
Influence of recrystallization and environment on tensile behavior of cold-rolled Ni3Al(Zr) alloys
LI Yu-fang(李玉芳)1, GUO Jian-ting(郭建亭)2, SHEN Yi-fu(沈以赴)1
1. College of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics,
Nanjing 210016, China;
2. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 5 July 2005; accepted 12 December 2005
Abstract:
The effects of recrystallization and environment (vacuum versus air) on tensile properties of B-free Ni3Al (Zr) alloys were investigated. The results indicate that the incompletely recrystallized and stress-relieved specimens show the most desirable ductility and ultimate tensile strength, and that the recrystallization treatment promotes susceptibility to the test environment of the alloys. It is found that the amount of ductile fracture is reduced by air for completely recrystallized specimens. The Auger analyses show that Zr atoms do not segregate to the grain boundaries(GBs) for specimens heat-treated at 1 100 ℃, however Zr atoms segregate to the GBs for specimens heat-treated at 900 ℃. These results imply that Zr-doping cannot suppress environmental embrittlement.
Key words:
Ni3Al; Ni3Al(Zr); Zr-doping; recrystallization; tensile properties; environmental embrittlement;
1 Introduction
Intermetallic compounds have long been the subjects of considerable interest for high temperature applications. In particular, Ni3Al is one of the more promising intermetallic compounds due to its anomalous temperature dependence of the yield strength, good oxidation resistance and lower density compared with superalloys[1-4]. However, it has a propensity to brittle intergranular fracture in polycrystalline forms under air environment. Some studies by LIU et al[5-7] have shown that low ductility and brittle fracture in Ni3Al is caused not only by intrinsic factors but also by extrinsic factors. Environmental degradation is found to be a major cause as an extrinsic factor in many ordered intermetallics including Ni3Al.
AOKI and IZUMI[8] found that the addition of a small amount of boron to polycrystalline Ni3Al significantly improves the intrinsic ductility and changes the fracture mode from intergranular to completely transgranular one, however low strength and low creep resistance at elevated temperature remains[9-11]. Fortunately, Mishima et al[12] found that zirconium increases the yield strength in B-doped Ni3Al by solid solution strengthening, and study showed that the creep rupture life of Ni3Al (B) increases with the Zr addition. It is now generally recognized that a small amount of Zr addition ductilizes Ni and Ni-base superalloys by suppressing segregation of impurities such as S to grain boundaries(GBs) through sulfide formation. Furthermore, GUO et al[13], George et al[6] found the ductilization of boron-free Ni3Al by Zr additions alone. Efforts are now underway to understand this ductilization mechanism better.
Heat treatment is another way to improving the intrinsic ductility and reducing the environmental embrittlement of intermetallics. SU et al [14] studied the effect of recrystallization on tensile properties of a Ni3Al-based alloy at room temperature and found that the best ductility was attained in specimens that had been heat-treated to complete recrystallization. The ductile fracture mode remained unchanged when the test environment changed from vacuum to air. For Ni3Al (Zr) alloy, information on the influence of recrystallization and environment on this material has not been reported yet.
The object of this paper is to describe the influence of recrystallization and environment on the tensile properties at room temperature for B-free Ni3Al (Zr)alloy, and to determine whether the heat treatment is an effective method for improving the intrinsic ductility. The ductility mechanism of Zr addition is also discussed.
2 Experimental
Four Ni3Al(Zr) alloys were prepared by melting and casting in a vacuum induction furnace, the ingots were homogenized at 1 100 ℃ for 4 h followed by cold- rolled repeatedly into around 2 mm-thick sheets with intermediate annealing at 1 050 ℃ for 30 min. The nominal compositions were based on the formula Ni77Al1-xZrx, where x is the mole fraction of Zr, it is 0.2, 0.6, 1.0 and 1.5 respectively. The agreement between the nominal and analyzed chemical composition was fairly good. Tensile specimens with a gage section of 2 mm×2.5 mm×16 mm were cut from the sheet with the gauge length parallel to the rolling direction. A set of samples were annealed isochronally for 2 h at temperatures ranging from 800 ℃ to 1 100 ℃, with an interval of 50 ℃. After these heat treatments, the gauge sections of the specimens were polished through 1 200 grit to remove the thin layer formed during annealing.
