DOI:10.19476/j.ysxb.1004.0609.2017.06.018
宽温域锂离子电池功能电解液的研究进展
洪 波1,闫霄林2,洪 树1,赖延清2,李 劼2,姜 锋1
(1. 中南大学 材料科学与工程学院,长沙 410083;
2. 中南大学 冶金与环境学院,长沙 410083)
摘 要:
分析锂离子电池在低温工作条件下的性能劣化机理,阐述溶剂物理性质对电解液低温性能的影响规律,总结目前通过低黏度及低熔点的溶剂组分、低阻抗的成膜添加剂以及新型锂盐来改善电池低温性能的研究工作。同时,探讨锂离子电池在高温工作条件下容量衰减机制,综述目前改善锂离子电池高温性能的主要方法,包括采用高温成膜添加剂、耐高温锂盐以及锂盐稳定剂。在此基础上指出目前宽温域锂离子电池发展面临的主要挑战,展望锂离子宽温域电解液的发展趋势。
关键词:
锂离子电池;宽温域电解液;低温性能;高温性能;线性羧酸酯;添加剂;
文章编号:1004-0609(2017)-06-1208-14 中图分类号:TF813 文献标志码:A
20世纪90年代初,日本索尼公司率先推出钴酸锂/石墨锂离子电池[1]。如今,锂离子电池虽然在小型移动电源领域(如手机、笔记本电脑、数码相机)已获得广泛的应用,但其对低温和高温环境的耐受性仍然无法满足极端条件下的应用要求[2-3]。当温度过低时,电解液的电导率会大大的降低、SEI膜阻抗增大,锂离子在电极中的传递阻抗也会增大[4-5]。温度较高时,电极又极容易与电解液发生各种复杂的界面反应,如正极材料的氧化和SEI膜成分的变化等[6-7]。如果温度继续增高,电池内部的剧烈反应将导致热失控,从而引发安全问题[8]。纯电动车(EV)和混合动力汽车 (HEV)领域对锂离子电池使用温度要求是能在-20 ℃低温下存储并进行低倍率放电,能在60 ℃高温下存储性能稳定且满足较长时间的高温循环(200圈容量保持率>80%);而国防军工等极端条件应用场合,则要求锂离子电池能在-40 ℃下低温存储并进行低倍率(0.2C)放电,且能在70 ℃下高温存储稳定(70 ℃存储48 h容量保持率>80%,软包电池膨胀率<5%);在航空航天领域,有时甚至要求电池能在-50 ℃低温下进行低倍率充放电[9]。这些要求都是目前锂离子电池所达不到的。因此,可同时兼顾低温性能和高温性能的“宽温域型锂离子电池”已经成为业内重大挑战和研发热点。本文作者将从改善电池低温性能及提高电池高温性能两方面综述宽温域锂离子电池电解液的研究进展及未来发展趋势。
1 低温电解液的研究进展
锂离子电池低温电解液的性能主要受限于3个方面[10]。首先,常用的EC基电解液在低温条件下黏度增大甚至凝固,使得电解液离子电导率变差[11];其次,低温下电极界面膜(SEI)电荷迁移阻抗增加,使充放电过程电极极化加大[12];再次,在低温下条件下,锂离子电池在恒流充电的后期易产生析锂现象,导致SEI膜状况恶化,电池循环性能变差。因此,改善锂离子电池低温性能的研究主要从以下几个方面入手:1) 溶剂成分优化。包括采用熔点较低的PC溶剂部分取代EC溶剂降低电解液熔点,以及通过低熔点、低黏度的共溶剂降低EC基电解液熔点并提高电解液低温电导率;2) 锂盐优化。加入低温性能较好的锂盐,也可以一定程度上改善电池低温性能;3) 低温添加剂。通过加入效果优于EC的成膜添加剂降低低温下电极界面电荷传递阻抗,或加入锂盐沉积改善剂,防止低温下锂支晶生长,改善电池低温循环稳定性。
1.1 低温溶剂的研究
根据电解液的性能要求,理想的锂离子电池电解液低温共溶剂应具有以下条件:1) 熔点低(Tm),沸点高(Tb),蒸气压低,在提高低温性能的同时尽量不对电池高温性能产生不利影响;2) 具有较低的黏度(η)及较高的介电常数(ε),从而使电导率高,有利于锂离子传输。此外,还应该具有化学性能稳定、与电池内各组件不发生反应、安全无毒、价格便宜等特点。但是实际上很难同时满足这两个要求,如大部分熔点低的溶剂沸点也低,而介电常数高的溶剂一般黏度较大,因此,真正能用于锂离子电池的低温溶剂有限,主要是碳酸丙烯酯(PC)和一些链状羧酸酯类(见表1)。如碳酸二甲酯(DMC)、碳酸二乙酯(DEC)、碳酸甲乙酯(EMC)、丁酸甲酯(MB)和丁酸乙酯(EB)等。
DING[13]研究碳酸酯二元(见图1)及三元(见图2)混合溶剂的相图,发现混合溶剂熔点与溶剂组分、各组分熔点及相对含量有关,在进行电解液设计时,可根据应用要求通过溶剂组分的选择及含量调整,配制出不同熔点的多元混合溶剂。
1) PC溶剂改善锂离子电池低温性能
PC的熔点(-48.8 ℃)比EC的熔点(36.6 ℃)低,而且它能够有效地抑制EC在低温时结晶析出,从而有效提高电池的低温性能。但目前PC很少用作锂离子电池电解液,主要原因是在锂离子电池中,PC溶剂容易同Li+一起向石墨负极共嵌,使石墨层发生剥离,导致电池循环性能下降。但是PC的这些缺点可以通过调整电解液混合溶剂的配比或加入适当的电解液添加剂来改善[14]。ZHANG等[15]比较了1 mol/L LiPF6 EC/EMC(EC和EMC的体积比为3:7)和1 mol/L LiPF6 PC/EC/EMC(PC、EC和EMC的体积比为1:1:3)电解液体系的低温性能,发现PC的加入虽然使电解液在低温下的黏度增大,致使离子电导率稍微减小,却能大大提高锂离子电池的低温性能。这一实验结果同时也证明了电解液的离子导电率不是影响锂离子电池低温性能的主要因素。
表1 锂离子电池电解液常用有机溶剂的物理参数
Table 1 Physical parameters of solvent in electrolyte of Li-ion battery
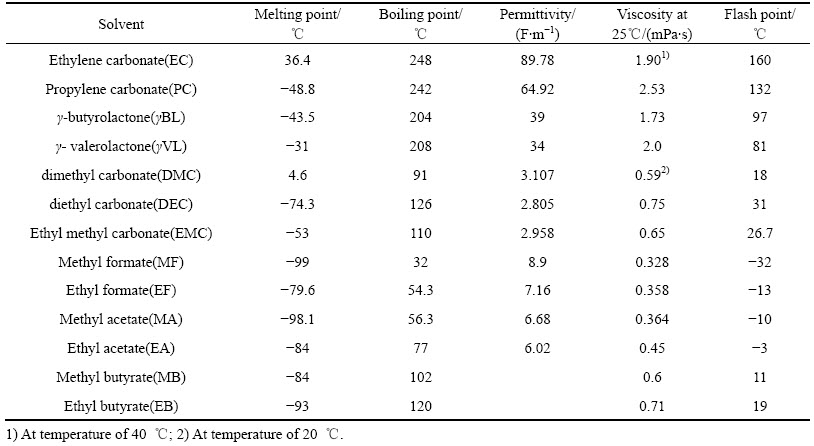
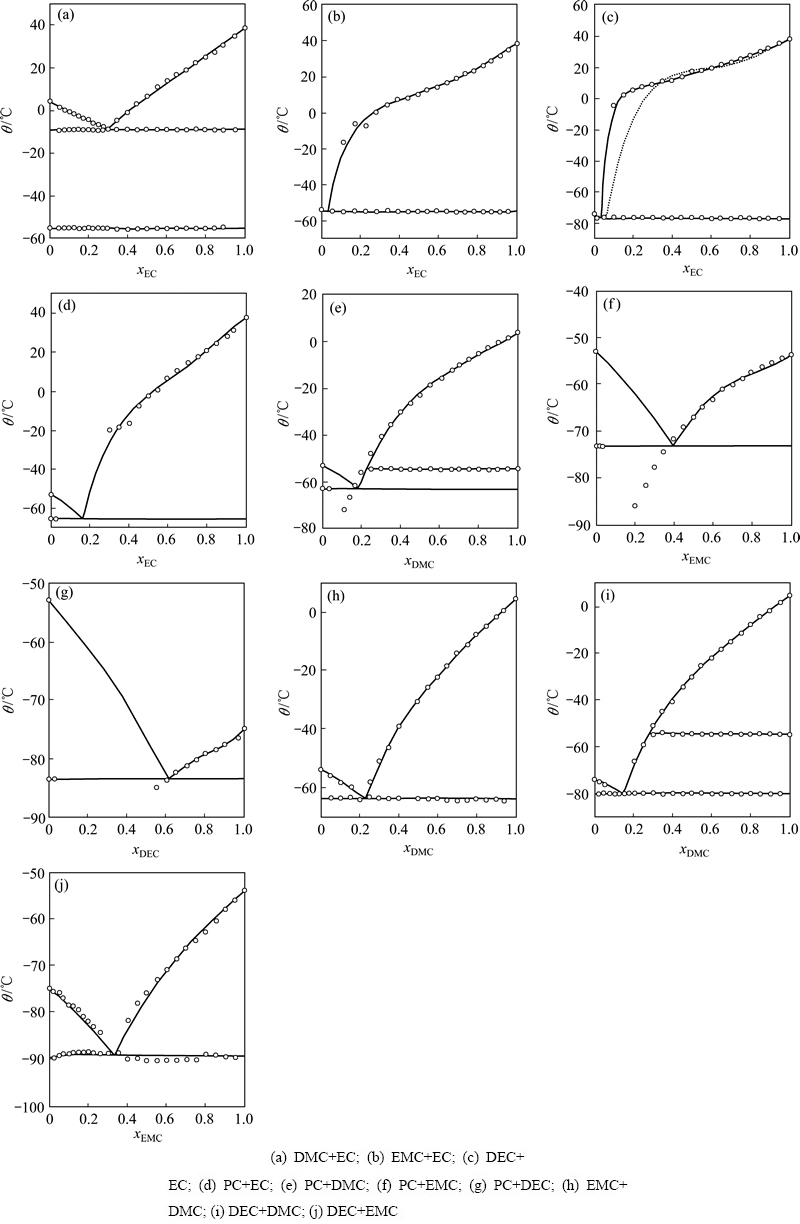
图1 EC、PC、DMC、EMC以及DEC的二元相图(圆点为实验测试数据,拟合线为理论计算数据)[13]
Fig. 1 Thermodynamic phase diagram for all binaries of EC, PC, DMC, EMC and DEC (Open dots determined by experiments and fitting curves from theoretical calculation)[13]
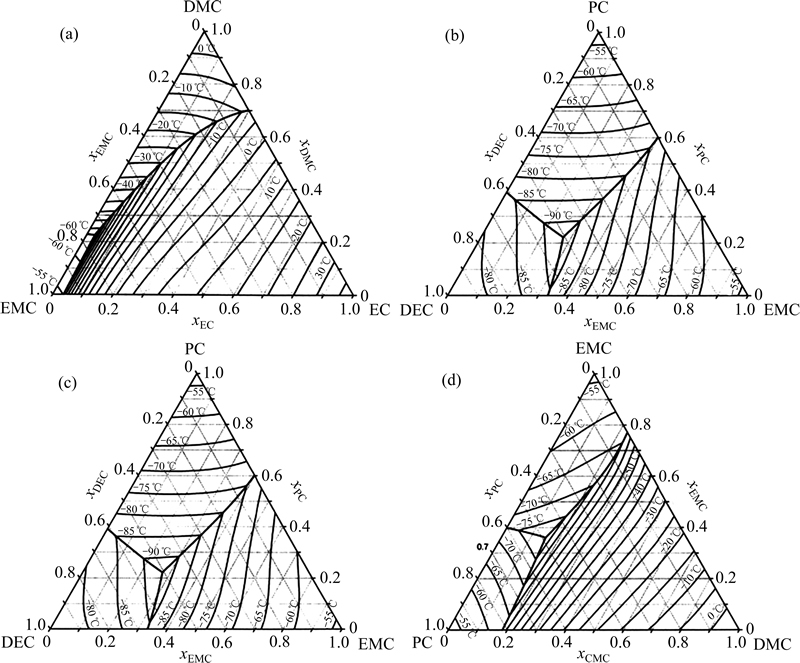
图2 三元碳酸酯溶剂EC-EMC-DMC、PC-EMC-DEC、PC-DEC-EMC和PC-EMC-DMC的液相面等温线投影图[13]
Fig. 2 Contour plot of temperature of liquidus surface of EC-EMC-DMC(a), PC-EMC-DEC(b), PC-DEC-EMC(c) and PC-EMC- DMC(d) ternary carbonate[13]
2) 碳酸酯共溶剂改善锂离子电池低温性能
