Trans. Nonferrous Met. Soc. China 28(2018) 2592-2598
Simultaneous determination of trace Cu2+, Cd2+, Ni2+ and Co2+ in zinc electrolytes by oscillopolarographic second derivative waves
Juan DU1, Hong-qiu ZHU2, Yong-gang LI2, Tai-ming ZHANG1, Chun-hua YANG2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Information Science and Engineering, Central South University, Changsha 410083, China
Received 5 December 2017; accepted 16 April 2018
Abstract:
Simultaneous determination of impurity metal ions in high concentration zinc solution is very important for zinc hydrometallurgy, and the purpose is to establish a method for determining the trace Cu2+, Cd2+, Ni2+ and Co2+ in zinc electrolytes at the same time using the second derivative waves of single sweep oscillopolarography. Factors affecting the derivative waves of the ions were researched in a medium of dimethylglyoxime (DMG)-sodium citrate-sodium tetraborate. The results indicated that the interferences of a high concentration of Zn2+ and most other coexisting ions on the determination can be eliminated; when the Cu2+, Cd2+, Ni2+ and Co2+ are in the ranges of 1×10-7-3×10-4, 6×10-7-2×10-4, 2×10-8-1×10-5 and 1×10-8-3×10-5 mol/L, respectively, the relationships between the peak currents of the second derivative waves and the concentrations are linear; the detection limits to determine the Cu2+, Cd2+, Ni2+ and Co2+ are 8×10-8, 2×10-7, 6×10-9 and 4×10-9 mol/L, respectively. Without any sample pretreatment, the method was used to directly determine the trace Cu2+, Cd2+, Ni2+ and Co2+ in actual zinc electrolytes with satisfactory results. The method is simple, sensitive and rapid.
Key words:
zinc electrolyte; copper; cadmium; nickel; cobalt; second derivative wave;
1 Introduction
In hydrometallurgy industry, the direct and simultaneous determination of trace amounts of Cu2+, Cd2+, Ni2+ and Co2+ in high concentrations of Zn2+ solutions is still a challenging task for analysts so far [1]. The concentrations of impurity ions in the zinc electrolyte must be strictly controlled because their excessive concentrations, particularly Cu2+, Cd2+, Co2+ and Ni2+ can cause a decrease in current efficiency and decrease the quality and morphology of cathode zinc [2]. Most removal methods in various hydrometallurgical plants are based on zinc powder replacement and chemical precipitation by adjusting the amount of reagents to be added depending on the impurity content. Therefore, a real-time and accurate monitoring of the contents of the impurities in the zinc electrolyte at the inlet and outlet of the relevant removal section is required, which can provide effective feedback information [3].
Traditional analytic methods to determine low concentrations of heavy metals in zinc electrolyte are ICP-AES, GF-AAS, ICP-MS, etc. However, their instruments are expensive and require a good work environment. For ICP-AES, its matrix effect is notably serious; in particular, when the impurity concentration decreases, the accuracy of the determination results also decreases. Thus, it is difficult to use the methods in the rapid online detection in production fields. Electro- chemical detection methods such as oscillopolarography and stripping voltammetry have been widely used to determine metal ions in complex matrices because of their low cost, high sensitivity, good selectivity and simple operation [4-6]. Among these methods, oscillopolarography uses a mercury drop electrode as the working electrode, which can be constantly updated; therefore, it is independent of its past history compared with other methods [7].
A high concentration of Zn2+ (130-170 g/L) in the electrolyte causes the matrix effect, decreases the sensitivity, and interferes with the determination; in particular, the proximity of the reduction potentials between Zn2+ and Co2+ makes the Co2+ signal easily covered by Zn2+ [8]. Furthermore, the direct and simultaneous determination of certain impurity ions in the zinc electrolyte is not always feasible because of inadequate resolution. To solve the problems, many researchers have constantly improved the voltammetric methods to determine the trace impurity ions in zinc solutions [9-16]. However, the improved method cannot still be used to determine the trace cobalt in high concentrated zinc electrolytes or determine various impurity metals including cobalt in it at the same time.
In this study, an sensitive and accurate voltammetric method for determining the trace Cu2+, Cd2+, Ni2+ and Co2+ in high concentrated zinc electrolytes at the same time has been established. In tetraborate buffer medium, the interference of Zn2+ with the determination of Co2+ can be eliminated completely under the complexation of sodium citrate.