Tensile tests were performed at room temperature with an initial strain rate of 1.04×10-3 s-1 on a Gleeble 1500 test machine equipped with a vacuum chamber. Environmental effects were investigated under two conditions: ambient room air and a vacuum of about 5×10-3 Pa. The microstructures and fracture surfaces of the tensile specimens were examined by optical and scanning electron microscopes(SEM), respectively. The studies of GBs were conducted using an EM420 transmission electron microscope(TEM) equipped with EDAX and LAS3000 auger electron spectroscopy(AES). The recrystallized fraction was estimated under a metallurgical microscope, and the grain sizes were measured by intercept method.
3 Recrystallization effect
3.1 Microstructure
Some typical optical micrographs of samples annealed for 2 h at temperatures ranging from 850 ℃ to 1 100 ℃ are given in Fig.1. It has been observed that the microstructure evolution is dependent on temperature strongly.
The original GBs and deformation bands preferentially serve as effective nucleation sites at the early stage of recrystallization. In these regions new recrystallized grains can be observed(Fig.1(c)). With the proceeding of recrystallization, the volume fraction of recrystallized structure increases, and the whole microstructure changes from the deformation bands introduced by cold rolling to complete recrystallization by annealing at 1 050 ℃. After annealing at 1 100 ℃(Figs.1(b) and (f)), the equiaxed grain structure is obtained. The volume fraction of the recrystallized zone and recrystallized grain size under different treatment conditions are shown in Table 1. It shows that, with increasing the zirconium content, the recrystallization temperature decreases and the recrystallized grain size decreases.
It is noticed that in alloys C and D, a small amount of the Zr-rich phase is found to be present in the γ′ matrix (Fig.2). These inclusions appear to be fragile and have a deleterious effect on ductility, but can be reduced by heat treatment[15].
3.2 Mechanical properties
Fig.3 illustrates the dependence of the yield strength on the heat-treatment temperature of four alloys. The yield strength decreases with increasing heat-treatment temperature (also recrystallization degree) for all four alloys. The maximum yield strength is attained for the unrecrystallized material, while the completely recrystallized material possesses the lowest yield strength. The high yield strength can be attributed to the intrinsic effect of strain-hardening arising from an increased dislocation density in the matrix due to the extrinsic influence of cold deformation. The course of recrystallization relieves the strain hardening.
It is also shown that the yield strength increases with an increase in Zr content except for alloy A (0.2%Zr, mole fraction), which is related to the solute strength. For alloy A, the degree of strain-hardening effect is probably very high and it is difficult to relieve in the course of heat treatment.
Fig.4 illustrates the variations of the elongation (Fig.4(a)) and the ultimate tensile strength(UTS) (Fig.4(b)) of four alloys with heat-treatment temperatures. The elongation begins to increase with an increase in heat-treatment temperature (recrystallization degree), and reaches a maximum (peak), and then tends to decrease with further increasing heat-treatment temperature (Fig.4(a)). The ultimate tensile strength basically decreases with increasing recrystallization degree.
Table 1 Volume fraction of recrystallized zone and recrystallized grain size for four Ni3Al-Zr alloys at different heat treatment temperatures(HTT)(%)
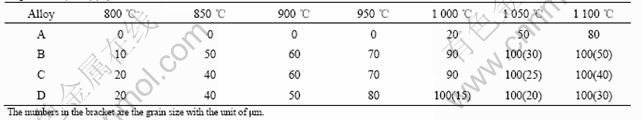
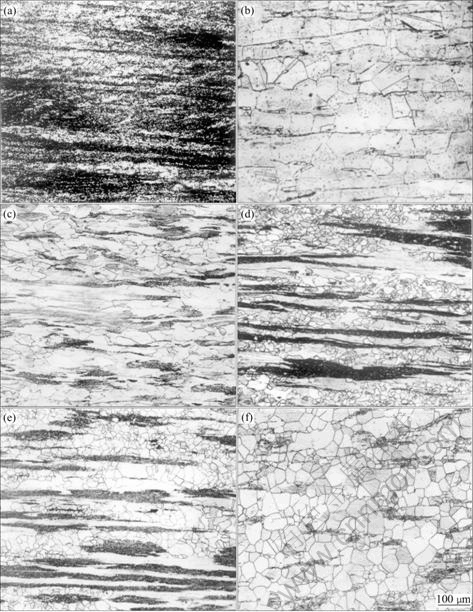
Fig.1 Microstructures of Ni3Al-Zr alloys at different heat treatments: (a) Alloy A, 900 ℃, 2 h, AC; (b) Alloy A, 1 100 ℃, 2 h, AC; (c) Alloy B, 850 ℃, 2 h, AC; (d) Alloy B, 900 ℃, 2h, AC; (e) Alloy B, 950 ℃, 2 h, AC; (f) Alloy B, 1 100 ℃, 2 h, AC
Fig.2 SEM micrograph of alloy B
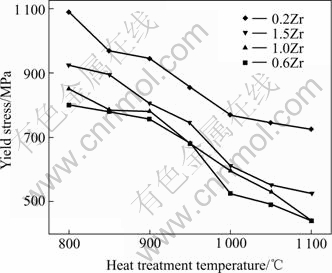
