DOI: 10.11817/j.ysxb.1004.0609.2021-37733
Cu2+、Fe2+和Fe3+对中等嗜热混合菌浸出黄铜矿的影响
田祖源1,李浩东1,魏 茜1,焦 芬1, 2,覃文庆1, 2,杨聪仁1, 2
(1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 战略含钙矿物资源清洁高效利用湖南省重点实验室,长沙 410083)
摘 要:
在50 ℃、pH 1.6的条件下,研究了Fe2+、Fe3+和Cu2+对中等嗜热混合菌浸出黄铜矿的影响。结果表明:添加低质量浓度Fe2+时,在浸出前期能够促进黄铜矿的浸出;而添加较高质量浓度Fe2+时,铜的浸出率反而降低;当添加不同质量浓度Fe3+时,由于形成黄钾铁矾而导致总铁质量浓度降低,但铜的浸出率并没有明显变化;添加不同质量浓度Cu2+对黄铜矿的浸出没有显著的影响。同时,添加Fe2+和Cu2+与单独添加Fe2+对黄铜矿的浸出影响是相似的。因此,在生物浸出过程中,可以通过调节浸矿微生物组成降低微生物的亚铁氧化活性,提高硫氧化能力来控制溶液电位,从而强化黄铜矿的生物浸出。
关键词:
文章编号:1004-0609(2021)-01-0171-10 中图分类号:TD952;TF18 文献标志码:A
引文格式:田祖源, 李浩东, 魏 茜, 等. Cu2+、Fe2+和Fe3+对中等嗜热混合菌浸出黄铜矿的影响[J]. 中国有色金属学报, 2021, 31(1): 171-180. DOI: 10.11817/j.ysxb.1004.0609.2021-37733
TIAN Zu-yuan, LI Hao-dong, WEI Qian, et al. Effects of Cu2+, Fe2+, and Fe3+ on bioleaching of chalcopyrite by moderate thermophilic mixed bacteria[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(1): 171-180. DOI: 10.11817/j.ysxb.1004.0609.2021-37733
黄铜矿是最重要也是最丰富的含铜矿物,约占地球铜资源的70%[1-2]。随着铜矿资源开采量的增加,铜矿品位不断下降[3],传统的选矿方法难以处理复杂低品位铜矿,而生物冶金技术具有操作简单、生产成本低、以及环境友好等特点,在处理低品位矿方面具有很好的前景[4-7]。许多微生物能都能够氧化或协助氧化黄铜矿,其中绝大部分浸矿微生物属于古菌和细菌。根据浸矿微生物的最佳生长温度条件,又可将其分为三类[3, 8-12]:中温菌,其最适生长温度为25~45 ℃;中等嗜热菌,其最适生长温度为45~50 ℃;嗜热菌,其最适生长温度大于65 ℃以上。在生物浸出过程中,浸矿菌种的选择是影响浸矿效果的一个关键因素[13]。QIN等[9]研究了中温菌(Acidithiobacillus ferrooxidans)和中等嗜热混合菌(Sulfobacillus thermosulfidooxidans和Ferroplasma sp.)对黄铜矿浸出的影响,在pH 1.6、温度30 ℃条件下浸出30 d,黄铜矿的中温菌浸出率为52%;在pH 1.6、温度50 ℃条件下浸出30 d,黄铜矿的中等嗜热菌浸出率达到了92%。使用中等嗜热菌浸出黄铜矿,不仅可以显著改善浸出的反应动力学,加快反应速率,缩短浸出周期,而且浸出过程中产生的硫化产物则会溶解,从而提高铜的浸出速率和浸出率[14-18]。另有研究表明,与单菌浸出相比,采用混合菌进行浸出能极大地提高浸出率[19-24]。