碳酸甲乙酯(EMC)由于其熔点低(-55 ℃),黏度较小(0.65 mPa·s),作为共溶剂可改善电池低温性能[16-17]。CAPIGLIA[18]研究了LiPF6、LiBF4及二(全氟乙基磺酰)亚胺锂(LiBETI,LiN(C2F5SO2)2)与EC/EMC(EC和EMC的体积比为2:8)溶剂配制的电解液低温性能,发现各种电解液冰点均降至-40 ℃以下。ZHANG等[19]采用 EC与MC按体积比为3:7比例制成的混合溶剂具有较好的低温稳定性和较高离子电导率。PLICHTA[20]研究了1 mol/L LiPF6 EC/DMC/ EMC(EC、DMC和EMC的体积比为1:1:1)电解液的电导率和化学稳定性,在Li/LiCoO2电池和石墨/ LiCoO2锂离子电池体系中使用,工作温度可降至-40 ℃,电导率约为1 mS/cm。XIAO等[21-22]研究EMC对电池低温性能的影响,发现三元溶剂体系1 mol/L LiPF6 EC/DMC/EMC(EC、DMC和EMC的质量比为1:3:8)在-40 ℃下拥有很好的离子电导率和放电容量(90%常温放电容量)。XIAO等[21-22]通过优化溶剂配比来提高电解液低温性能,获得了最佳电解液1 mol/L LiPF6 EC/DMC/EMC(EC、DMC和EMC的体积比为8.3:25:66.7),用于锂离子电池,在-40 ℃下以0.1C倍率放电到2.0 V容量能保持常温下的90.3%。王夏芬[23]研究了EC+EMC及EC+PC基电解液电池低温性能,发现SEI膜电阻增加幅度大是电池低温性能变差的主要原因。1 mol/L LiPF6 PC/EC/EMC(EC、DMC和EMC的体积比为1:1:4)电解液-20 ℃下放电容量保持率为96.7%,但PC电解液中必须加入其他的电解液添加剂阻止其对石墨电极的破坏。SMART等[24-25]针对含EMC的低温电解液体系做了大量的研究工作,发现1 mol/L LiPF6 EC/DEC/DMC/EMC(EC、DMC和EMC的质量比为1:1:1:3)体系电解液-40 ℃的离子电导率可达到1.32 mS/cm,-60 ℃下不凝固。制得的LiNi0.8Co0.2O2/ MCMB电池在-20 ℃放电时可得到80%额定容量,在-40 ℃放电时可得70%额定容量,电池还可以在-70 ℃下以C/50放出35%的容量。EMC作为共溶剂有效拓宽了电解液的温度范围,但是需要注意的是,EMC热稳定性差,受热和碱性条件下易发生酯交换反应,生成DEC和DMC。
3) 链状羧酸酯共溶剂改善锂离子电池低温性能
从表1中可以看到,短链的线性羧酸酯一般具有较低的熔点及黏度,作为低温共溶剂与EC或PC共用,可有效降低电解液冰点及低温下黏度,从而提高锂离子电池低温性能。韩景立等[26]研究了MA对电解液低温电导率的影响,发现1 mol/L LiPF6 EC/MA(EC和MA的体积比为1:1)体系低温电导率很好,-40 ℃为1.53 mS/cm,-45 ℃为1.25 mS/cm,-50 ℃为0.93 mS/cm,但MA对负极成膜有不利影响。SHIAO等[27]测试含甲酸甲酯(MA)及甲苯(tol)的不同组分电解液的低温性能,发现1 mol/L LiPF6 EC/EMC/MA/tol (LiPF6 EC、EMC、MA和tol的体积比为1:1:1:1)电解液-50 ℃下电导率大于1 mS/cm,-40 ℃放电容量保持率约60%,且甲苯可使SEI膜更稳定从而提高电池循环性能。美国NASA喷射推进实验室的M.C. Smart课题组针对锂离子电池低温电解液做了大量的研究工作,指出一些线性酯类如乙酸乙酯(EA)、丁酸乙酯(EB)、丙酸乙酯(EP)等作为共溶剂能够提高低温下电池的电导率[28]。链状羧酸酯的熔点要比EMC低很多,在电解液中同时添加EMC和链状羧酸酯并研究其最佳配比组合将可更一步提高电解液的低温性能。其中,1 mol/L LiPF6 EC/EMC/MB (LiPF6 EC、EMC和MB的体积比为1:1:1) 体系锂离子电池,可在-60 ℃下以C/20放出常温容量的80%,代表国际低温锂离子电池的领先水平。HONG等[29]对比研究了采用1 mol/L LiPF6 EC/EMC/EA(EC、EMC和EA的质量比为1:1:2)、1 mol/L LiPF6 EC/EMC/ EP(EC、EMC和EP的质量比为1:1:2)、1 mol/L LiPF6 EC/EMC/ EB(EC、EMC和EB的质量比为1:1:2)电解液的LiMn2O4/AG电池的低温性能,发现EA、EP、EB的加入均可改善电池低温放电性能,采用1 mol/L LiPF6 EC/EMC/EA电解液的18650锂离子电池低温性能最好,其在-40 ℃及-60 ℃下放电容量保持率分别为90%及44.4%,但EA的低沸点可能影响软包电池在高温下的性能(图3)。
谢晓华等[30]研究乙酸丙酯(PA)对LiCoO2/AG电池低温性能的影响,发现1 mol/L LiPF6 EC/DEC/PA (EC、DEC和PA的体积比为1:1:4)锂离子电池在-40 ℃时离子电导率达到3.24 mS/cm,25、0、-10、-20、-30和-40 ℃时放电相对容量分别为99.8%、100.3%、99.9%、99.2%、96.9%和89%。通过进一步加入成膜添加剂与PA配合使用,使电池-40、-50和-60 ℃相对放电容量分别达到91.1%、81.3%和58.0%,但文中并未给出成膜添加剂成分。
YAMAKI等[31]发现采用氟代乙酸甲酯(MFA)或氟代乙酸乙酯(EFA)替代MA或EA用于锂离子电池,可以改善溶剂与负极材料的相容性,提高电池循环性能。这是由于F原子半径小,电负性强,F取代后链状羧酸酯冰点基本不变甚至略有降低,同时多氟代酯通常具有较高的闪点,对电池高温安全性能有利,因此,氟代链状羧酸酯溶剂在改善锂离子电池温度性能方面具有较好的应用前景。SMART等[32]研究了乙基-2,2,2三氟代乙酸酯(ETFEC)等对Li1-xNi0.8Co0.2O2/ MCMB电池低温性能的影响,发现ETFEC的加入可以减小负极SEI膜阻抗,提高了电池低温下充放电容量。
1.2 低温锂盐的研究
锂离子电池的锂盐可分为无机阴离子锂盐和有机阴离子锂盐两大类。
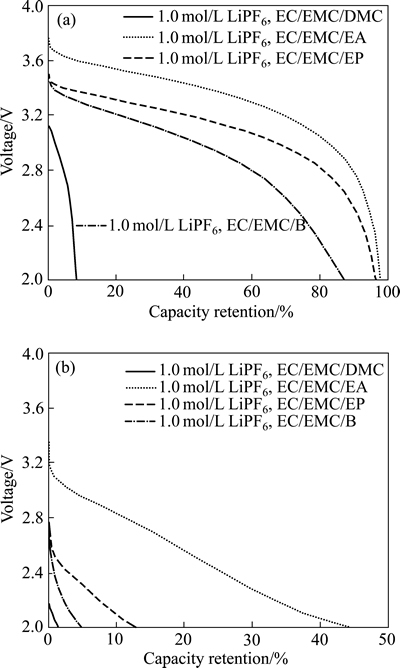
图3 采用不同电解液的锰酸锂/石墨电池在-40 ℃和-60 ℃下的放电曲线[29]
Fig. 3 Discharge curves of LiMn2O4/graphite cells containing different electrolytes at -40 ℃(a) and -60 ℃(b)[29]
在无机锂盐中,LiAsF 热稳定性好,不易水解,但价格昂贵,且有毒性;LiClO4具有电导率较高,热稳定好,价格低廉,易于制备和纯化等特点,但其较强的氧化性会带来安全隐患;LiBF4其阴离子半径较小,容易缔合,使得电解液电导率较低,故电池倍率性能较差。另外,LiBF4单独使用时不能在石墨负极表面形成稳定的SEI膜,使得电池循环性能很差。但其在高温或低温情况下性能优于LiPF6。综合来讲,LiPF6是目前性能最优的锂盐,电导率较大、电化学稳定性好,不腐蚀铝集流体,但其价格较贵,抗热和抗水解性能不够理想。有机锂盐主要包括LiCF3SO3(三氟甲磺酸锂)、LITFSI(二(三氟甲基磺酰)亚胺锂)、LiB(C2O4)2(双乙二酸硼酸锂,LiBOB)、LiODFB(二氟草酸硼酸锂)、Li(C2H5)3PF3等。其中,LiCF3SO3、LITFSI或LiC(CF3SO2)3制备成本相对较高且不易纯化,磷酸盐Li(C2H5)3PF3是用C2H5基团取代LiPF6中的氟原子形成的,与LiPF6相比,Li(C2H5)3PF3的热稳定性、耐水解性能和闪燃点均显著提高,增加了电池的安全性,与LiPF6混合使用能显著提高电解液的高温性能,具有良好的发展前景。