2 Experimental
2.1 Apparatus and agents
A polarograph (JP-303; Chengdu Instrument Factory, Chengdu, China) was used, which was equipped with a three-electrode system consisted of a dropping mercury electrode as the working electrode, a saturated calomel electrode as a reference and an auxiliary platinum electrode. A pH-meter (pHS-3C; ShanghaiLeiz Chong Yi Instrument Co. Ltd., Shanghai, China) was used for the pH measurements.
The main agents were sodium hydroxide solution (2.0 mol/L), sodium tetraborate buffer (0.30 mol/L; the salt is dissolved by heating, and then the solution pH was adjusted to 9.3 with sodium hydroxide), sodiumcitrate solution (1.0 mol/L), DMG ethanol solution (11 g/L) prepared by dissolving 1.1 g DMG in 100 mL absolute ethanol, sulfuric acid solution (concentrated sulfuric acid mixed with water in a 1:2 ratio). The Zn2+ (2.6 mol/L), Cu2+ (0.010 mol/L), Cd2+ (0.010 mol/L), Co2+ (0.010 mol/L) and Ni2+ (0.010 mol/L) standard stock solutions were prepared by dissolving the corresponding sulphate in an appropriate amount of water, and diluted to the required concentration before use. The sulfates of the metal ions were guaranteed reagents, and other reagents were analytical reagents. The water was redistilled water.
2.2 Procedure
The testing process is divided into two steps: (1) An appropriate amount of the zinc electrolyte sample (or the metal standard solution to be measured and 1.0 mL ZnSO4 solution) was transferred into a 10 mL volumetric flask; then, 0.15 mL DMG solution was added, mixed well; afterwards, 3.0 mL sodium citrate solution was added; after adjusting the solution to a weak acidity (namely the color of methyl red just transformed from red to yellow) using sodium hydroxide, 4.0 mL sodium tetraborate buffer was added into the flask. The mixture was diluted to 10 mL with redistilled water and thoroughly mixed. The mixture was let to stand for 5 min and transferred into an electrolytic cell. The polarographic second derivative waves were recorded from -200 to -1400 mV (Fig. 1(a)), and the peaks of Cu2+, Ni2+ and Co2+ appeared at approximately -366, -1050 and -1180 mV, respectively. (2) After reading the data, 0.50 mL of sulfuric acid was added to the cell and mixed well. The second derivative wave was recorded from -400 to -800 mV using the continuous measurement function of the instrument (Fig. 1(b)), and the derivative wave of Cd2+ was obtained at approximately -600 mV. The parameters in the JP-303 system remained invariant during the testing process with a scanning rate of 0.5 V/s and a dropping mercury cycle of 9.0 s. The concentration of metallic ions was calculated using the standard addition method. In addition, all determinations were performed at (20±2) °C, and all vessels were soaked with 10% nitric acid and washed with redistilled water.
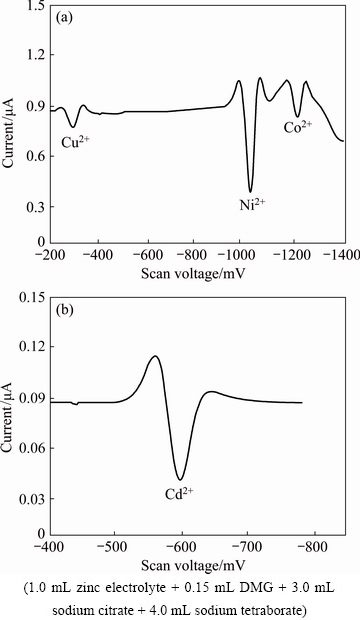
Fig. 1 Second derivative waves of Cu2+, Cd2+, Ni2+ and Co2+ solutions
3 Results and discussion
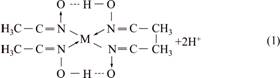
According to the literatures and experiments [17,18], Cu2+, Co2+ and Ni2+ can react with DMG with a coordination ratio of 1:2, as expressed in Eq. (1) (M2+ is Cu2+, Co2+ or Ni2+). From the equation, we observe that DMG is more likely to lose H+ in an alkaline condition, so the reaction can more rapidly proceed.
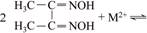
However, Cd2+ has a low sensitivity in the alkaline condition because it hardly reacts with DMG, which does not satisfy the test requirement. Nonetheless, when the solution pH decreased to approximately 4.0, the sensitivity was remarkably improved.
Because of the difference between determining the Cu2+, Co2+ and Ni2+ and determining the Cd2+, we finally determined the test system: the Cu2+, Co2+ and Ni2+ were determined in the alkaline buffer with DMG as a complexing agent; then, the Cd2+ was determined in the buffer whose pH=4.0.
