When proper precipitator was added into the process of precipitation, it would accelerate the precipitation speed and shorten the precipitation time. But it should be considered that precipitator has no disadvantages for biosorption process.
3.3 Analysis
In the condition of adsorbing for 30 min with cadmium concentration in cadmium-containing electroplating wastewater about 26 mg/L, waste saccharomyces cerevisiae 16.25 g/L, temperature 18 ℃, pH 6.0, the result was analyzed as follows.
3.3.1 Surface characteristic of waste saccharomyces cerevisiae before and after biosorption
The SEM photographs of waste saccharomyces cerevisiae before and after biosorption are shown in Fig.7.

Fig.7 SEM images of waste saccharomyces cerevisiae (a) and waste saccharomyces cerevisiae contacting with cadmium (b)
As shown in Fig.7, there are some buds on the surface of cell, and it is slippery before biosorption; while there are some tiny holes on the surface of cell after biosorption, perhaps because some solutes, such as amylose on the surface of cell, go into the liquid from the cell. And on the surface of cell, there are also some gaps or holes, which proves that the structure of the cell is damaged in the process of biosorption. Probably, cadmium is adsorbed on the surface of the cell, and there are some pellets after biosorption.
3.3.2 FT-IR analysis
The representative FT-IR spectra of waste saccharomyces cerevisiae before and after biosorption are shown in Figs.8(a) and (b), respectively.
As shown in Fig.8(a), the broad strong band at about 3 411 cm-1 is assigned to the stretching vibrations of the amidocyanogen —NH2 and hydroxyl groups―OH in amylose. At about 2 925 cm-1, there is an asymmetric―CH2― stretching and symmetric―CH2― stretching vibration. 1 644 cm-1 band is the result of the stretching vibration of —C=O— and —NH— (amideⅠ) peptidic bond of protein, while 1 536 cm-1 band shows bending vibration of ―O=C―NH― (amide Ⅱ) peptidic bond of protein. The band at 1 457 cm-1 represents cutting vibration peak of ―CH2― overlaping an asymmetric bending vibration peak of ―CH3, while the band at 1399 cm-1 results in a bending vibration of ―COOH and ―OH. The band at about 1 241 cm-1 is assigned to the vibration of the hydroxyl group ―OH in amylase[16-17].
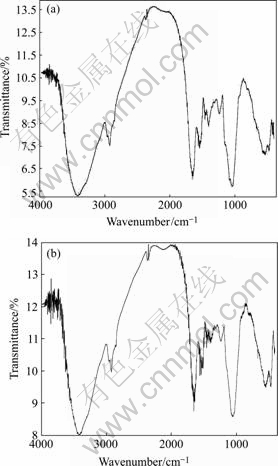
Fig.8 FT-IR spectra of waste saccharomyces cerevisiae before and after biosorption: (a) Waste saccharomyces cerevisiae; (b) Waste saccharomyces cerevisiae contacting with cadmium
Typical spectrum for waste saccharomyces cerevisiae contacting with cadmium is given in Fig.8(b). Compared with the waste saccharomyces cerevisiae before adsorbing cadmium, the band at 3 411 cm-1 shifts to 3 419cm-1, the band at 1 644 cm-1 to 1 648 cm-1 and the band at 1 536 cm-1 to 1 523 cm-1, which indicates that cadmium interacts with ―C=O―, ―OH, and―NH 2. And this might be attributed to the single-twin electron provided by N atom in the functional groups, such as —NH2—,—C=O—NH— and —NHCOCH3, resulting in the change of the polarity of groups. The band at 1 457 cm-1 shifts to 1 445 cm-1, which indicates that hydrogen bonding and van der Waals force all work in the adsorption. The band at 1 399 cm-1 shifts to 1 391 cm-1 and 1 241 cm-1 to 1 246 cm-1, which shows that―COOH and —OH groups are concerned with the biosorption of cadmium. So, it is obvious that —NH2—, —C=O—, —C=O—NH—, —CH3 and ―OH all function in the adsorption.