耐低温型锂盐研究较少,主要有四氟硼酸锂(LiBF4),二氟草酸硼酸锂(LiODFB)和三氟甲基黄酰亚胺锂(LiTFSI)。
ZHANG等[33]对比研究了两种锂盐(LiBF4及LiPF6)对电池低温性能的影响,电解液为1 mol/L锂盐+ PC/EC/EMC(PC、EC和EMC的质量比为1:1:3)体系。发现LiBF4虽然降低了电导率,但减小了低温下电池电化学反应阻抗,从而提高电池放电容量(见图4)。采用LiBF4电解液体系的电池-30 ℃下首次放电容量达到常温的86%,而LiPF6体系只有72%。但是,LiBF4电池成膜性能较差,常温下循环容量衰减很快。为了改善LiBF4锂盐体系循环性能,ZHANG等[34]在 LiBF4体系电解液中加入1%~5%的LiBOB,发现LiBOB可以显著改善LiBF4的成膜性能,从而提高电池常温下的循环性能。但是LiBOB形成的膜层较厚且阻抗较大,可能对电池低温性能带来不利影响。
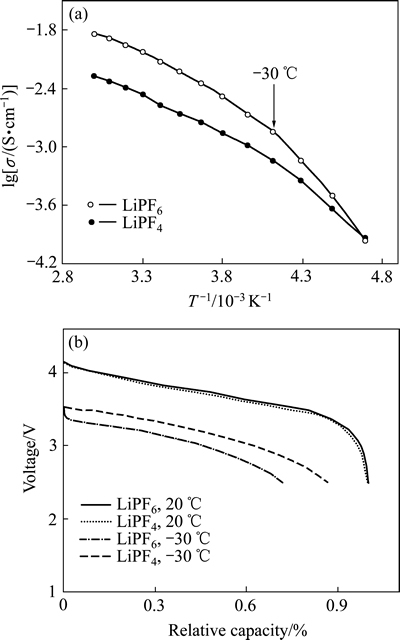
图4 锂盐对电解液电导率及相应电池低温放电性能的影响[33]
Fig. 4 Effect of lithium electrolyte salts on conductivity of electrolyte(a) and discharge performance of corresponding batteries at low temperature(b)[33]
结合LiBF4和LiBOB的结构特点,人们设计并合成新型锂盐草酸二氟硼酸锂LiODFB,该盐具备了两种盐的优点。ZHANG等[35]将LiPF6与LiODFB按照不同比列混合制备了一系列电解液1 mol/L锂盐 EC:PC:DMC(EC、PC和DEMC的体积比为1:1:3)。组装LiFePO4/AG、LiFePO4/Li和Li/AG电池进行测试。在LiFePO4/Li电池中,含有LiODFB的电池电化学性能略微低于含纯LiPF6的电池。在Li/AG电池中含有LiODFB的电池相比含纯LiPF6的电池在电流效率和容量保留量方面有显著的优势。向容[36]研究了LiODFB基锂离子电池的性能,发现1 mol/L LiODFB EC/PC/DMC/EMC (EC、PC、DMC和EMC的体积比为4:1:3:2)电解液低温-20 ℃下电导率为3.15 mS/cm2,制作的LiFePO4/AG电池-30 ℃下放电容量保持率为56%左右。ZHANG等[37]以PC+EC+EMC混合溶液为溶剂,研究了LiBOB、LiBF4、LiODFB为锂盐对电解液电导率的影响,发现当在温度低于-30 ℃时,3种电解液的导电率顺序为LiBF4=LiODFB>LiBOB,但当温度高于-10 ℃时,3种电解液的导电率由大到小依次为LiBOB、LiODFB、LiBF4。以1.0 mol/L (1-x)LiBF4xLiBOB PC+EC/EMC(PC、EC、EMC质量比为1:1:3)为电解液,x=0.1时,Li/LiFePO4 半电池在-50 ℃,以1C放电3 V的电压放电容量保持约30%,而且电池能够在90 ℃下稳定循环。LAZAR等[38]在采用1 mol/L LiODFB代替LiPF6,溶剂为EC/DMC/DEC (EC、DMC和DEC的体积比为1:1:1),发现LiODFB加入会降低电解液电导率(-20~60 ℃),从而降低电池比容量,说明LiODFB并不能完全取代LiFP6。
在对室温熔融盐的研究中发现,阴离子为BF4-、CF3COO-、CF3SO3-、N(CF3SO2)2-、N(C2F5SO2)2-的室温熔融盐均可一定程度降低电解液体系熔点,提高低温性能,尤其以N(C2F5SO2)2-阴离子降低熔点效果最为显著。HU等[39-40]研究了由3种具有不同阴离子半径、结构类似的锂盐:二(三氟甲基磺酰)亚胺锂(LiTFSI,LiN(CF3SO2)2)、二(全氟乙基磺酰)亚胺锂(LiBETI,LiN(C2F5SO2)2)、三氟甲基磺酸锂(LiCF3SO3)与乙酰胺(熔点81℃)组成的电解液体系物理化学性能,发现LiTFSI+乙酰胺(1:4)混合体系熔点低至-67 ℃, LiBETI+乙酰胺(1:4)体系熔点低至-57 ℃,因此,此类锂盐具有较大的低温开发潜力。MANDAL等[41]对二(三氟甲基磺酸)亚胺锂(LiTFSI)的低温性能进行研究,发现0.9 mol/L LiTFSI EC/DMC/EMC (EC、DMC和EMC的体积比为15:37:48)电解液体系在-40 ℃下电导率接近2 mS/cm,Li/LiNiCoO2半电池-40 ℃放电容量保持率约30%。需要注意的是,LiTFSI电解液中Cu2+、Al3+和Fe2+均具有较高的溶解度,使得电池循环过程中集流体腐蚀严重[42]。通过加入LiBF4或LiPF6使铝箔钝化形成AlF3可以缓解腐蚀[43-44]。ZHANG 等[45]发现0.2 mol/L LiTFSI加入1 mol/L LiPF6基电解液中,可显著降低Li/AG半电池的电荷转移阻抗,并提高电池循环稳定性、倍率性能,但文中没有对全电池性能进行系统研究。
LiC(CF3SO2)3结构及性能与LiTFSI相似,分解温度为340 ℃,略低于LiTFSI的,但该盐的EC/DMC电解液在-20 ℃的低温下电导率达到1.1~3.5 mS/cm,低温性能优于LiTFSI的[46]。而且,LiC(CF3SO2)3在电压高于4.5 V时,才会对铝箔产生腐蚀,与铝箔的相容性优于LiTFSI(4.0 V)。
1.3 低温成膜添加剂
1) 降低膜层阻抗
路婷婷[47]研究氟代碳酸酯(FEC)、乙酸乙酯(EA)及添加剂r-丁内酯(GBL)对LiFePO4电池低温性能的影响,发现EC+EMC+PC+EA体系加入FEC有利于形成致密稳定且阻抗低的SEI膜,减少电池常温及低温下的阻抗,在与3% GBL混合使用时,可使电解液-40 ℃电导率达到2.875 mS/cm,电池-20 ℃和-40 ℃容量保持率分别为86.2%和60%。刘兵晓[48]研究石墨负极及负极成膜添加剂FEC对锂离子电池低温性能的影响,发现-20℃下放电容量由大到小依次为AGP-8、天然石墨168、MCMB,这是由于AGP-8的粒径较小,电荷转移阻抗小。FEC加入可在石墨表面形成低阻抗SEI膜,降低低温脱锂电位,提高低温倍率性能。含2%FEC的Li/石墨电池-20 ℃放电容量保持率为80%,高于不加FEC电解液(50%)。少量的碳酸亚乙烯酯(VC)加入,能提高Li迁移速率及负极界面的稳定性,从而提高电池低温性能。MOLLER等[49]发现N,N-二甲基三氟乙酰胺(DTA)具有较低黏度及在石墨负极良好的成膜性能,其添加入PC溶剂中,可使电解液凝固点降到-40 ℃以下。
