
Low-temperature performance and high-rate discharge capability of AB5-type non-toichiometric hydrogen storage alloy
LU Yan-jing(陆延静), ZHU Lei(朱 磊), CHENG Yan(成 艳),
CHEN Hui(陈 晖), JIAN Xu-yu(简旭宇), WANG Zhong(王 忠)
Energy Material and Technology Research Institute, General Research Institute for Nonferrous Metals,Beijing 100088, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Low-temperature performance and high-rate discharge capability of AB5-type non-stoichiometric hydrogen storage are studied. X-ray diffraction(XRD), pressure-composition-temperature(PCT) curves and electrochemical impedance spectroscopy(EIS) are applied to characterize the electrochemical properties of ABx (x=4.8, 4.9, 5.0, 5.1, 5.2) alloys. The results show that the non-stoichiometric alloys exhibit better electrochemical properties compared with that of the AB5 alloy.
Key words:
AB5-type hydrogen storage alloy; non-stoichiometry; low-temperature performance; high-rate discharge capability;
1 Introduction
In recent years, nickel-metal hydride(Ni-MH) secondary batteries have been widely used in various portable electronic devices, electric tools and vehicles because of their excellent electrochemical performance and environmental compatibility[1]. However, the low-temperature performance of Ni-MH batteries is unsatisfied. As we known, hydrogen storage alloys play a key role in the discharging process at low temperature for Ni-MH battery. At present, the commercialized negative electrode materials are mainly AB5-type alloys due to its long-term cycling stability, high-rate capacity, and good charge-discharge kinetics[2-5]. Therefore, it is necessary to improve the low-temperature electro- chemical performances of hydrogen storage alloy.
To our knowledge, no systematically investigation has been done for the AB5-type non-stoichiometric alloys under high rate and low temperature. In this work, the performance of the AB5-type non-stoichiometric alloys are investigated.
2 ExperimentalBased upon the AB5-type (LaCePr)(NiCoMnAl)5 hydrogen alloy, (LaCePr)(NiCoMnAl)x (x=4.8, 4.9, 5.0, 5.1, 5.2) non-stoichiometric alloys were designed and prepared by induction melting under argon shield of pure elements in a water-cooled copper crucible. The ingots were melted and turned over three times for homogeneity Then the ingots were crushed and mechanically grounded to a particle size of less than 74 μm.
The crystal structures of (LaCePr)(NiCoMnAl)x (replaced by ABx in the following) were analyzed by X-ray diffractogram.
2.1 Preparation of the MH electrode
0.1 g alloy powder was mixed uniformly with 0.3 g nickel powder in proportion of 1?3, and then they were pressed by 20 MPa into the negative plate 13 mm in diameter and 0.5 mm in thickness. The counter electrode was formed by the NiOOH/Ni(OH)2, whereas the reference electrode was Hg/HgO electrode filled with 6 mol/L KOH solution.
2.2 Electrochemical measurements
After activation at room temperature (20 ℃), the electrodes were charged for 7 h at 60 mA/g (0.2C), then some were kept for 30 min at room temperature and the others were placed in the low temperature cabinets for 4 h and 6 h at the temperature of -20 ℃ and -40 ℃,respectively, then the samples were discharged at various rates (0.2C, 0.5C, 1C and 3C) to -0.6, -0.6, -0.5, -0.4 voltage with respect to Hg/HgO reference electrode.
Pressure-composition-temperature(PCT) curves of the alloys were determined by electrochemical methods[6]. The thermodynamics parameters including the equilibrium pressure of the hydrogen absorption or desorption and the change of enthalpy were calculated.
The electrochemical impedance spectroscopies of the 50%DOD (depth of discharge) electrodes were measured and the scan frequency was from 100 kHz to 1 mHz.
3 Results and discussion3.1 Discharge capacity of electrode
Fig.1 shows the variation of the discharge capacity, versus the value of x of the ABx alloy. It can be seen that with the increase of x, the discharge capacities curves of various rates (0.2C, 0.5C, 1C, 3C) at 20 ℃ and -20 ℃, as well as that of 0.2C at -40 ℃, present the shape of character M. Over-stoichiometric alloys and under- stoichiometric alloys exhibit better high-rate discharge capability and low-temperature performance compared with that of the stoichiometric alloy (x=5.0). The alloy with x=5.1 shows the excellent electrochemical capability, of which the 3C discharge capacity reaches 284 mA?h/g at 20 ℃, and 0.2C also reaches 233 mA?h/g at -40 ℃.