3.1 Selection of conditions to determine Cu2+, Co2+ and Ni2+
3.1.1 Effect of pH on determining Cu2+, Co2+ and Ni2+
To select the optimum acidity, the effect of pH in the medium on the peak currents of the second derivative waves of Cu2+, Co2+ and Ni2+ was studied in the range of 6.0 to 10.5. The pH was adjusted using sodium hydroxide. When the pH is less than 8.2, the Zn2+ wave appears at a more positive potential, which causes the overlap of the waves of Co2+ and Zn2+. Because of the complexation of Zn2+ with citrate, the Zn2+ wave gradually moves towards a more negative potential with the increase of pH. When the pH is greater than 10.5, Zn2+ begins to precipitate because the hydroxide concentration is too large, and the peak currents of the ions to be measured decrease due to the co-precipitation. When the pH is 8.2-10.3, there is neither the polarographic derivative wave of zinc nor the hydroxide precipitation of zinc in the test interval, which facilitates the simultaneous on-line determination of the Cu2+, Co2+, Ni2+. The relationships between the peak currents of Cu2+, Co2+, Ni2+ and the pH in the range of 8.2-10.3 are shown in Fig. 2. The peak current of Cu2+ increases with the increase of pH, whereas those of Co2+ and Ni2+ first increase and subsequently decrease, and at approximately 8.8, reach their maximum values. It is obvious from Fig. 2 that the sensitivities of the Co2+ and Ni2+ are higher than that of the Cu2+ in the presence of DMG. In general, the Cu2+ concentration in the zinc electrolyte is much higher than that of the Co2+ and Ni2+, therefore, to determine the three ions simultaneously, we finally selected pH=9.3 in the further study.
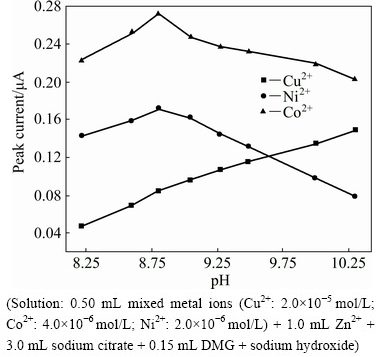
Fig. 2 Effect of pH on peak currents of Cu2+, Co2+ and Ni2+ (Solution: 0.50 mL mixed metal ions
3.1.2 Choice of buffer system to determine Cu2+, Co2+ and Ni2+
Both NH3-NH4Cl and sodium tetraborate may be used as buffers to control the solution pH at 9.3 because the negative logarithms of their relevant dissociation equilibrium constants (pKa) are 9.25 and 9.24, respectively. However, on using DMG as the complexing agent to determine the Co2+ and Ni2+ in the zinc electrolyte under the alkaline condition, sodium tetraborate as the buffer is better than NH3-NH4Cl. When the Co2+ and Ni2+ in NH3-NH4Cl buffer solution are determined using DMG, the reduction potential of Zn2+ will be more negative due to the complexation of the Zn2+ and ammonia, which weakens the effect of Zn2+ on the determination of Co2+ and Ni2+ but cannot completely eliminate the effect of a large quantity of Zn2+ [19-22]. However, on determining the Cu2+, Co2+and Ni2+ in the buffer solution of sodium tetraborate, the Zn2+ does not produce any polarographic derivative wave in the range of scan interval. It is a major advantage for determining the impurities in the zinc electrolyte, particularly the Co2+ and Ni2+. Thus, we selected sodium tetraborate as the buffer. The principle to form the buffer system by the hydrolysis of sodium tetraborate may be shown by Eq. (2).
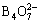 +5H2O=2H3BO3+
+5H2O=2H3BO3+ (2)
(2)
It is obvious from Eq. (2) that the hydrolysis of sodium tetraborate forms a buffer solution consisting of H3BO3 and NaH2BO3. The molar ratio of conjugated acid to conjugated base is 1:1. Therefore, when the buffer concentration is constant, the buffer capacity is the largest, and the solution pH is 9.24, which equals pKa1. The pH can be adjusted to 9.3 using sodium hydroxide. In addition, it is obvious from Eq. (3) that the addition of sodium hydroxide not only adjusts the buffer solution pH but also increases the solubility of sodium tetraborate.