3.3.3 Potential analysis
The cell wall of the waste saccharomyces cerevisiae was made up of special yeast cellulose with polysaccharide, amrita polysaccharide and protein as main components. And the structure determined that when the pH of waste saccharomyces cerevisiae changed, the surface of the cell would adsorb or ionize some H+, so that the surface of the waste saccharomyces cerevisiae had some charges. Zeta potential of waste saccharomyces cerevisiae and waste saccharomyces cerevisiae adsorbing cadmium under different pH is shown in Fig.9.
The surface of cell is charged due to the presence of functional groups such as carboxyl (―COOH), amino (―NH2) and hydroxyl (―OH), originated from the cell wall components of lipopolysacharides, lipoproteins and bacterial surface proteins. The Iso-electric point of waste saccharomyces cerevisiae accounted for the balance between the acid-alkali groups of negative ions and positive ions.
As shown in Fig.9, Zeta potential of waste saccharomyces cerevisiae is about 2.4, and its surface is made mainly of amylose[18]. When pH<2.4, the surface of waste saccharomyces cerevisiae has positive electricity, while pH>2.4, the surface of waste saccharomyces cerevisiae has negative electricity. When pH is lower than 6.0, the potential decreases with the increase of pH. Fig.2 shows that when pH<6.0, the adsorption rate of cadmium by waste saccharomyces cerevisiae increases with the increase of pH. Electrostatic attraction works in the adsorption process between waste saccharomyces cerevisiae whose surface loads negatively with Cd2+. With the increase of static attraction, the adsorption rate of positive ion Cd2+ rises up and the static attraction is one of the adsorption form.
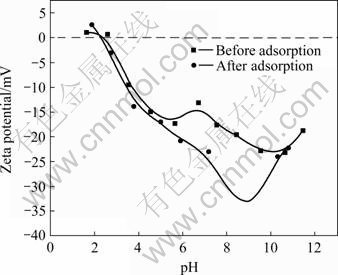
Fig.9 Zeta potential before and after waste saccharomyces cerevisiae adsorbing cadmium
Fig.9 also shows that at pH 2.4-11, Zeta potential of waste saccharomyces cerevisiae after adsorbing cadmium becomes smaller, in other words, the absolute value of negative potential becomes larger. The main cause is that the soluble metal positive ion in the waste saccharomyces cerevisiae such as K+, Na+, Ca2+ and Mg2+ dissolves and enters liquid; at the same time, waste saccharomyces cerevisiae adsorbs metal positive ion as cadmium. The dissolving content of positive ion is larger than its adsorbing content by waste saccharomyces cerevisiae, so absolute value of negative potential on the surface of waste saccharomyces cerevisiae becomes larger. For example, when pH=6, concentrations of K+, Na+, Ca2+ and Mg2+ in wastewater are 9.13, 338.06, 22.82 and 20.27 mg/L, respectively, and they are 402.2, 282.6, 16.00 and 34.95 mg/L after being adsorbed by waste saccharomyces cerevisiae. The change valves of concentration of K+, Na+, Ca2+ and Mg2+ are +393.1, -55.2, -6.8, +14.7 mg/L, while cadmium concentration in waste water decreases to 1.9 mg/L from 26 mg/L. The overall positive ion in wastewater increases obviously, and the overall positive ion in the waste saccharomyces cerevisiae decreases reverse, so absolute value of negative potential on the surface of waste saccharomyces cerevisiae becomes larger than that before adsorption.
4 Conclusions
1) The adsorption rate of cadmium in electroplating wastewater can reach 89.85% by waste saccharomyces cerevisiae under the optimum condition.
2) The waste saccharomyces cerevisiae is precipitable. When it is precipitated for 1 h, the precipitation rate is over 92%, when it is precipitated for 4 h, precipitation rate gets to 98%, and the removal rate of cadmium is over 88%. The wastewater can reach domestic discharge standard after three-step treatment.
3) The mechanism analysis shows that the chemical chelating is the main adsorption form. Electrostatic attraction hydrogen bonding and van der Waals force all function in the process of adsorption.
4) Removal of cadmium from cadmium-containing electroplating wastewater by biosorption-precipitation using waste saccharomyces cerevisiae as sorbent has the advantages of simple process, low running cost, energy saving and environmental protection.