Fig.3 Yield strength at room temperature as function of heat- treatment temperature for Zr-doped Ni3Al alloys
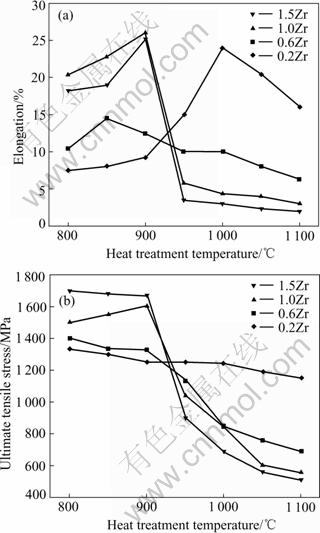
Fig.4 Variations of elongation(a) and ultimate tensile stress(b) at different heat-treatment temperatures for Zr-doped Ni3Al alloys
It was found that the greatest ductility corresponded to stress-relieved specimens with incompletely recrystallized microstructure, and the completely recrystallized microstructures coincided with the least desirable properties for all alloys.
It should be pointed out that, when the heat-treatment temperature is higher than 950 ℃, the elongation and UTS decrease with the increase of Zr amount. That perhaps relates to the increase of the Zr-rich phase, or the increase of the transverse grain boundaries where hydrogen atoms diffuse more easily.
3.3 Fracture behavior
Fig.5 illustrates the variations of the fractograph patterns observed in alloy B treated at different temperatures. The transgranular fracture with dimple-like patterns is dominant when the volume fraction of recrystallization is small (Figs.5(a) and (b)). The intergranular fracture patterns tend to be more dominant with increasing heat-treatment temperatures (Fig.5(c)). The specimens show complete intergranular fracture patterns when heated to complete recrystallization (Fig.5(d)).
Reminding that, the fracture patterns are basically correlated to the elongation (or the UTS) itself. Related to the Fig.4, as the elongation (or the UTS) value increases the fracture patterns change from intergranular to transgranular.
4 Environmental effect
4.1 Tensile properties
The mechanical properties of alloy C were examined by tensile tests at room temperature in vacuum and air (Fig.6). The yield strength decreases with an increase in heat-treatment temperature, regardless of atmosphere. These results show that the yield strength is not susceptible to air. The ultimate tensile strength and the elongation are reduced by air (versus vacuum). In the other words, the completely recrystallized specimen exhibits a more serious susceptibility to environmental embrittlement. These results might be ascribed to the fact that, a number amount of GBs are parallel to the tensile axis and there is little normal stress on GBs in the partially recrystallized specimens used in this study. For the completely recrystallized polycrystalline sample, the equiaxed grain structure not only gives rise to a normal stress on GBs but also provides transverse boundaries which enhance the entry rate of hydrogen atoms into the alloy, since grain boundary diffusion is much faster than bulk one.
4.2 Fracture behavior
Fig.7 shows the fracture patterns of samples that were tested at room temperature in vacuum and air respectively. For the specimens heat-treated at 900 ℃, 950 ℃ and 1 100 ℃, these two fracture patterns at vacuum and air are quite similar and show mostly ductile transgranular fracture patterns (Figs.7(a) and (b)). The fracture surfaces of specimens are slanted at about 45? to the tensile axis, approximately corresponding to the plane of maximum shear stress. These results correspond well with the result that two samples show almost identical and high values of the elongation and UTS, as shown in Fig.6. Figs.7(e) and (f) show very interesting fracture patterns of samples that were treated at 950 ℃ and 1 100 ℃ in vacuum and in air. The specimens tested in vacuum exhibit a mixture of intergranular and transgranular fracture patterns (Figs.7(a) and (e)). However, the samples tested in air show almost all intergranular fracture ones, and the grain-boundary facets are smooth and free from deformation marks, indicating the brittleness of grain boundaries (Figs.7(d) and (f)). The fracture surface of the specimens is perpendicular to the tensile axis. These consist with the relatively high elongation (and UTS) in vacuum, and the low elongation (and UTS) in air. All these results verify the conclusion mentioned before that Ni3Al (Zr) alloy is embrittled by air at room temperature.