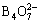 +5H2O=2H3BO3+
+5H2O=2H3BO3+
 H3BO3+
H3BO3+ +H2O
+H2O … (3)
… (3)
3.1.3 Effect of sodium tetraborate concentration
To examine the effects of the buffer concentration on the peak currents of Cu2+, Co2+, and Ni2+ waves, their polarographic derivative waves in the test solutions were determined by only changing the volume of the added buffer. The results are shown in Fig. 3. The peak currents of the Cu2+ and Ni2+ are not affected by the sodium tetraborate concentration, and the peak current of the Co2+ increases with its increase. When the added sodium tetraborate volume exceeds 3.4 mL, the peak current is substantially constant. In addition, by the comparison of Fig. 2 and Fig. 3, it is obvious that when the concentrations of the ions to be measured and the solution pH are constant, all of the peak currents of the ions decrease slightly after adding sodium tetraborate to the solution. Therefore, the volume of the added sodium tetraborate buffer must be constant, and is selected as 4.0 mL.
3.1.4 Effect of sodium citrate concentration
To select the best amount of sodium citrate, the effect of its concentration on the derivative waves of Cu2+, Co2+ and Ni2+ was studied by only changing its added volume in the 1.0-5.0 mL range. The relationships between the peak currents and the added volume are shown in Fig. 4. Sodium citrate was used as a zinc-complexing agent in this study. When the volume of sodium citrate was less than 3.0 mL, a white precipitate formed in the solution, which was produced from the hydrolysis of Zn2+, and the peak currents of metal ions to be measured decrease due to their co-precipitation. However, when the added volume was too large, the peak currents would also decrease due to the complexation of the metal ions with citrate. It is obvious from Fig. 4 that the appropriate volume of sodium citrate added to the test solution is 3.0 mL. Therefore, for all subsequent parts in the study, 3.0 mL sodium citrate solution was used.
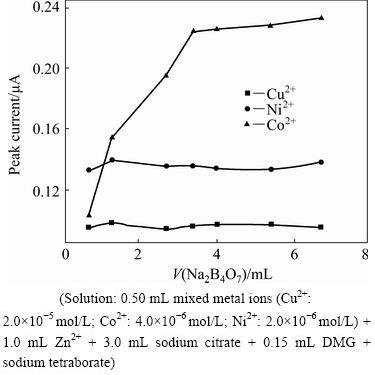
Fig. 3 Effect of sodium tetraborate volume on peak currents of Cu2+, Co2+ and Ni2+
Fig. 4 Effect of sodium citrate volume on peak currents of Cu2+, Co2+ and Ni2+ (Solution: 0.50 mL mixed metal ions
3.1.5 Effect of DMG concentration
DMG is present as a ligand in the system. The effects of DMG concentration on the second derivative waves of the Cu2+, Co2+ and Ni2+ were studied by only changing the volume of the added DMG solution. The relationship between the peak current and the DMG volume is shown in Fig. 5. The increase of DMG concentration causes the peak current variation, and the variation trends of peak currents of the three ions are similar. When the DMG volume is lower than 0.05 mL, their peak currents rapidly increase with the increase of DMG concentration, and subsequently slowly increase. When the added DMG volume is higher than 0.15 mL (0.12 mL for the Ni2+), the peak currents reduce instead because too much ethanol in the test solution, which is introduced from the added DMG ethanol solution, competes with the ion complexes for the adsorption on the mercury electrode. Therefore, the peak current will become weak [17]. Therefore, 0.15 mL DMG ethanol solution should be selected.
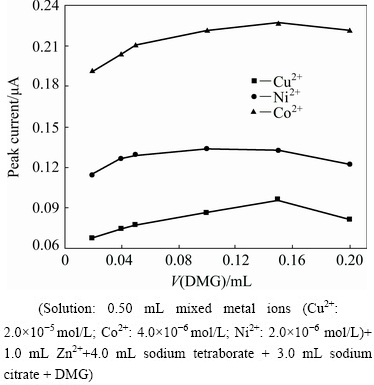
Fig. 5 Effect of DMG volume on peak currents of Cu2+, Co2+ and Ni2+ (Solution: 0.50 mL mixed metal ions
3.2 Selection of conditions to determine Cd2+
Cd2+ cannot produce the complex adsorption wave in the test solution because it does not react with DMG, so it cannot be determined simultaneously with Cu2+, Co2+, Ni2+in the alkaline medium. However, in a weak acidic solution, Cd2+ can produce a sensitive second derivative wave. Therefore, the Cd2+ in zinc electrolyte can be determined just choosing a suitable acidity and buffer system.