3.2. Structural analysis
The XRD patterns of ABx metal-hydride alloys are given in Fig.2. The results show that all alloys keep the typical single phase of CaCu5.The lattice parameters and lattice volume are calculated and compiled in Table 1. It can be seen that with the increase of the value of x, The lattice parameters c first increase and then decrease, which is similar to the behaviour of the low-temperature discharge capacity. On the contrary, the lattice parameters a exhibit the opposite phenomenon. The variation of lattice parameters and volume as a function of x is showed in Fig.3. The alloys with smaller lattice parameter a and larger lattice parameter c show better low-temperature performances and high-rate discharge capabilities.
Table 1 Smaller (a) and bigger (b) lattice parameters and lattice volume(V) of ABx metal- hydride alloys

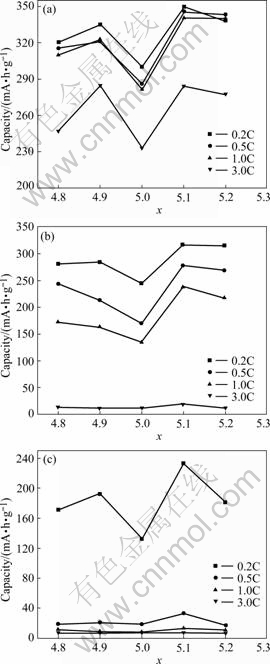
Fig.1 Variation of different rate discharge capacities with x for ABx alloys at different temperatures: (a) 20 ℃; (b) -20 ℃; (c) -40 ℃
3.3 Thermodynamic properties
The pressure-composition-temperature(P—C—T) curves measured at 20, -20 and -40 ℃ are shown in Fig.4.
The equilibrium pressure of hydrogen absorption or desorption (Peq) are defined as following equation[7]:
![]() (1)
(1)
where P2 and P1 are the pressures of inflection points of curves in Fig.4. The variation of Peq versus the value of x is given in Fig.5. According to Peq, Van’t Hoff curves of ABx alloys is presented in Fig.6, and the variation of the change of enthalpy(ΔH) of ABx alloys as a function of x is given in Fig.7.
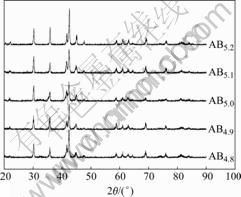
Fig.2 XRD patterns of of ABx metal-hydride alloys
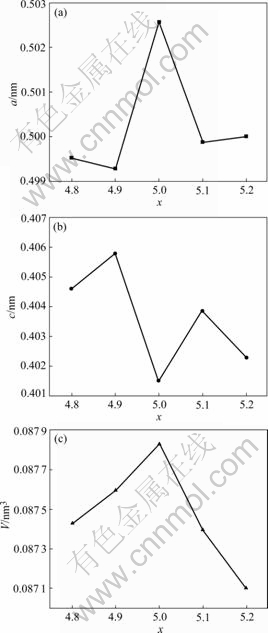
Fig.3 Variation of lattice parameters (a, c) and lattice volume (V) of ABx metal-hydride alloys as function of x: (a) a; (b) c; (c) V
The results show that over-stoichiometric alloys and under-stoichiometric alloys present higher equilibrium pressure (Peq) and less absolute value of the change of enthalpy (|ΔH|). The alloy with x=5.0 exhibit the lowest Peq and the highest |ΔH|, while the alloy with x=5.1 shows the higher Peq and the least |ΔH|, and its |ΔH| reaches 28.9 kJ/mol. With the increase of the value of x, the change of enthalpy (ΔH) shows the same tendency as that of the discharge capacities, which indicates that there is a close relationship between the electrochemical property and the thermodynamic performance for non-stoichiometric alloys. The alloys with higher equilibrium pressure and less absolute value of enthalpy display higher high-rate discharge capacities and low- temperature capabilities, which may be ascribed to the poor stability of metal hydrides materials.
3.4 Electrochemical impedance spectroscopy
Electrochemical impedance is a powerful tool for characterization of metal-hydride electrodes, and has been widely used[8-10]. According to the mathematical model developed by WANG et al[11], the charge-transfer resistance, Rt, could be calculated from the electro- chemical impedance spectroscopy(EIS) by constructing the equivalent circuit, and the exchange current density (I0) could be defined as following equation[12]:
![]() (2)
(2)
where R, T, m and F are the gas constant, the absolute temperature, the effective mass of material and the Faraday constant, respectively.
Fig.8 shows the Nyquist plot of ABx metal-hydride alloys with 50%DOD, and the calculated Rt and I0 are listed in Table 2 and Table 3.