2) 改善锂盐沉积
低温下充放电会引起锂盐沉积,从而降低电池寿命,因此,对于长期在低温下工作的电池,需要重点考虑锂盐沉积问题。早期的研究发现在LiClO4-PC溶液中加入低浓度的无机物如SO2[50]、多硫化物、CO2、甚至是水都可以有效改善锂在钝化金属基底上的循环性能。由于这些化合物可以应用在形成导Li+薄膜过程中,使电极表面形貌更加圆滑。同样地,在LiClO4/PC或PC-DME中加入一些有机化合物如2-甲基四氢呋喃、2-甲基噻吩和硝基甲烷也能够提高锂循环的库伦效率。这些结果是基于这些有机化合物能够在析锂前发生还原,积累的还原产物用于形成SEI膜,从而抑制了树突状锂的形成。更有趣的是这些有机物还可以降低电极-电解液界面的导电阻抗。
除了上述这些方法,还有人提出酸性HF也能改善锂沉积的形貌。由于锂表面SEI膜的不均匀会导致电流分布的不均匀,进而造成锂沉积为树突状。而锂表面组成SEI膜的成分都是强碱,酸性的HF可以与SEI膜的碱性成分如烷基碳酸锂烃氧基锂和Li2CO3发生反应,来形成更多含LiF的SEI膜。电解液中含5×10-3~20×10-3 mol/L的HF就可以形成LiF含量丰富的SEI膜,有效抑制树突状锂的生长。MOGI等[51]发现在1 mol/L LiClO4 PC电解液中加入5%(质量分数)的氟代乙烯基碳酸酯可以极大的提高锂的循环效率,用原子力显微镜(AFM)对锂表面进行原位检测,发现锂表面有一个由许多直径100~150 nm的颗粒状沉积物堆积而成的薄片,并且该薄片的阻抗低于没有添加剂时形成的SEI膜阻抗。
2 锂离子电池高温电解液的研究进展
目前,锂离子电池常用锂盐LiPF6的热力学不稳定,在温度较高时会分解释放出强Lewis酸PF5(80 ℃下明显反应)。PF5与有机溶剂有很强的反应活性,能与高介电常数溶剂组分发生复杂反应;同时,PF5与电解液中的微量水反应生成HF,HF对SEI膜层具有较强的腐蚀破坏作用;此外,PF5即使是在H2O和其它酸性杂质不存在的情况下也会与SEI膜组分如LiCO3、RCO2Li和ROCO2Li发生一系列的反应,从而使SEI膜在石墨表面的稳定性降低[52-53],如
LiPF6→PF5+2LiF+CO2 (1)
PF5+H2O→POF3+2HF (2)
2HF+2LiCO3→2LiF+H2CO3 (3)
Li2CO3+PF5→POF3+2LiF+CO2 (4)
R CO2Li+PF5→RCOF+LiF+POF3 (5)
ROCO2Li+PF5→RF+LiF+CO2+POF3 (6)
这些反应会导致LiF的含量升高,电池内阻增大,同时生成气体产物使电池内压增大,导致电池胀气鼓包。所以,目前对提高锂离子电池高温稳定性的研究主要集中在3个方面:1) 开发高温性能优异,能替代LiPF6的高温锂盐;2) 寻找性能优良的锂盐稳定剂,抑制高温下LiPF6分解;3) 寻找高温成膜添加剂,提高SEI膜的高温稳定性。
2.1 高温锂盐
人们研究的新型耐高温锂盐主要集中在磷酸锂配合物及硼酸锂配合物上,这两种类型锂盐由于以B或P原子为中心,其吸电子能力较强,容易形成大的共轭π键,以分散中心离子的负电荷,使阴离子更加稳定,研究较多的为LiBOB、LiODFB、LITSFI。
LiBOB的阴离子以B为中心,与草酸根两个氧原子相连,使其虽不含氟离子及苯环,但仍能形成稳定的大π键共轭体系,电荷分布比较分散,由于不含—F、—SO3、—CH等降低分子热稳定性的基团,其分解温度达到302 ℃,使其配成的电解液具有非常好的高温稳定性。XU等[54]研究发现,LiBOB用于PC电解液,可在石墨负极表面形成稳定的SEI膜,使电池在70 ℃时都具有很好的稳定性。配制了1 mol/L LiBOB EC/EMC(EC和EMC的体积比为1:1)的电解液并用于LiNiO2/石墨电池体系中,发现这种电池的容量利用率、充放电效率以及首次效率均大于LiPF6电池。WANG等[55]以LiMn2O4/Li作为半电池对比了0.7 mol/L LiBOB EC/EMC(EC和EMC的体积比为1:1)和1 mol/L LiPF6 EC/EMC/DMC (EC、EMC和DMC的体积比为1:1:1)两种电解液,发现LiBOB相比LiPF6电导率较低,使得LiBOB基电解液循环前期放电比容量较低。但LiBOB高温性能明显优于LiPF6,在65 ℃两种电解液起始容量基本相同,但是由于Mn的溶解致使LiPF6基电解液循环5圈后容量下降19%,而LiBOB基电解液在高温下没有锂盐分解产生HF从而有较高的容量保持率。FU等[56]通过ICP比较了1 mol/L LiPF6 EC/PC/EMC(EC、PC和EMC的体积比为1:1:3)和1 mol/L LiODFB EC/PC/EMC (EC、PC和EMC的体积比为1:1:3)电解液中从尖晶石LiMn2O4正极中溶解出来的Mn2+的含量,结果表明:ODFB-能够捕获溶解的锰离子并且在LiMn2O4表面形成一个更紧密更稳定的薄膜。相较于LiPF6,LiODFB能在25 ℃和60 ℃时显著的提高100次循环后材料的容量保持率。WANG等[57]研究1 mol/L LiPF6+LiTFSI EC/EMC(EC和EMC的体积比为1:1)混合盐电解液对Li(Ni1/3Mn1/3Co1/3)O2/石墨电池性能的影响,发现与1 mol/L LiPF6电解液相比,LiTFSI的存在降低了电池电荷转移阻抗及自放电率,减少了高温存储下的析气量,对电池高低温性能均有一定改善。但是,当充电电压高于4.45 V时,电解液中Ni,Mn,Co金属离子含量升高,说明LiTFSI会加剧过渡金属溶解。车海英等[58]研究三氟甲基磺酰亚胺锂盐(LiTFSI)对磷酸铁锂电池高温行为的影响,发现LiTFSI取低LiPF6可以减少高温循环过程中Fe的溶出,而且通过LiBF4与LiTFSI的配合使用可以有效抑制LiTFSI对铝箔集流体的腐蚀。采用0.8 mol/L LiTFSI+0.2 mol/L LiBF4 EC:EMC(EC和EMC的体积比为1:1)电解液的LiFePO4电池在55 ℃下1C循环40圈放电容量保持率为97.3%,而采用LiPF6的电池容量保持率只有81%。
2.2 锂盐稳定剂
有研究表明[59],少量路易斯碱性添加剂可以削弱PF5的反应活性和酸性。由于PF5是呈缺电子性的,所以富电性化合物可以很好的达到削弱PF5的反应活性和酸性的目的。理想情况下这些化合物如果带有轻微的路易斯碱性,则可以使LiPF6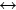 LiF+PF5反应的平衡不会向分解方向移动。比如说三(2,2,2-三氟乙基)磷酸酯(TTFP)可以与PF5产生较弱的结合,从而达到稳定LiPF6的效果。很多酰胺基化合物包括1-甲基-2-吡咯烷酮(35)、氟代氨基甲酸酯和六甲基磷酰胺也与TTFP作用原理相似,化合物中的>C=0或>P=0官能团对氮的孤对电子有强的吸电子能力,可作为一种较弱的碱性中心来使PF5失去活性,起到LiPF6稳定剂的作用[60-61]。