3.2.1 Effect of pH on determining Cd2+
The experiment has found that the sensitivity of Cd2+ derivative wave is particularly affected by the Zn2+ in the alkaline buffer. For example, when the concentration of the Zn2+ is 0.26 mol/L, the cadmium peak current is reduced by approximately 100 times. However, the cadmium current in acidic solutions are hardly affected by zinc. In the experiment, the sulfuric acid was used to adjust the pH, and we studied the change of the Cd2+ peak current in the range of pH 3.3-5.5 (Fig. 6). The peak current first increases and subsequently decreases with the increase of pH; when pH=4.0, the maximum peak current is obtained.
3.2.2 Choice of buffer system to determine Cd2+
If a proper amount of sulfuric acid is added to the solution after determining the Cu2+, Co2+ and Ni2+, the citrate (Cit3-) may be converted into an amphoteric acid-base compound, namely bi-hydrogen citrate (H2Cit-) buffer. Citric acid (H3Cit) as the conjugated acid is a weak acid containing three protons, and its ionization equilibriums and the negative logarithms of the equilibrium constants are as follows:
H3Cit=H++H2Cit-, pKa1=3.13 (4)
H2Cit-=H++HCit2-, pKa2=4.76 (5)
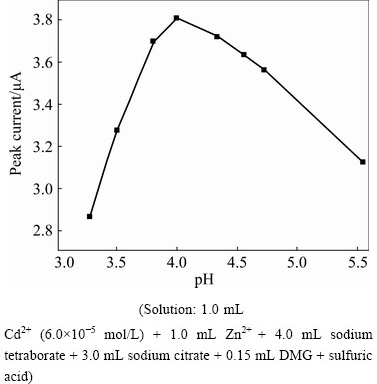
Fig. 6 Effect of pH on peak current of Cd2+
HCit2-=H++Cit3-, pKa3=6.40 (6)
According to principles of the material balance and the acid-base dissociation equilibrium, the formulas to calculate the distribution fractions of the four forms of citrate existing in the solution can be obtained. The formulas and the calculation results at pH=4.0 are as follows:
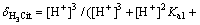
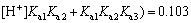 (7)
(7)

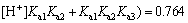 (8)
(8)
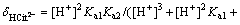
 (9)
(9)

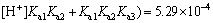 (10)
(10)
It is obvious that when pH=4.0, the amphoteric substance, H2Cit- is the main form of existence, which can form a very good buffer system. The sensitive and stable peak current of Cd2+ may be determined in the buffer system. At pH=9.3, the calculation result of the distribution fraction of the trivalent anion, namely the citrate (Cit3-) is 1.00. This shows that the citrate in the test solution to determine the Cu2+, Co2+ and Ni2+exists completely in the form of Cit3-. Therefore, before determining the cadmium, a proper amount of the sulfuric acid should be added to the solution to form the buffer system whose pH=4.0. The experiment has shown that when 0.50 mL of the sulfuric acid is added to the solution, the desired pH is obtained.
3.3 Linear ranges, detection limits and repeatability of method
Under the optimal conditions, the linear relationships between the peak currents and the metal concentrations in the presence of 0.26 mol/L Zn2+ were researched. The results indicate that the current- concentration relationships are linear in the concentration ranges of 1×10-7-3×10-4, 6×10-7-2×10-4, 2×10-8- 1×10-5, and 1×10-8-3×10-5 mol/L for Cu2+, Cd2+, Ni2+ and Co2+, respectively. The equations to indicate the linear relationships among the Cu2+, Cd2+, Ni2+ and Co2+ peak currents (Ip) and the concentrations (C) are Ip=13.5C(Cu2+)+0.135, Ip=16.1C(Cd2+)+0.392, Ip= 1.56C(Ni2+)+0.011 and Ip=1.32C(Co2+)+0.016, respectively. The detection limits (3σ) of the Cu2+, Cd2+, Ni2+ and Co2+ are 8×10-8, 2×10-7, 6×10-9 and 4×10-9 mol/L, respectively. The correlation coefficients are 0.9990, 0.9995, 0.9991 and 0.9992, respectively. Six repeated determinations have shown that the relative standard deviations for 5×10-7 mol/L Cu2+, 1×10-6 mol/L Cd2+, 2×10-7 mol/L Ni2+ and 2×10-7 mol/L Co2+ are 1.2%, 0.73%, 1.2% and 1.6%, respectively, namely the method is of satisfactory precision.