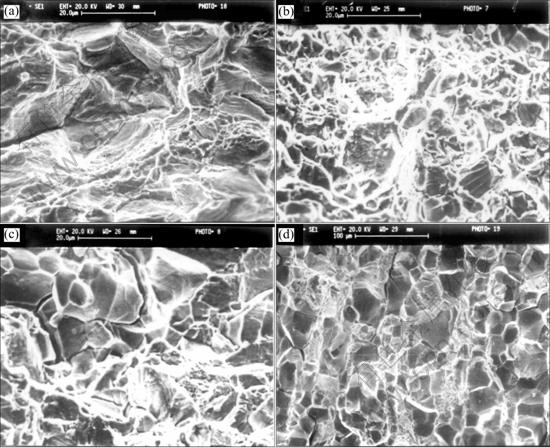
Fig.5 Fractographs of alloy B after different heat treatments tested at room temperature: (a) 850℃, 2 h, AC; (b) 900 ℃, 2 h, AC; (c) 950 ℃, 2 h, AC; (d) 1 100 ℃, 2 h, AC
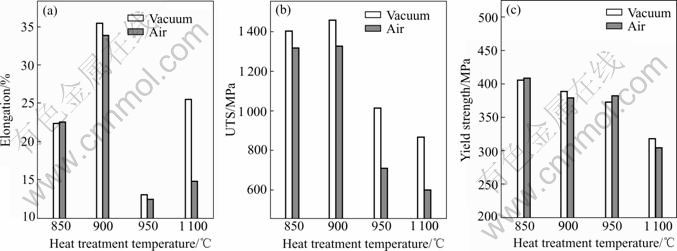
Fig.6 Variations of elongation(a), ultimate tensile strength(b) and yield strength(c) of alloy C versus heat treatment temperature in vacuum and air
Recent studies have revealed that many ordered intermetallics containing aluminum are very susceptible to environmental embrittlement. The phenomenon of environmental embrittlement in aluminides[16, 17], as well as in aluminum-based alloys is explained by following chemical reaction:
2Al+3H2O→Al2O3+6H++6e-

Fig.7 Fractographs of alloy C deformed at room temperature in vacuum and air: (a), (b) 900 ℃, 2 h, AC; (c), (d) 950 ℃, 2 h, AC; (e), (f) 1 100 ℃, 2 h, AC
Aluminum atoms in the aluminides react with moisture in air, resulting in the generation of atomic hydrogen that causes embrittlement, which has been borne out by test results of YIN et al[18] in 1991. Because Ni3Al(Zr) alloy also contains the reactive element Al, we believe that above reaction should occur in this alloy also.
Our results indicate that the stress-relieved, incompletely recrystallized microstructure in some way minimizes the environmental effect, and elongated grains or unrecrystallized rolled bands may disrupt the entrance of hydrogen by providing a minimum number of transverse grain boundaries. We can also conclude that the Ni3Al(Zr) alloy still has a tendency for environ- mental embrittlement.
Similar results have been reported by GAO et al[19] for B-free binary Ni-22.6Al(mole fraction, %) alloys. SU et al[14] have studied the effect of recrystallization on environmental embrittlement of a multicomponent Ni3Al-base alloy doped with 0.02%B(mass fraction). It was found that the ductility increases and the susceptibility to environmental embrittlement decreases with increasing the recrystallized fraction. The fracture modes were ductile and transgranular, and no intergranular fracture was observed. These are quite different from the results obtained in the present study. The differences might be explained in terms of the boron effect. Boron can reduce the diffusion of atomic hydrogen in Ni3Al[20]. Therefore, the recrystallized Ni3Al alloy doped with boron is less sensitive to the test environment and shows a fracture mode other than intergranular fracture. For the B-free Ni3Al(Zr) alloy used in this study, the recrystallization treatment generates many transverse grain boundaries that are susceptible to hydrogen embrittlement, bringing on the fact that the amount of intergranular fracture increases with increasing the degree of recrystallization. It is concluded that the addition of Zr cannot reduce the environmental embrittlement of Ni3Al alloy.