Fig.9 shows the variation of the exchange current density (I0) of ABx metal-hydride alloys (50%DOD) as a function of x. With the increase of x, the exchange current densities of ABx alloys present the same tendency as that of the discharge capacities, and the exchange current densities of the non-stoichiometric alloys are higher than that of AB5 alloy. The high-rate discharge capacities increase gradually with the increase of the exchange current density[13-15]. It is well known that the high exchange current density indicates not only the high reaction rate of the electrode, i.e. high rate charge-discharge capability, but also a low degradation rate of electrode performance[16]. Thus, we can conclude that the non-stoichiometry is beneficial to the increase of electrodes exchange current density, which can improve the low-temperature performance and high-rate discharge ability.
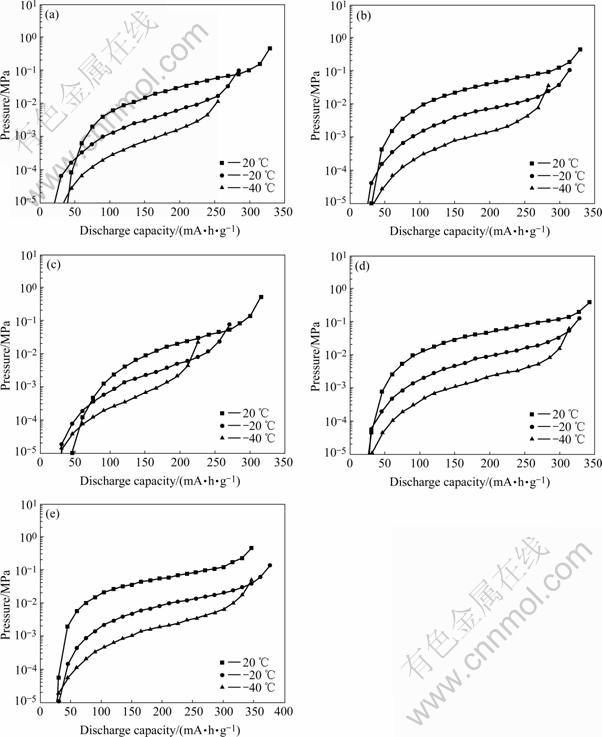
Fig.4 P—C—T curves measured at different temperatures of ABx metal- hydride alloys: (a) AB4.8; (b) AB4.9; (c) AB5.0; (d) AB5.1; (e) AB5.2
Table 2 Charge-transfer resistance (Rt) of ABx metal-hydride alloys (50%DOD) at different temperatures
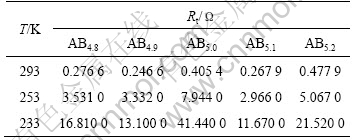
Table 3 Exchange current density (I0) of ABx metal-hydride alloys (50%DOD) at different temperatures

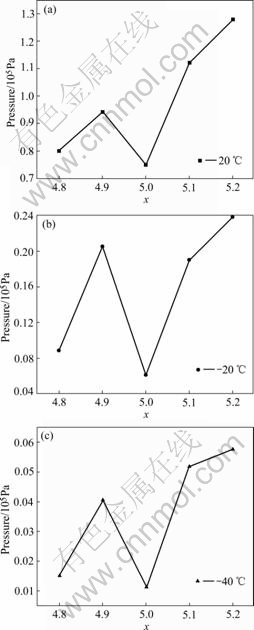
Fig.5 Variation of equilibrium pressures of ABx metal-hydride alloys as function of x: (a) 20 ℃; (b) -20 ℃; (c) -40 ℃
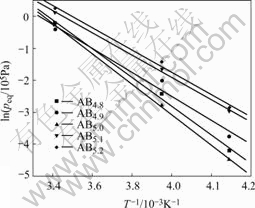
Fig.6 Van’t Hoff curves of ABx metal-hydride alloys

Fig.7 Variation of enthalpy (ΔH) of ABx metal-hydride alloys as function of x

Fig.8 Nyquist plots of ABx metal-hydride alloys (50%DOD)

Fig.9 Variation of exchange current density (I0) of ABx metal-hydride alloys as function of x: (a) 20 ℃; (b) -20 ℃; (c) -40 ℃
4 Conclusions
1) With the increase of x, the discharge capacities of ABx electrodes present the shape of “M”. Under- stoichiometric and over-stoichiometric alloys present the better low-temperature performance and the higher high-rate discharge ability compared with that of the stoichiometric alloy (x=5.0).
2) Non-stoichiometric alloys possess smaller lattice parameter a and bigger lattice parameter c, which may be beneficial to their low-temperature performance and high-rate discharge capability.