3.4 Interference study
Special attention was paid to the interference of Zn2+. It was observed that Zn2+ whose concentration was lower than 0.30 mol/L did not affect the determination. The interference of other foreign ions was studied for a solution that contained 0.26 mol/L Zn2+, 5×10-7 mol/L Cu2+, 1×10-6 mol/L Cd2+, 2×10-7 mol/L Ni2+ and Co2+ by adding the foreign ions to the solution. The results show that 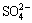 ,
, 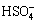 ,
, 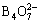 ,
,  ,
, 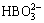 , Cit3-, HCit2-, H2Cit-,
, Cit3-, HCit2-, H2Cit-,  ,
,  ,
, 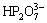 , Br-, Cl-, F– and NH4+, Li+, Na+, K+, Sr2+, Ba2+ do not interfere with the determination. The tolerance limits of other coexisting ions (namely the mass times corresponding to 0.2 mg/L of Cu2+, Cd2+ and 0.04 mg/L of Ni2+, Co2+ (5% relative error)) are, respectively, Al3+, W(VI), Mo(VI) (90000 and 38000), Mn2+ (48000 and 20000), Ca2+ (39000 and 16000), Mg2+ (11500 and 4900), Ge2+, Hg2+ (9500 and 4100), Sn(IV) (8000 and 3100), Si(IV) (5200 and 2100), Bi3+ (3800 and 1600), As(V) (950 and 400), As3+ (100 and 45), Fe3+ (230 and 95), In3+ (200 and 75), Sb3+ (13 and 6), Ti3+, Be2+, Ag+ (10 and 4), Cr3+, V(V) (9 and 4). It is obvious that the method to determine the Cu2+, Cd2+, Ni2+ and Co2+in the zinc electrolyte is of satisfactory selectivity.
, Br-, Cl-, F– and NH4+, Li+, Na+, K+, Sr2+, Ba2+ do not interfere with the determination. The tolerance limits of other coexisting ions (namely the mass times corresponding to 0.2 mg/L of Cu2+, Cd2+ and 0.04 mg/L of Ni2+, Co2+ (5% relative error)) are, respectively, Al3+, W(VI), Mo(VI) (90000 and 38000), Mn2+ (48000 and 20000), Ca2+ (39000 and 16000), Mg2+ (11500 and 4900), Ge2+, Hg2+ (9500 and 4100), Sn(IV) (8000 and 3100), Si(IV) (5200 and 2100), Bi3+ (3800 and 1600), As(V) (950 and 400), As3+ (100 and 45), Fe3+ (230 and 95), In3+ (200 and 75), Sb3+ (13 and 6), Ti3+, Be2+, Ag+ (10 and 4), Cr3+, V(V) (9 and 4). It is obvious that the method to determine the Cu2+, Cd2+, Ni2+ and Co2+in the zinc electrolyte is of satisfactory selectivity.
3.5 Application of method
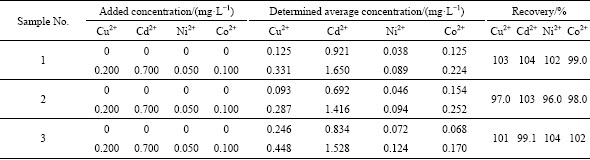
The proposed method was applied to determining the Cu2+, Cd2+, Ni2+ and Co2+ in some actual zinc electrolytes from Zhuzhou Smelter Group Company Limited, Zhuzhou, China, which were collected at the third purification process section at different time. To quantify the four ions, 1.0 mL of zinc electrolytes was added to the test solution. Three parallel determinations were performed for each sample. The results are shown in Table 1. The recoveries are 96.0%-104%, the accuracy is satisfactory.
4 Conclusions
1) A new method to determine the Cu2+, Cd2+, Ni2+ and Co2+ in zinc electrolytes at the same time is offered. In a solution whose pH=9.3, the complexes formed by the reactions of Cu2+, Ni2+, Co2+ with DMG will produce sensitive second derivative waves; however, under the action of citrate anion, Zn2+ does not produce any derivative wave in the scan interval, which may completely eliminate the interference of the Zn2+ with the determination of the Ni2+ and Co2+. In addition, a sensitive derivative wave of Cd2+ will emerge in a weak acidic solution (pH=4.0). Therefore, the Cu2+, Ni2+ and Co2+ can be determined in the alkaline solution containing DMG and Cit3-, and the Cd2+ can be determined under the acidic condition, using sodium tetraborate and sodium bi-hydrogen citrate solutions to control the pH under the alkaline and acidic conditions, respectively.
2) The method has satisfactory precision and accuracy, is simple, requires a low cost, and can quickly and directly achieve the simultaneous determination of Cu2+, Cd2+, Ni2+ and Co2+.
Table 1 Results to determine Cu2+, Cd2+, Ni2+ and Co2+ in actual zinc electrolytes collected thrice
References
[1] ZHU Hong-qiu, WANG Guo-wei, YANG Chun-hua, CAO Yu, GUI Wei-hua. Overlapped peaks resolution for linear sweep polarography using Gaussian-like distribution [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 2181-2186.