5 Zr alloying effect
From the obtained results we know that the elongation of the completely recrystallized alloy A is around 16.4% in air (Fig.4(b)), which is similar to the result achieved by GEORGE et al[6] in polycrystalline specimens produced by deforming and recrystallizing a single crystal of Ni-22.7Al-0.3Zr. The ductility is considerably higher than that of binary Ni3Al (about 3.1%[21]). The result mentioned above confirms that Zr significantly improves the ductility of the Ni3Al alloy. While the exact mechanism is not clear, we might suggest that the mechanism by which Zr improves the ductility of the Ni3Al alloy is different from that by boron.
In literatures, several ideas have been suggested to account for the increasing ductility by Zr addition. One assumption, proposed by GEORGE et al[7], is that Zr appears to both alleviate environmental embrittlement and enhance GB cohesion, but they could not clearly indicate the mechanism. However, we have confirmed that Zr cannot alleviate environmental embrittlement in this study. CHUANG et al[22] proposed that Zr eliminates the segregation of harmful impurity element including sulphur to GBs. It is argued that even if the GBs are relatively free of embrittlement impurities, polycrystalline Ni3Al still exhibits brittle intergranular fracture.
To investigate whether Zr improves the ductility of the Ni3Al alloy by segregating to GBs, we performed Auger analyses on GBs of alloy B. We found that Zr does not segregate to GBs when treated at 1 100 ℃(Fig.8(b)), however, for the specimen treated at 900 ℃, Zr does segregate to GBs though the amount is small (Fig.8(a)). Therefore, if Zr does strengthen GBs in the Ni3Al alloy, its mechanism is not clear. The other possibility is that, instead of strengthening GBs, Zr somehow changes the slip behavior of Ni3Al, so reduces the effective stress concentration at GBs, or Zr contributes to an increase of the fraction of special boundaries, such as low angle boundary(LAB), ∑3 GBs, which are more resistant to fracture[23]. The last possibility is that, Zr increases the lattice parameter of the Ni3Al alloy tremendously, and thereby the activation energy decreases. All possibilities require further investigation.
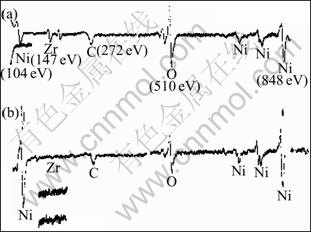
Fig.8 AES spectra taken from intergranular(IG) region for alloy B at 900 ℃, 2 h, AC(a) and 1 100 ℃, 2 h, AC(b)
6 Conclusions
1) The yield stress decreases with an increase in the degree of recrystallization. The completely recrystallized specimens have the lowest yield stress. The highest elongation is obtained in the stress-relieved specimens with incompletely recrystallized microstructure.
2) The amount of ductile fracture reduces for completely recrystallized specimens, and the ductile fracture mode remains unchanged for the incompletely recrystallized samples when tested from vacuum to air.
3) The increase in recrystallization degree results in an increase in the susceptibility to environmental embrittlement, and the completely recrystallized samples exhibit the most serious environmental embrittlement.
4)The Ni3Al (Zr) alloys still have a tendency for environmental embrittlement, and Zr appears to have no effect on suppressing environmental embrittlement.
References
[1] Horton J A, Liu C T, Santella M. Microstructures and mechanical properties of Ni3Al alloyed with iron additions[J]. Metall Trans, 1987, 18A: 1265-1277.
[2] Lall C, Chin S, Pope D P. Plastic behaviour in Ni3(Al, X) single crystal[J]. Metall Trans, 1979, 10A: 1323-1326.
[3] Takeyama M, Liu C T. Effect of grain and test temperature on the ductility and fracture behavior of a B-doped Ni3Al alloys[J]. Acta Metall, 1988, 36: 1241-1243.
[4] Masahashi N, Takasugi T, Izumi O. Electronic and structural studies of grain-boundary strength and fracture in L12 ordered alloy[J]. Acta Metall, 1988, 36: 1823-1836.
[5] LIU C T. Environmental embrittlement and grain boundary fracture in Ni3Al alloy[J]. Scr Metall, 1992, 27: 25-28.
[6] George E P, Liu C T, Pope D P. Environmental embrittlement: the major cause of RT brittlement in polycrystalline Ni3Al[J]. Scr Metall, 1992, 27: 365-370.
[7] George E P, Liu C T, Pope D P. Intrinsic ductility and environmental embrittlement of binary Ni3Al[J]. Scr Metall, 1993, 28: 857-860.