3) With the increase of x, the change of enthalpy (ΔH) and the exchange current density (I0) of ABx alloys show the same tendency as that of the discharge capacities, which indicate that there exists a close relationship between the ΔH, I0, low-temperature performance and high-rate discharge ability.
4) The AB5.1 alloy, with the least |ΔH| and highest I0, shows the best discharge capability. Its 3C discharge capacity reaches 284 mA?h/g at 20 ℃, while 0.2C reaches 233 mA?h/g at -40 ℃.
References
[1] TLIHA M, KHALDI C, MATHLOUTHI H, LAMLOUMI J, PERCHERON-GUEGAN A. Electrochemical investigation of the iron-containing and no iron-containing AB5-type negative electrodes [J]. J Alloys Compd, 2007, 440(1/2): 323-327.
[2] RIVERA M A, PAL U, WANG Xian-you, GONZALEZ- RODRIGUEZ J G, GAMBOA S A. Rapid activation of MmNi5-xMx based MH alloy through Pd nanoparticle impregnation [J]. J Power Sources, 2006, 155: 470-474.
[3] LI Rong, WU Jian-min, SU Hang, ZHOU Shao-xiong. Micro- structure and electrochemical performance of vanadium-containing AB5-type low-Co intermetallic hydrides [J]. J Alloys Compd, 2006, 421: 258-267.
[4] YUAN Xian-xia, XU Nai-xin. Determination of hydrogen diffusion coefficient in metal hydride electrode by cyclic voltammetry [J]. J Alloys Compd, 2001, 316(1/2): 113-117.
[5] LI Shang, PAN Gui-ling, ZHANG Ying, GAO Xue-ping, QU Jing-qiu, YAN Jie, WU Feng, SONG De-ying. Electrochemical properties of MmNi3.6Co0.7Al0.3Mn0.4 alloy containing carbon nanotubes [J]. J Alloys Compd, 2003, 353: 295-300.
[6] IWAKURA C, OURA T, INOUE H, MATSUOKA M, YAMAMOTO Y. Effect of alloy composition on hydrogen diffusion in the AB5-type hydrogen storage alloys [J]. J Electroanal Chem, 1995, 398(1/2): 37-41.
[7] YU X B, FENG S L, WU Z, XIA B J, XU N X. Hydrogen storage performance of Ti-V-based BCC phase alloys with various Fe content [J]. J Alloys Compd, 2005, 393: 128-134.
[8] VAL?EN L O, LASIA A, JENSEN J O, TUNOLD R. The electrochemical impedance of metal hydride electrodes [J]. Electrochimica Acta, 2002, 47: 2871-2884.
[9] YUAN Xian-xia, XU Nai-xin. Determination of hydrogen diffusion coefficient in metal hydride electrode by modified Warburg impedance [J]. J Alloys Compd 2001, 329: 115-200.
[10] RAJU M, MANIMARAN K, ANANTH M V, RENGANATHAN N G. An EIS study on the capacity fades in MmNi3.6Al0.4Mn0.3Co0.7 metal-hydride electrodes [J]. Int J Hydrogen Energy, 2007, 32: 1721-1727.
[11] WANG Chun-sheng, SORIAGA M P, SRINIVASAN S. Determination of reaction resistances for metal-hydride electrodes during anodic polarization [J]. J Power Sources 2000, 85: 212-223.
[12] NOTTEN P H L, HOKKELING P. Double-phase hydride forming compounds: A new class of highly electrocatalytic materials [J]. Journal of the Electrochemical Society, 1991, 138(7): 1877-1885.
[13] IWAKURA C, MATSUOKA M, ASAI K, KOHNO T. Surface modification of metal hydride negative electrodes and their charge/discharge performance [J]. J Power Sources, 1992, 38: 335-343.
[14] MATSUOKA M, ASAI K, ASAI K, FUKUMOTO K, IWAKURA C. Electrochemical characterization of surface-modified negative electrodes consisting of hydrogen storage alloys [J]. J Alloys Comp, 1993, 192: 149-151.
[15] IWAKURA C, FUKUMOTO Y, MATSUOKA M, KOHNO T, SHINMOU K. Electrochemical characterization of hydrogen storage alloys modified with metal oxides [J]. J Alloys Comp, 1993, 192: 152-154.
[16] FENG F, NORTHWOOD D O. Effect of surface modification on the performance of negative electrodes in Ni/MH batteries [J]. J Hydrogen Energy, 2004, 29: 955-960.
(Edited by LAI Hai-hui)
Foundation item: Project(2006AA11A151) supported by the National Hi-Tech Research and Development Program of China
Corresponding author: LU Yan-jing; Tel: +86-10-82241241; E-mail: luyanjing82@126.com