LiF+PF5反应的平衡不会向分解方向移动。比如说三(2,2,2-三氟乙基)磷酸酯(TTFP)可以与PF5产生较弱的结合,从而达到稳定LiPF6的效果。很多酰胺基化合物包括1-甲基-2-吡咯烷酮(35)、氟代氨基甲酸酯和六甲基磷酰胺也与TTFP作用原理相似,化合物中的>C=0或>P=0官能团对氮的孤对电子有强的吸电子能力,可作为一种较弱的碱性中心来使PF5失去活性,起到LiPF6稳定剂的作用[60-61]。
2.3 高温成膜添加剂
在锂离子电池首次循环过程中电解液组分发生分解,在石墨化负极表面形成一层对电池性能有很大影响的固态电解质界面,即为SEI膜。SEI膜的主要成分是电解质溶剂和锂盐的分解产物,包括LiCO3、烷基碳酸锂、烷氧基锂和其他一些盐类,如LiPF6基电解质的会有LiF等[62-63]。SEI成膜促进剂主要分还原性添加剂和反应性添加剂两种。
还原型添加剂的还原电位通常高于电解质溶剂的还原电位。在电解质溶剂还原之前,这些添加剂能够被较好的还原生成一种不溶的固态产物,包裹在负极表面作为一层预备薄膜,减少石墨与有机溶剂反应生成气体的几率并提高SEI膜的稳定性,如碳酸亚乙烯酯(VC)[64-66]、乙烯基碳酸乙烯酯[67]等一些含有碳碳双键的有机物、SO2[68-69]、CS2[70]、多硫化物(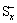 )[71-72]等一些含硫化合物、含氟化合物[73]。反应性添加剂不一定会在嵌锂的整个电压范围发生电化学还原,但他们可以作为溶剂还原反应的中间化合物来捕获自由基负离子,或者与烷基碳酸锂、烷氧基锂等最终产物结合形成更稳定的SEI膜成分,如CO2[74-75]、二烷基焦碳酸酯[76]等能够直接或间接提供CO2的化合物、一系列含磷、氮化合物[77-78]和硼基化合物[79]。WANG等[80]研究Li2CO3作为添加剂加入对LiMn2O4/Li电池高温性能的影响,发现Li2CO3加入可以在LiMn2O4电极表面形成一层具有良好离子导电性的正极电解液界面膜(CEI),从而显著提高电池高温循环性能。
)[71-72]等一些含硫化合物、含氟化合物[73]。反应性添加剂不一定会在嵌锂的整个电压范围发生电化学还原,但他们可以作为溶剂还原反应的中间化合物来捕获自由基负离子,或者与烷基碳酸锂、烷氧基锂等最终产物结合形成更稳定的SEI膜成分,如CO2[74-75]、二烷基焦碳酸酯[76]等能够直接或间接提供CO2的化合物、一系列含磷、氮化合物[77-78]和硼基化合物[79]。WANG等[80]研究Li2CO3作为添加剂加入对LiMn2O4/Li电池高温性能的影响,发现Li2CO3加入可以在LiMn2O4电极表面形成一层具有良好离子导电性的正极电解液界面膜(CEI),从而显著提高电池高温循环性能。
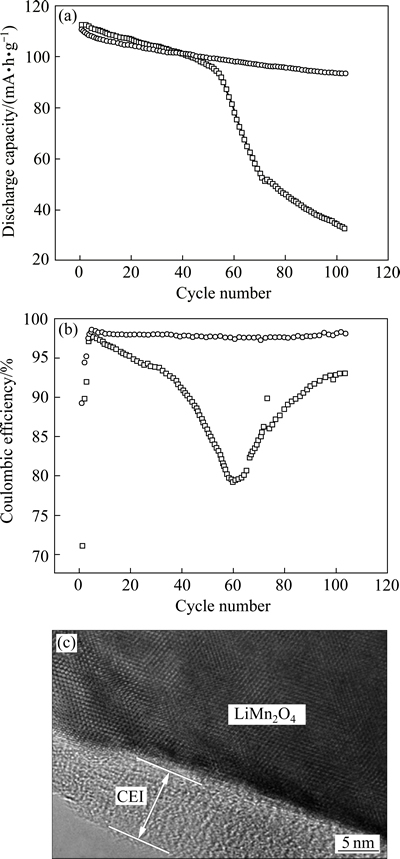
图5 采用不同电解液的LiMn2O4/Li电池在55 ℃下0.5C循环性能和库伦效率以及100次循环后LiMn2O4颗粒TEM像[80]
Fig. 5 Cycling performances(a) and coulombic efficiencies(b) of LiMn2O4/Li cells with different electrolytes at 55 ℃ and 0.5C and TEM image of LiMn2O4 particles after 100 cycles(c)[80]
KANG等[81]以碳酸亚乙烯酯(VC)、丁二腈(SN)、1,3-丙烷磺酸内酯(PS)为高温成膜添加剂,研究了其对Li[Ni0.6Mn0.2Co0.2]O2电池高温性能的影响,发现几种添加剂均可提高电池60℃下的高温循环性能(见图6)。不含添加剂的电池50圈循环后容量保持率只有78.2%,加入VC添加剂的电池50圈循环容量保持率为90.4%,而加入SN及PS添加剂的电池容量保持率分别为94.6%和98.9%。
丙烯基-1,3-磺酸内酯(PES)是近年来发现的具有较为优异的高温性能的成膜添加剂。XIA[82]和KANG等[83]对比研究了VC和PES添加剂对Li[Ni1/3Mn1/3Co1/3]O2/石墨电池性能的影响,发现含有PES的电池比含VC电池产生更少的气体,且在高温下PES表现出较好的性能,其在55 ℃下循环1000圈后,容量损失低于20%。LI等[84-85]用PC基电解液1.0 mol/L LiPF6 PC/EMC(PCt EMC的质量比为1:1)添加PES或PS,通过对LiCoO2/石墨半电池进行CV和充放电测试发现,在PES还原的过程中形成了具有保护性的SEI膜且比PS更加有效,避免了PC和锂离子共同嵌入石墨层。含3%(质量分数)PS添加剂的电池表现出127 (mA·h)/g的放电比容量和79%的库伦效率,而含3% PES(质量分数)表现出136 (mA·h)/g的放电比容量和85%的库伦效率。同时两种添加剂在电池循环50圈后,PES添加剂表现出14%的容量损失,低于PS的20%。
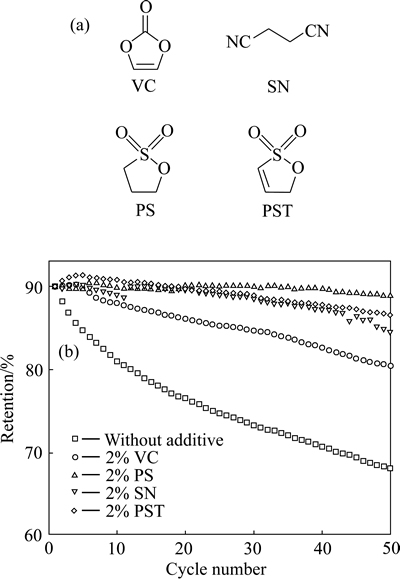
图6 添加剂的分子结构以及采用这些添加剂的Li[Ni0.6Mn0.2Co0.2]O2电池在60 ℃下的放电容量保持率[81]
Fig. 6 Molecular structure of additives(a) and discharge retention of Li[Ni0.6Mn0.2Co0.2]O2 batteries depending on additives at 60 ℃(b)[81]
3 锂离子宽温域电解液的发展趋势
目前,改善锂离子电池温度特性的研究工作多是单独针对低温性能或高温性能,虽然在这两方面均取得了一定的进展,但是这些研究成果并不能简单拼凑进一步拓宽锂电池工作温度范围。一方面,大部分线性羧酸酯类溶剂熔点较低,沸点也较低,蒸气压较大,虽然能有效改善电池低温性能,但同时会对电池高温性能带来不利影响。如MA(沸点56 ℃)用于软包锂离子电池,会使电池50 ℃便开始膨胀变形而无法使用。即使沸点较高的EMC(沸点110 ℃),由于其蒸气压较大,在要求高温存储(>70 ℃)的电池中,也需要严格限制其用量。再如能够显著改善锂电池低温性能的成膜添加剂FEC,其形成的膜层在高温下易分解,且FEC加入会增加电解液中HF含量,这均会降低电池高温稳定性。另一方面,改善电池高温性能的添加剂(LiBOB、PS、MEC等)虽然能在正极或负极表面形成稳定的SEI膜抵挡电解液侵蚀并改善电池高温性能,但是此种膜层一般较厚且阻抗较大,会严重恶化电池低温性能。鉴于以上种种原因,目前开发的低温锂电池普遍高温性能不好,而高温锂电池则常常低温性能较差。针对军用或民用极端工作需求,开发同时兼顾高低温的锂离子电池电解液将是未来的发展趋势。
REFERENCES
[1] KOKSBANG R, BARKER J, SHI H, SAIDI M Y. Cathode materials for lithium rocking chair batteries[J]. Solid State Ionics, 1996, 84(1/2): 1-21.
[2] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2011, 414(6861): 359-367.