References
[1] WANG P, MIN X B, CHAI L Y. The status of treatment technology on wastewater containing cadmium and the development of its bio-treatment technology [J]. Industrial Safety and Dust Control, 2006, 32(8): 14-16.
[2] China National Enrollment Committee. Universal tutorial of basic knowledge about ISO14000 environment management system by national enrollment assessor [M]. Beijing: China Computation Press, 2000.
[3] QIU T S, CHENG X X, HAO Z W, LOU X P. Present situation and development for wastewater containing cadmium treatment technology [J]. Sichuan Nonferrous Metals, 2002, (4): 38-41.
[4] WU J, LI Q H, DENG X, LU Y H. Studies on biosorption of Pb2+ by phanerochaete chrysosporium [J]. Acta Microbiologica Sinica, 1999, 19(1): 87-90.
[5] ZHOU D Q, WEI D Z. On the antagonism against of heavy metals to biosorption of Cu2+, Pb2+ and Hg2+ by gordona amarae [J]. Journal of Northeastern University (Natural Science), 2005, 26(3): 304-306.
[6] VALENTINA V. Umrania, 2006. Bioremediation of toxic heavy metals using acidothermophilic autotrophies [J]. Bioresource Technology, 97(10): 1237-1242.
[7] V?TOR J P V, CID?LIA M S B, Boaventura R A R. Equilibrium and kinetic modelling of Cd(II) biosorption by algae gelidium and agar extraction algal waste [J]. Water Research, 2006, 40(2): 291-302.
[8] LODEIRO P, REY-CASTRO C, BARRIADA J L, SASTRE DE VICENTE M E, HERRERO R. Biosorption of cadmium by the protonated macroalga sargassum muticum: Binding analysis with a nonideal, competitive, and thermo- dynamically consistentadsorption (NICCA) model [J]. Journal of Colloid and Interface Science, 2005, 289(2): 352-358.
[9] ZHANG X L, LIU Y Y. Biosorption of precious and heavy metals [J]. Chinese Journal of Applied and Environmental Biology, 2002, 8(6): 668-671.
[10] QIU T S, CHENG X X. Study on cadmium biosorption by saccharomyces cerevisiae [J]. Environmental Pollution, Prevention and Cure, 2004, 26(2): 95-97.
[11] ZHOU D Q, WEI D Z. Biosorptive-flotation and desorption operation of heavy metals from wastewater effluents by gordona amarae [J]. Environmental Science, 2006, 27(5): 961–964.
[12] YANG J, VOLESKY B. Cadmium biosorption rate in protonated sargassum biomass [J]. Environmental Science and Technology, 1999, 33: 751-757.
[13] WU X Q, ZHENG L. Research and application of fungi in environment protection [J]. Journal of Nanjing Forestry University(Natural Sciences Edition), 2002, 26(5): 76-80.
[14] SADOWSKI Z. Effect of biosorption of Pb(Ⅱ), Cu(Ⅱ) and Cd(Ⅱ) on the zeta potential and flocculation of nocardia sp.[J]. Minerals Engineering, 2001, 14(5): 547-552.
[15] VOLESKY B. Sorption and biosorption, Chapter 6, Evaluation of Biosorption Performance[M]. BV-Sorbex, Inc, St. Lambert, Quebec, 2003: 103-116.
[16] ZHU H W. Spectrum analysis of organic molecule structure[M]. Beijing: Chemical Technical Press, 2005.
[17] WANG F L. Organic chemistry experiment [M]. Wuhan: Wuhan University Publishing Company, 2001: 319-320.
[18] DAI S J, ZHOU D Q, WEI D Z, LIU W G. Study on using Bacillus mucilaginosus adsorption to treat Pb2+ in water by biosorption- flotation process [J]. Metals Mine, 2007(5): 70-74.
Foundation item: Project(50174014) supported by the National Natural Science Foundation of China; Project(20042021) supported by the Natural Science Foundation of Liaoning Province, China; Project(2006223002) supported by the High Science and Technology Plan of Liaoning Province, China
Corresponding author: DAI Shu-juan; Tel: +86-24-83678534; E-mail: shujuandai@163.com
(Edited by YANG Bing)