[2] MORGAN S W K. Zinc and Iron alloys [M]. England Mac Donald and Evans: Industrial Metals Series, 1977.
[3] BOROVKOV G A, MONASTYRSKAYA V I. Voltammetric determination of cobalt (II) in zinc sulfate solution [J]. Russian Journal of Applied Chemistry, 2001, 74(8): 1310-1317.
[4] RAJABI M, ASGHARI A, MOUSAVI H Z. Trace amounts determination of lead, zinc and copper by adsorptive stripping voltammetry in the presence of dopamine [J]. Journal of Analytical Chemistry, 2010, 65(5): 511-517.
[5] HERRERO E, ARANCIBIA V, ROJAS–ROMO C. Simultaneous determination of Pb2+, Cd2+, and Zn2+, by adsorptive stripping voltammetry using Clioquinol as a chelating-adsorbent agent [J]. Journal of Electroanalytical Chemistry, 2014, 729(26): 9-14.
[6] NASCIMENTO D S, INSAUSTI M, BAND B S F, LEMOS S G. Simultaneous determination of Cu, Pb,Cd, Ni, Co and Zn in bioethanol fuel by adsorptive stripping voltammetry and multivariate linear regression [J]. Fuel, 2014, 137(6): 172-178.
[7] SOMER G, SUKRU K, SENDIL O. A new and direct method for the determination of trace elements in spinach using differential pulse polarography [J]. Journal of Electroanalytical Chemistry, 2016, 778: 49-52.
[8] WANG Guo-wei, YANG Chun-hua, ZHU Hong-qiu, LI Yong-gang, GUI Wei-hua. Reagent optimization for on-line simultaneous polarographic determination of trace amounts of Cu2+, Cd2+, and Co2+, in the presence of anextremely large excess of Zn2+ [J]. Journal of Central South University, 2016, 23(9): 2199-2204.
[9] KOROLCZUK M. Voltammetric determination of nickel in the flow system in the presence of an extremely large excess of cobalt and zinc [J]. Electroanalysis, 2015, 12(18): 1502-1504.
[10] BOND A M, KNIGHT R W, NEWMAN O M G. Flow-through cell for continual on-line monitoring of cadmium, copper, antimony, and lead by anodic stripping voltammetry in highly dense zinc plant electrolyte [J]. Analytical Chemistry, 1988, 60(21): 2445-2448.
[11] MRZLJAK R I, BOND A M, CARDWELL T J, CATTRALL R W, KNIGHT R W, NEWMAN O M G, CHAMPION B R. On-line and off-line voltammetric methods for the determination of nickel in zinc plant electrolyte [J]. Analyst, 1994, 119(119): 1057-1061.
[12] MRZLJAK R I, BOND A M, CARDWELL T J, CATTRALL R W, KNIGHT R W, NEWMAN O M G, CHAMPION B R, HEY J, BOBROWSKI A. On-line monitoring of cobalt ion zinc plant electrolyte by differential pulse adsorptive stripping voltammetry [J]. Analytica Chimica Acta, 1993, 281(2): 281-290.
[13] GEIBLER M, RUI D M. Determination of cobalt in the presence of high concentrations of zinc by differential pulse polarography [J]. Analytical and Bioanalytical Chemistry, 1988, 330(7): 624-626.
[14] SCHMIDT T, GEIBLER M, WERNER G, EMONS H. Polarographic cobalt determination in the presence of high zinc concentrations [J]. Analytical and Bioanalytical Chemistry, 1988, 330(8): 712-713.
[15] BOBROWSKI A, BOND A M. Catalytic adsorptive stripping voltammetric determination of cobalt as an α-benzildioxime complex in the presence of an extremely large excess of zinc [J]. Electroanalysis, 1991, 3(3): 157-162.
[16] KOROLCZUK M, GRABARCZYK M, MOROZIEWICZ A. Determination of traces of cobalt in zinc matrix by catalytic adsorptive stripping voltammetry at bismuth film electrode [J]. Electroanalysis, 2007, 19(19-20): 2155-2158.
[17] BOBROWSKI A. The nature of voltammetric waves of copper complexes with dimethylglyoxime in ammonia and borate buffer solutions [J]. Electroanalysis, 1996, 8(1): 79-88.