[3] NISHI Y. Lithium ion secondary batteries; past 10 years and the future[J]. Journal of Power Sources, 2001, 100(1): 101-106.
[4] MIKHAYLOV A A, MEDVEDEV A A, MASON C W, NAGASUBRAMANIAN A, MADHAVI S, BATABYAL S K, ZHANG Q, GUN J, PRIKHODCHENKO P V, LEV O. Graphene oxide supported sodium stannate lithium ion battery anodes by the peroxide route: Low temperature and no waste processing[J]. Journal of Materials Chemistry A, 2015, 3(41): 20681-20689.
[5] WANG Chao-yang, ZHANG Guang-sheng, GE Shan-hai, XU Terrence, JI Yan, YANG Xiao-guang, LENG Yong-jun. Lithium-ion battery structure that self-heats at low temperatures[J]. Nature, 2016, 529(7587): 515-518.
[6] XIANG H F, LIN H W, YIN B, ZHANG C P, GE X W, CHEN C H. Effect of activation at elevated temperature on Li-ion batteries with flame-retarded electrolytes[J]. Journal of Power Sources, 2010, 195(1): 335-340.
[7] SCROSATI B, GARCHE J. Lithium batteries: Status, prospects and future[J]. Journal of Power Sources, 2010, 195(9): 2419-2430.
[8] 程 昀, 李 劼, 贾 明, 汤依伟, 宋文锋, 张冶安, 张 凯. 动力锂离子电池模块散热结构仿真研究[J]. 中国有色金属学报, 2015, 25(6): 1607-1616.
CHENG Yun, LI Jie, JIA Ming, TANG Yi-wei, SONG Wen-feng, ZHANG Zhi-an, ZHANG Kai. Simulation research of heat dissipation structure for automotive lithium-ion battery packs[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(6): 1607-1616.
[9] LU Wei, XIE Kai, CHEN Zhong-xue, XIONG Shi-zhao, YI Pan, ZHENG Chun-man. A new co-solvent for wide temperature lithium ion battery electrolytes: 2,2,2-Trifluoroethyl n-caproate [J]. Journal of Power Sources, 2015, 274:676-684.
[10] ZHANG S S, XU K, JOW T R. The low temperature performance of Li-ion batteries[J]. Journal of Power Sources, 2003, 115(1): 137-140.
[11] 张丽娟, 李法强, 诸葛芹, 任齐都, 王连亮, 史一飞. 锂离子二次电池低温电解液的研究进展[J]. 盐湖研究, 2009, 17(2): 57-61.
ZHANG Li-juan, LI Fa-qiang, ZHU Ge-qin, REN Qi-du, WANG Lian-liang, SHI Yi-fei. Research progress of low temperature electrolytes for Li-ion batteries[J]. Journal of Salt Lake Research, 2009, 17(2): 57-61.
[12] ZHANG S S, XU K, JOW T R. Electrochemical impedance study on the low temperature of Li-ion batteries[J]. Electrochimica Acta, 2004, 49(7): 1057-1061.
[13] DING M S. Liquid-solid phase diagrams of ternary and quaternary organic carbonates[J]. Journal of the Electrochemical Society, 2004, 151(5): A731-A738.
[14] 许梦清, 左晓希, 李伟善, 周豪杰, 刘建生, 袁中直. PC作电解质组分的锂离子蓄电池高低温性能[J]. 电源技术, 2006, 30(1): 14-17.
XU Meng-qing, ZUO Xiao-xi, LI Wei-shan, ZHOU Hao-jie, LIU Jian-sheng, YUAN Zhong-zhi. High and low temperature performance of Li-ion battery using PC as electrolyte component[J]. Chinese Journal of Power Sources, 2006, 30(1): 14-17.
[15] ZHANG S S, XU K, ALLEN J L, JOW T R. Effect of propylene carbonate on the low temperature performance of Li-ion cells[J]. Journal of Power Sources, 2002, 110(1): 216-221.
[16] UE M, MORI S. Mobility and Ionic association of lithium salts in a propylene carbonate-ethyl methyl carbonate mixed solvent[J]. Journal of the Electrochemical Society, 1995, 142(8): 2577-2581.
[17] DING M S, XU K, ZHANG S S, AMINE K, HENRIKSEN G L, JOW T R. Change of conductivity with salt content, solvent composition, and temperature for electrolytes of LiPF6 in ethylene carbonate-ethyl methyl carbonate[J]. Journal of the Electrochemical Society, 2001, 148(10): A1196-A1204.
[18] CAPIGLIA C, SAITO Y, KAGEYAMA H, MUSTARELLI P, LWAMOTO T, TABUCHI T, TUKAMOTO H. 7Li and 19F diffusion coefficients and thermal properties of non-aqueous electrolyte solutions for rechargeable lithium batteries[J]. Journal of Power Sources, 1999, 81/82: 859-862.
[19] ZHANG S S, JOW T R, AMINE K, HENRIKSEN G L. LiFP6-EC-EMC electrolyte for Li-ion battery [J]. Journal of Power Sources, 2002, 107(1): 18-23.
[20] PLICHTA E J, BEHL W K. A low-temperature electrolyte for lithium and lithium-ion batteries[J]. Journal of Power Sources, 2000, 88(2): 192-196.
[21] 肖利芬, 艾新平, 杨汉西, 曹余良. 锂离子电池用低温电解质溶液研究[J]. 电池, 2004, 34(1): 10-12.
XIAO Li-fen, AI Xin-ping, YANG Han-xi, CAO Yu-liang. Research on low temperature electrolyte for Li-ion batteries[J]. Battery Bimonthly, 2004, 34(1): 10-12.
[22] XIAO L F, CAO Y L, AI X P, YANG H X. Optimization of EC-based multi-solvent electrolytes for low temperature applications of lithium-ion batteries[J]. Electrochimica Acta, 2004, 49(27): 4857-4863.
[23] 王夏芬. 锂离子电池低温电解液的研究[D]. 哈尔滨: 哈尔滨工业大学, 2009.
WANG Xia-fen. Research on low temperature of lithium batteries[D]. Harbin: Harbin Institute of Technology, 2009.
[24] SMART M C, WHITACRE J F, RATNAKUMAR B V, AMINE K. Electrochemical performance and kinetics of Li1+x(Co1/3Ni1/3Mn1/3)1-xO2 cathodes and graphite anodes in low-temperature electrolytes[J]. Journal of Power Sources, 2007, 168(2): 501-508.
[25] SMART M C, RATNAKUMAR B V, WHITCANACK L D, CHIN K B, SURAMPUDI S, CROFT H, TICE D, STANIEWICZ R. Improved low-temperature performance of lithium-ion cells with quaternary carbonate-based electrolytes[J]. Journal of Power Sources, 2003, 119: 349-358.
[26] 韩景立, 于燕梅, 陈 健, 万春荣. 锂离子电池电解液低温导电性能的研究[J]. 电化学, 2003, 9(2): 222-227.
HAN Jing-li, YU Yan-mei, CHEN Jian, WANG Chun-rong. Conductance properties for electrolyte of lithium ion battery at low temperature[J]. Electro Chemistry, 2003, 9(2): 222-227.
[27] SHIAO H C A, CHUA D, LIN H, SLANE S, SALOMON M. Low temperature electrolytes for Li-ion PVDF cells[J]. Journal of Power Sources, 2000, 87(1): 167-173.
[28] SMART M C, RATNAKUMAR B V, SURAMPUDI S. Electrolytes for low-temperature lithium batteries based on ternary mixtures of aliphatic carbonates[J]. Journal of the Electrochemical Society, 1999, 146(2): 486-492.
[29] HONG Shu, LI Jie, WANG Guan-chao, ZHANG Zhi-an, LAI Yan-qing. Effect of linear carboxylic ester on low temperature performance of LiMn2O4-graphite cells[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(1): 206-210.
[30] 谢晓华. 锂离子电池电解液添加剂的性能研究[D]. 哈尔滨: 哈尔滨工程大学, 2004.
XIE Xiao-hua. Study on the performances of electrolyte additives for lithium ion batteries[D]. Harbin: Harbin Engineering University, 2004.
[31] YAMAKI J I, YAMAZAKI I, EGASHIRA M, OKADA S. Thermal studies of fluorinated ester as a novel candidate for electrolyte solvent of lithium metal anode rechargeable cells[J]. Journal of Power Sources, 2001, 102(1/2): 288-293.
[32] SMART M C, RATNAKUMAR B V, RYAN-MOWREY V S, SURAMPUDI S, PRAKASH G K S, HU J, CHEUNG I. Improved performance of lithium-ion cells with the use of fluorinated carbonate-based electrolytes[J]. Journal of Power Sources, 2003, 119/121: 359-367.
[33] ZHANG S S, XU K, JOW T R. A new approach toward improved low temperature performance of Li-ion battery[J]. Electrochemistry Communications, 2002, 4(11): 928-932.
[34] ZHANG S S, XU K, JOW T R. Enhanced performance of Li-ion cell with LiBF4-PC based electrolyte by addition of small amount of LiBOB[J]. Journal of Power Sources, 2006, 156(2): 629-633.
[35] ZHANG Zhian, CHEN Xu-jie, LI Fan-qun, LI Jie, LIU Ping, WANG Xin-yu. LiPF6 and lithium oxalyldifluoroborate blend salts electrolyte for LiFePO4/artificial graphite lithium-ion cells[J]. Journal of Power Sources, 2010, 195(21): 7397-7402.
[36] 向 容. LiODFB基锂离子电池的性能研究[D]. 合肥: 中国科学院大学, 2013.