[8] Aoki K, Izumi O. Ductility and fracture behavior of B-doped polycrystalline Ni3Al[J]. J Japan Inst Met, 1979, 43: 1190-1194.
[9] Liu C T, Sikka V K. Nickel. Aluminide for structure use[J]. J Metals, 1986, 38 (5): 19-21.
[10] Schneibel J H, Peterson G F, Liu C T. Creep behavior of a polycrystalline nickel aluminide: Ni-23.5at% Al-0.5at% Hf-0.2at% B[J]. J Mater Res, 1986, 1: 68-72.
[11] Hus S E, Hsu N S, Tong C H, LEE S Y. Mechanical properties of Ni3Al(B) alloys at high temperature[A]. STOLOFF N, KOCH C C, LIU C T, IZUMI O. High Temperature Ordered Intermetallic Alloy Ⅱ[C]. Pittsburgh, PA: MRS Symp Proc Publications, 1987, 81: 507- 512.
[12] Mishima Y, Ochiai S, Hanao N, YODOGAWA M, SUZUKI T. Effect on the mechanical properties in B-doped Ni3Al by alloying elements [J].Trans Jpn Inst Met, 1986, 27: 656-660.
[13] GUO Jian-ting, ZHENG Zhi, WU Wei-wen, SUN Chao, ZHU Yao-xiao. Effect of zirconium on the strength and the ductility of boron-free Ni3Al alloys [A]. SHI Chang-xu, LI Heng-de, SCOOT A. The First Pacific Rim International Conference on Advanced Materials and Processing[C]. Guilin: The Minerals, Metals & Material Society, 1992.805-810.
[14] SU Jian-qing, GAO Shu-jun,YU bing-xi, GUO Jian-ting. Effect of recrystallization on environmental embrittlement of a Ni3Al-based alloy [J]. J Mater Sci, 1997, 32: 1201-1204.
[15] GUO Jian-ting, GU Yue-feng, LIN Dong-liang. Effect of Zr on grain boundary and mechanical properties in Ni3Al alloys[J]. Acta Metall Sinica, 1995, 31(3): 120-123.(in Chinese)
[16] Liu C T, Lee E H, Mckamey C G. Comparison of grain boundary compositions in B-doped and B-free Ni3Al[J]. Scr Metall, 1989, 23: 875-880.
[17] Liu C T, Gorge E P. Environmental embrittlement in B-doped and B-free FeAl(40at%) alloys[J]. Scr Metall, 1990, 24: 1285-1290.
[18] YIN Wei-min, GUO Jian-ting, HU Zhuang-qi. Influence of testing environment on deformation and fracture behavior in intermetallics FeAl[J]. Journal of University of Science and Technology Beijing, 1991, 13: 102-105. (in Chinese)
[19] GAO Shu-jun, SU Jian-qing, HU Zhuang-qi. Environmental embrittlement of a Ni-22.6Al (at%) intermetallics[J]. Key Engineering Materials, 1998, 145-149: 1031-1034.
[20] Wan X J, Zhu J H, Jing K L, LIU C T. Hydrogen diffusivity in B-doped polycrystalline Ni3Al[J]. Scr Metall, 1994, 31: 677-681.
[21] Liu C T, White C L, Horton A. Effect of boron on grain boundaries in Ni3Al[J]. Acta Metall, 1985, 33(2): 213-229.
[22] Chuang T H. The mutual effect of boron, zirconium and aluminium on grain boundary segregation in Ni3Al intermetallic compounds[J]. Mater Sci Eng A, 1991,141: 169.
[23] HUI Lin, POPE D P. The influence of grain boundary geometry on intergranular crack propagaton in Ni3Al [J]. Acta Metall Mater, 1993, 41(2): 553-562.
Corresponding author: LI Yu-fang; Tel: +86-25-51905739; E-mail: lyf_msc@nuaa.edu.cn
Abstract: The effects of recrystallization and environment (vacuum versus air) on tensile properties of B-free Ni3Al (Zr) alloys were investigated. The results indicate that the incompletely recrystallized and stress-relieved specimens show the most desirable ductility and ultimate tensile strength, and that the recrystallization treatment promotes susceptibility to the test environment of the alloys. It is found that the amount of ductile fracture is reduced by air for completely recrystallized specimens. The Auger analyses show that Zr atoms do not segregate to the grain boundaries(GBs) for specimens heat-treated at 1 100 ℃, however Zr atoms segregate to the GBs for specimens heat-treated at 900 ℃. These results imply that Zr-doping cannot suppress environmental embrittlement.