[18] BAXTER L A, BOBROWSKI A, BOND A M, HEATH G A, PAUL R L, MRZLJAK R, ZAREBSKI J. Electrochemical and spectroscopic investigation of the reduction of dimethylglyoxime at mercury electrodes in the presence of cobalt and nickel [J]. Analytical Chemistry, 1998, 70(7): 1312-1323.
[19] BOBROWSKI A, BOND A M. Exploitation of the nitrite catalytic effect to enhance the sensitivity and selectivity of the adsorptive stripping voltammetric method for the determination of cobalt with dimethylglyoxime [J]. Electroanalysis, 1992, 4(10): 975-979.
[20] MU Peng-tao, SHEN Qing-feng, YU Xiao-hua, XU Shuang-quan, LIU Chun-xia. Determination of copper, lead, cadmium and nickel in zinc electrolyte by differential pulse stripping voltammetry [J]. Metallurgical Analysis, 2016, 36(10): 15-20. (in Chinese)
[21] REN Feng-lian, SHEN Fang, LI Yuan, CAO Fu-yue, PU Qiu-mei. Simultaneous determination of copper, cobalt and nickel in zinc electrolyte by differential pulse adsorptive stripping voltammetry [J]. Physical Testing and Chemical Analysis Part B(Chemical Analysis) , 2012, 48(9): 1033-1036. (in Chinese)
[22] ZHANG Ming-hao, LIANG Yi-zeng. Rapid and simultaneous determination of copper, cadmium, nickel, and cobalt in zinc electrolyte solutions by complex adsorption wave polarography [J]. Journal of Trace & Microprobe Techniques, 2002, 20(1): 1-14.
用示波极谱二阶导数波同时测定锌电解液中微量铜、镉、镍、钴
杜 娟1,朱红求2,李勇刚2,张泰铭1,阳春华2
1. 中南大学 化学化工学院,长沙 410083;2. 中南大学 信息科学与工程学院,长沙 410083
摘 要:同时测定高浓度锌电解液中多种杂质金属离子对湿法炼锌非常重要,旨在建立一种用单扫描示波极谱二阶导数波同时测定锌电解液中微量铜、镉、镍、钴的新方法。研究这四种金属离子在丁二酮肟-柠檬酸钠-四硼酸钠介质中极谱导数波的影响因素。结果表明:高浓度Zn2+和其他大多数共存离子不干扰Cu2+、Cd2+、Ni2+和Co2+的测定。锌电解液中Cu2+、Cd2+、Ni2+和Co2+的浓度分别在1×10-7~3×10-4、6×10-7~2×10-4、2×10-8~1×10-5和 1×10-8~3×10-5 mol/L范围时,其二阶导数波峰电流与浓度呈线性关系。Cu2+、Cd2+、Ni2+ 和Co2+的检出限分别为8×10-8、2×10-7、6×10-9和4×10-9 mol/L。该方法无需任何预处理直接测定锌电解液中的Cu2+、Cd2+、Ni2+和Co2+,结果令人满意。该方法简便、灵敏、快速。
关键词:锌电解液;铜;镉;镍;钴;二阶导数波
(Edited by Xiang-qun LI)
Foundation item: Projects (61533021, 61773403) supported by the National Natural Science Foundation of China
Corresponding author: Chun-hua YANG; Tel: +86-13975894807; E-mail: ychh@csu.edu.cn
DOI: 10.1016/S1003-6326(18)64906-4
Abstract: Simultaneous determination of impurity metal ions in high concentration zinc solution is very important for zinc hydrometallurgy, and the purpose is to establish a method for determining the trace Cu2+, Cd2+, Ni2+ and Co2+ in zinc electrolytes at the same time using the second derivative waves of single sweep oscillopolarography. Factors affecting the derivative waves of the ions were researched in a medium of dimethylglyoxime (DMG)-sodium citrate-sodium tetraborate. The results indicated that the interferences of a high concentration of Zn2+ and most other coexisting ions on the determination can be eliminated; when the Cu2+, Cd2+, Ni2+ and Co2+ are in the ranges of 1×10-7-3×10-4, 6×10-7-2×10-4, 2×10-8-1×10-5 and 1×10-8-3×10-5 mol/L, respectively, the relationships between the peak currents of the second derivative waves and the concentrations are linear; the detection limits to determine the Cu2+, Cd2+, Ni2+ and Co2+ are 8×10-8, 2×10-7, 6×10-9 and 4×10-9 mol/L, respectively. Without any sample pretreatment, the method was used to directly determine the trace Cu2+, Cd2+, Ni2+ and Co2+ in actual zinc electrolytes with satisfactory results. The method is simple, sensitive and rapid.