XIANG Rong. Performance research of LiODFB-based lithium ion battery[D]. Hefei: University of Chinese Academy of Sciences, 2013.
[37] ZHANG S S, XU K, JOW T R. An improved electrolyte for the LiFePO4 cathode working in a wide temperature range[J]. Journal of Power Sources, 2006, 159(1): 702-707.
[38] LAZAR M L, LUCHT B L. Carbonate free electrolyte for lithium ion batteries containing γ-butyrolactone and methyl butyrate[J]. Journal of the Electrochemical Society, 2015, 162(6): A928-A934.
[39] HU Yong-sheng, WANG Zhao-xiang, HUANG Xue-jie, CHEN Li-quan. Physical and electrochemical properties of new binary room-temperature molten salt electrolyte based on LiBETI and acetamide[J]. Solid State Ionics, 2004, 175(1/4): 277-280.
[40] HU Yong-sheng, WANG Zhao-xiang, LI Hong, HUANG Xue-jie, CHEN Li-quan. Ionic conductivity and association studies of novel RTMS electrolyte based on LiTFSI and acetamide[J]. Journal of the Electrochemical Society, 2004, 151(9): A1424-A1428.
[41] MANDAL B K, PADHI A K, SHI Zhong, CHAKRABORTY S, FILLER R. New low temperature electrolytes with thermal runaway inhibition for lithium-ion rechargeable batteries[J]. Journal of Power Sources, 2006, 162(1): 690-695.
[42] 胡锋波, 张庆华, 詹晓力, 陈丰秋. 双(氟代磺酰)亚胺及其盐的制备、性能与应用进展[J]. 化工进展, 2011, 30(10): 2097-2105.
HU Feng-bo, ZHANG Qing-hua, ZHAN Xiao-li, CHEN Feng-qiu. Synthesis, performance, and application progress of bis(fluoro-based sulfonyl)imides and their salts[J]. Chemical Industry and Engineering Progress, 2011, 30(10): 2097-2105.
[43] YAMADA Y, FURUKAWA K, SODEYAMA K, KIKUCHI K, YAEGASHI M, TATEYAMA Y, YAMADA A. Unusual stability of acetonitrile-based superconcentrated electrolytes for fast-charging lithium-ion batteries[J]. Journal of the American Chemical Society, 2014, 136(13): 5039-5046.
[44] TARASCON J M, ARMAND M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367.
[45] ZHANG Long-fei, CHAI Li-li, ZHANG Li, SHEN Ming, ZHANG Xian-lin, BATTAGLIA V S, STEPHENSON T, ZHENG Hong-he. Synergistic effect between lithium bis (fluorosulfonyl)imide (LiFSI) and lithium bis-oxalato borate (LiBOB) salts in LiPF6-based electrolyte for high-performance Li-ion batteries[J]. Electrochimica Acta, 2014, 127(5): 39-44.
[46] WALKER C W, COX J D, SALOMON M. Conductivity and electrochemical stability of electrolytes containing organic solvent mixtures with lithium tris(trifluoromethanesulfonyl) methide[J]. Journal of the Electrochemical Society, 1996, 143(4): L80-L82.
[47] 路婷婷. 磷酸铁锂电池低温电解液的应用研究[D]. 济南: 山东大学, 2014.
LU Ting-ting. Application research of the electrolyte used in lithium iro phosphate batteries at low temperature[D]. Ji’nan: Shandong University, 2014.
[48] 刘兵晓. 氟代碳酸乙烯酯对锂离子电池低温性能的影响及其机理研究[D]. 上海: 华东理工大学, 2012.
LIU Bing-xiao. Effects and mechanism of fluoroethylene carbonate on low temperature performance of Li-ion batteries[D]. Shanghai: East China University of Science and Technology, 2012.
[49] MOLLER K C, HODAL T, APPEL W K, WINTER M, BESENHARD J O. Fluorinated organic solvents in electrolytes for lithium ion cells[J]. Journal of Power Sources, 2001, 97: 595-597.
[50] RAUH R D, BRUMMER S B. The effect of additives on lithium cycling in propylene carbonate[J]. Electrochimica Acta, 1977, 22(1): 75-83.
[51] MOGI R, INABA M, JEONG S K, IRIYAM Y, ABE T, OGUMI Z. Effects of some organic additives on lithium deposition in propylene carbonate[J]. Journal of the Electrochemical Society, 2002, 149(12): A1578-A1583.
[52] SHIM J, KOSTECKI R, RICHARDSON T, SONG X, STRIEBEL K A. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature[J]. Journal of Power Sources, 2002, 112(1): 222-230.
[53] 胡传跃, 李新海, 郭 军, 汪形艳, 易 涛. 高温下锂离子电池电解液与电极的反应[J]. 中国有色金属学报, 2007, 17(4): 629-635.
HU Chuan-yue, LI Xin-hai, GUO Jun, WANG Xing-yan, YI Tao. Reactions between electrolyte and electrode of lithium ion batteries at elevated temperature[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(4): 629-635.
[54] XU K, LEE U, ZHANG S S, WOOD M, JOW T R. Chemical analysis of graphite/electrolyte interface formed in LiBOB-based electrolytes[J]. Electrochemical and Solid-State Letters, 2003, 6(7): A144-A148.
[55] WANG Sai, QIU Wei-hua, LI Tao, YU Bi-tao, ZHAO Hai-lei. Properties of lithium bis (oxatlato) borate (LiBOB) as a lithium salt and cycle performance in LiMn2O4 half cell[J]. Int J Electrochem Sci, 2006, 1: 250-257.
[56] FU M H, HUANG K L, LIU S Q, LIU J S, LI Y K. Lithium difluoro (oxalato) borate/ethylene carbonate+ propylene carbonate+ethyl (methyl) carbonate electrolyte for LiMn2O4 cathode[J]. Journal of Power Sources, 2010, 195(3): 862-866.
[57] WANG D Y, XIAO A, WELLS L, DAHN R. Effect of mixtures of lithium hexafluorophosphate (LiPF6) and lithium bis (fluorosulfonyl)imide (LiFSI) as salts in Li[Ni1/3Mn1/3Co1/3]O2/ graphite pouch cells[J]. Journal of the Electrochemical Society, 2015, 162(1): A169-A175.
[58] 车海英, 杨 军, 吴 凯, 王久林, 努丽燕娜. 二(三氟甲基磺酰)亚胺埋对磷酸铁锂正极高温行为的影响[J]. 化学学报, 2011, 69(11): 1287-1292.
CHE Hai-ying, YANG Jun, WU Kai, WANG Jiu-lin, NULI Yan-na. Effect of lithium bis(trifluoromethylsulfonyl)imide on the high-temperature behavior of LiFePO4 positive electrode[J]. Acta Chimica Sinica, 2011, 69(11): 1287-1292.
[59] XU Jin-qiu, THOMAS H R, FRANCIS R W, LUM K R, WANG Jing-wei, LIANG Bo. A review of processes and technologies for the recycling of lithium-ion secondary batteries[J]. Journal of Power Sources, 2008, 177(2): 512-527.
[60] ZHANG S S, XU K, JOW T R. A thermal stabilizer for LiPF6-based electrolytes of Li-ion cells[J]. Electrochemical and Solid-state Letters, 2002, 5(9): A206-A208.
[61] NAM T H, SHIM E G, KIM J G, KIM H S, MOON S I. Diphenyloctyl phosphate and tris(2, 2, 2-trifluoroethyl) phosphite as flame-retardant additives for Li-ion cell electrolytes at elevated temperature[J]. Journal of Power Sources, 2008, 180(1): 561-567.
[62] NITTA N, WU Fei-xiang, LEE J T, YUSHIN G. Li-ion battery materials: Present and future[J]. Materials Today, 2015, 18(5): 252-264.
[63] SHI S J, TU J P, TANG Y Y, ZHANG Y Q, LIU X Y, WANG X L, GU C D. Enhanced electrochemical performance of LiF-modified LiNi1/3 Co1/3Mn1/3O2 cathode materials for Li-ion batteries[J]. Journal of Power Sources, 2013, 225: 338-346.
[64] AURBACH D, GAMOLSKY K, MARKOVSKY B, GOFER Y, SCHMIDT M, HEIDER U. On the use of vinylene carbonate (VC) as an additive to electrolyte solutions for Li-ion batteries[J]. Electrochimica Acta, 2002, 47(9): 1423-1439.
[65] CONTESTABILE M, MORSELLI M, PARAVENTI R, NEAT R J. A comparative study on the effect of electrolyte/additives on the performance of ICP383562 Li-ion polymer (soft-pack) cells[J]. Journal of Power Sources, 2003, 119/121: 943-947.
[66] SASAKI T, ABE T, IRIYAMA Y, INABA M, OGUMI Z. Suppression of an alkyl dicarbonate formation in Li-ion cells[J]. Journal of the Electrochemical Society, 2005, 152(10): A2046-A2050.
[67] HU Yong-sheng, KONG Wei-he, WANG Zhao-xiang, LI Hong, HUANG Xue-jie, CHEN Li-quan. Effect of morphology and current density on the electrochemical behavior of graphite electrodes in PC-based electrolyte containing VEC additive[J]. Electrochemical and Solid-state Letters, 2004, 7(11): A442-A446.
[68] EIN-ELI Y, THOMAS S R, KOCH V R. New electrolyte system for Li-ion battery[J]. Journal of the Electrochemical Society, 1996, 143(9): L195-L197.
[69] EIN-ELI Y, THOMAS S R, KOCH V R. The role of SO2 as an additive to organic Li-ion battery electrolytes[J]. Journal of the Electrochemical Society, 1997, 144(4): 1159-1165.
[70] EIN-ELI Y. Dithiocarbonic anhydride (CS2)-a new additive in Li-ion battery electrolytes[J]. Journal of Electroanalytical Chemistry, 2002, 531(1): 95-99.
[71] WAGNER N W, LIEBENOW C, BESENHARD J O. Effect of polysulfide-containing electrolyte on the film formation of the negative electrode[J]. Journal of power sources, 1997, 68(2): 328-332.
[72] BESENHARD J O, WAGNER N W, WINTER M, JANNAKOUDAKIS A D, JANNAKOUDAKIS P D, THEODORIDOU E. Inorganic film-forming electrolyte additives improving the cycling behaviour of metallic lithium electrodes and the self-discharge of carbon—lithium electrodes[J]. Journal of Power Sources, 1993, 44(1): 413-420.
[73] AURBACH D, GNANARAJ J S, GEISSLER W, SCHMIDT M. Vinylene carbonate and Li salicylatoborate as additives in LiPF3(CF2CF3)3 solutions for rechargeable Li-ion batteries[J]. Journal of the Electrochemical Society, 2004, 151(1): A23-A30.
[74] EIN-ELI Y, MARKOVSKY B, AURBACH D, CARMELI Y, YAMIN H, LUSKI S. The dependence of the performance of Li-C intercalation anodes for Li-ion secondary batteries on the electrolyte solution composition[J]. Electrochimica Acta, 1994, 39(17): 2559-2569.
[75] SIMON B, BOEUVE J P, BROUSSELY M. Electrochemical study of the passivating layer on lithium intercalated carbon electrodes in nonaqueous solvents[J]. Journal of power sources, 1993, 43(1): 65-74.
[76] ZHUANG G V, YANG H, BLIZANAC B, ROSS P N. A study of electrochemical reduction of ethylene and propylene carbonate electrolytes on graphite using ATR-FTIR spectroscopy[J]. Electrochemical and Solid-state Letters, 2005, 8(9): A441-A445.
[77] CHEN Zong-hai, REN Yang, JANSEN A N, LIN Chi-kai, WENG Wei, AMINE K. New class of nonaqueous electrolytes for long-life and safe lithium-ion batteries[J]. Nature Communications, 2013, 4: 1513.
[78] YAN Guo-chun, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, WANG Jie-xi. Effect of fluoroadditives on the electrode characteristics of graphite for secondary lithium battery[J]. Journal of New Materials for Electrochemical Systems, 2006, 9(3): 181.
[79] ZHANG S S, XU K, JOW T R. Enhanced performance of Li-ion cell with LiBF4-PC based electrolyte by addition of small amount of LiBOB[J]. Journal of Power Sources, 2006, 156(2): 629-633.
[80] WANG Ren-heng, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, HOU Tao, YAN Guo-chun, HUANG Bin. Lithium carbonate as an electrolyte additive for enhancing the high-temperature performance of lithium manganese oxide spinel cathode[J]. Journal of Alloys and Compounds, 2015, 618: 349-356.
[81] KANG K S, CHOI S, SONG J, WOO S G, JO Y N, GHOI J, YIM T, YU J S, KIM Y J. Effect of additives on electrochemical performance of lithium nickel cobalt manganese oxide at high temperature[J]. Journal of Power Sources, 2014, 253: 48-54.
[82] XIA J, MA L, AIKEN C P, NELSON K J, CHEN L P, DAHN J R. Comparative study on prop-1-ene-1, 3-sultone and vinylene carbonate as electrolyte additives for Li (Ni1/3Mn1/3Co1/3)O2/ graphite pouch cells[J]. Journal of the Electrochemical Society, 2014, 161(10): A1634-A1641.
[83] KANG K S, CHOI S, SONG J H, WOO S G, JO Y N, CHOI J, YIM T, YU J S, KIM Y J. Studies of the effect of varying prop-1-ene-1, 3-sultone content in lithium ion pouch cells[J]. Journal of the Electrochemical Society, 2014, 161(12): A1884-A1889.
[84] LI Bin, XU Meng-qing, LI Tian-tian, LI Wei-shan, HU She-jun. Prop-1-ene-1, 3-sultone as SEI formation additive in propylene carbonate-based electrolyte for lithium ion batteries[J]. Electrochemistry Communications, 2012, 17: 92-95.
[85] LI Bin, XU Meng-qing, LI Ben-zhen, LIU Yan-lin, LI Wei-shan, HU She-jun. Properties of solid electrolyte interphase formed by prop-1-ene-1, 3-sultone on graphite anode of Li-ion batteries[J]. Electrochimica Acta, 2013, 105: 1-6.
Review of functional electrolyte for lithium-ion battery working in wide temperature scope
HONG Bo1, YAN Xiao-lin2, HONG Shu1, LAI Yan-qing2, LI Jie2, JIANG Feng1
(1. School of Materials Science and Engineering, Central South University, Changsha 410083, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: The degradation mechanism of lithium ion battery under low temperature was analyzed. The effect regularity of physical properties of solvent on low temperature performance of electrolyte was also described. Then, the research works of improving the low temperature performances of batteries by using low melting point and low viscosity solvent, low-impedance film-forming additives, new lithium salts were summarized. Meanwhile, the capacity attenuation mechanism of lithium ion battery under elevated temperature working conditions was also discussed. The main methods of improving the high temperature stability of lithium ion battery were reviewed, containing using high temperature film additive, high temperature lithium salt and lithium salt stabilizer. On these basis, the domain challenges and the development trend of lithium ion battery working in a wide temperature scope was prospected.
Key words: lithium-ion battery; wide-temperature electrolyte; low-temperature performance; high-temperature performance; linear carboxylic acid esters; additive
Foundation item: Project(51222403) supported by the National Natural Science Foundation of China; Project(13JJ1003) supported by the Natural Science Foundation of Hunan Province, China; Project(2016) supported by Postdoctoral Research Foundation Plan of Central South University, China
Received date: 2016-04-21; Accepted date: 2016-10-25
Corresponding author: LAI Yan-qing; Tel: +86-731-88876454; E-mail: laiyanqing@csu.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(51222403);湖南省自然科学基金资助项目(13JJ1003);中南大学博士后基金资助项目(2016)
收稿日期:2016-04-21;修订日期:2016-10-25
通信作者:赖延清,教授,博士;电话:0731-88876454;E-mail: laiyanqing@csu.edu.cn
摘 要:分析锂离子电池在低温工作条件下的性能劣化机理,阐述溶剂物理性质对电解液低温性能的影响规律,总结目前通过低黏度及低熔点的溶剂组分、低阻抗的成膜添加剂以及新型锂盐来改善电池低温性能的研究工作。同时,探讨锂离子电池在高温工作条件下容量衰减机制,综述目前改善锂离子电池高温性能的主要方法,包括采用高温成膜添加剂、耐高温锂盐以及锂盐稳定剂。在此基础上指出目前宽温域锂离子电池发展面临的主要挑战,展望锂离子宽温域电解液的发展趋势。
[8] 程 昀, 李 劼, 贾 明, 汤依伟, 宋文锋, 张冶安, 张 凯. 动力锂离子电池模块散热结构仿真研究[J]. 中国有色金属学报, 2015, 25(6): 1607-1616.
[11] 张丽娟, 李法强, 诸葛芹, 任齐都, 王连亮, 史一飞. 锂离子二次电池低温电解液的研究进展[J]. 盐湖研究, 2009, 17(2): 57-61.
[14] 许梦清, 左晓希, 李伟善, 周豪杰, 刘建生, 袁中直. PC作电解质组分的锂离子蓄电池高低温性能[J]. 电源技术, 2006, 30(1): 14-17.
[21] 肖利芬, 艾新平, 杨汉西, 曹余良. 锂离子电池用低温电解质溶液研究[J]. 电池, 2004, 34(1): 10-12.
[23] 王夏芬. 锂离子电池低温电解液的研究[D]. 哈尔滨: 哈尔滨工业大学, 2009.
[26] 韩景立, 于燕梅, 陈 健, 万春荣. 锂离子电池电解液低温导电性能的研究[J]. 电化学, 2003, 9(2): 222-227.
[30] 谢晓华. 锂离子电池电解液添加剂的性能研究[D]. 哈尔滨: 哈尔滨工程大学, 2004.
[36] 向 容. LiODFB基锂离子电池的性能研究[D]. 合肥: 中国科学院大学, 2013.
[42] 胡锋波, 张庆华, 詹晓力, 陈丰秋. 双(氟代磺酰)亚胺及其盐的制备、性能与应用进展[J]. 化工进展, 2011, 30(10): 2097-2105.
[47] 路婷婷. 磷酸铁锂电池低温电解液的应用研究[D]. 济南: 山东大学, 2014.
[48] 刘兵晓. 氟代碳酸乙烯酯对锂离子电池低温性能的影响及其机理研究[D]. 上海: 华东理工大学, 2012.
[53] 胡传跃, 李新海, 郭 军, 汪形艳, 易 涛. 高温下锂离子电池电解液与电极的反应[J]. 中国有色金属学报, 2007, 17(4): 629-635.
[58] 车海英, 杨 军, 吴 凯, 王久林, 努丽燕娜. 二(三氟甲基磺酰)亚胺埋对磷酸铁锂正极高温行为的影响[J]. 化学学报, 2011, 69(11): 1287-1292.