为了提高黄铜矿的浸出率,学者们还研究了溶液组成(如Fe2+、Fe3+、Cu2+)对黄铜矿浸出的影响。HIROYOSHI等[25-28]发现,当溶液电位维持在310~ 400 mV (Ag/AgCl)范围内时,溶液中共存的Cu2+和Fe2+能促进黄铜矿的浸出;同时,在硫酸亚铁溶液中比在硫酸铁溶液中能更有效地浸出黄铜矿[29]。为了解释这种现象,HIROYOSHI等[25]提出了黄铜矿在低电位条件下的两步溶出模型:黄铜矿首先与Cu2+和Fe2+反应生成辉铜矿(见式(1));然后,辉铜矿再被Fe3+氧化为Cu2+和单质硫(见式(2))。与黄铜矿(静电位为0.52V vs SHE)相比,辉铜矿(静电位为0.44 V vs SHE)更容易被氧化,因为它的静电位较 低[30]。JIANG等[31]研究发现,Fe3+能在0.06 mol/L的浓度下促进硫化铜矿的生物浸出。张卫民等[32]在原生硫化矿细菌浸出中添加Fe3+,可以提高浸出液的氧化还原电位,但不会提高铜的浸出速度和浸出率。NICOL等[33]在氯化物媒介中浸出黄铜矿时,向浸出媒介中添加少量的Cu2+能促进铜的浸出。HIROYOSHI等[25-27]通过研究Fe2+和Cu2+对黄铜矿浸出的影响发现,当溶液电位在310~400 mV (Ag/AgCl)范围内时,Cu2+的添加能够促进黄铜矿的溶解。因此,本文在50 ℃、pH 1.6的条件下,考察了Fe2+、Fe3+和Cu2+对中等嗜热混合菌浸出黄铜矿的影响,探究离子的组成及质量浓度对中等嗜热混合菌浸出黄铜矿的影响。
CuFeS2+3Cu2++3Fe2+→2Cu2S+4Fe3+ (1)
2Cu2S+8Fe3+→4Cu2++8Fe2++2S0 (2)
1 实验
1.1 实验矿样
黄铜矿样品取自湖北大冶铜绿山铜矿。用于浸出试验的黄铜矿样品粒度小于0.074 mm。矿样的XRD分析仅检测到黄铜矿的特征峰,如图1所示。
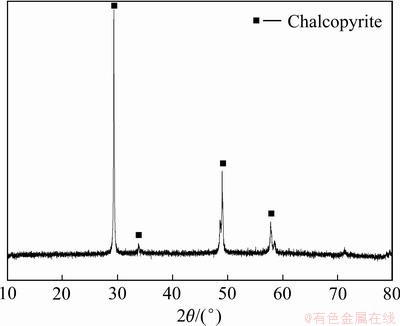
图1 样品的XRD谱
Fig. 1 XRD pattern of sample
矿样的化学分析显示矿样中含有33.91% Cu、30.62% Fe、32.90% S、0.039% Pb和0.018% Zn。
1.2 细菌培养
中等嗜热混合菌采自内蒙古某硫化铜矿山的浸出液,该混合菌主要由Sulfobacillus thermosulfidooxidans、Acidithiobacillus caldus和Ferroplasma sp.组成。用于微生物培养的基本盐溶液组成如下:3.0 g/L (NH4)2SO4、0.1 g/L KCl、0.5 g/L MgSO4·7H2O、0.5 g/L K2HPO4、0.01 g/L Ca(NO3)2。取90 mL基本盐溶液加入250 mL的锥形瓶中,同时,添加2%的矿粉,并调节溶液pH至1.6,加入10 mL的浸出液或者菌液,将锥形瓶置于空气恒温振荡器中进行培养,设定转速为160 r/min,培养温度为50 ℃。培养时向培养液中添加0.02%的酵母提取物。
1.3 浸出试验
将95 mL基本盐溶液添加到250 mL的锥形瓶中,然后添加2 g黄铜矿矿粉,并调节溶液pH至1.6,之后加入5 mL的菌液,培养液中的初始细胞浓度为1.0×107 cell/mL,此时记录浸出体系的总质量m,最后把锥形瓶放置在空气恒温振荡器中进行培养,振荡器转速为160 r/min。浸出温度为50 ℃,同时向培养液中添加0.02%的酵母提取物。平行的无菌浸出试验是在相同条件下进行的。
浸出一定时间后,向浸出体系中补加蒸馏水至浸出体系的质量m,并使用BPP-922型pH/ORP计监测浸出液的pH和ORP(vs. Ag/AgCl),用稀硫酸调节浸出液pH,避免浸出液的pH值过高导致Fe3+沉淀。然后使用无菌移液枪取出1 mL的浸出液用于成分分析,同时要补加1 mL的基本盐溶液。浸出结束后,在室温条件下对浸出渣进行过滤,并用pH为1.6的酸液反复清洗,在真空干燥箱中进行干燥,干燥温度为40 ℃。样品干燥后立即转移到密封袋中待测。
1.4 分析方法
浸矿微生物培养及浸出过程中细胞浓度是通过血细胞计数板直接计数的。浸出液中的Cu和Fe质量浓度通过原子吸收光谱(Atomic absorption spectroscopy,AAS)分析。浸出液的pH和ORP (Oxidation-Reduction Potential)利用BPP-922型(BELL)pH/ORP计监测,其中pH测量使用的是65-1型玻璃pH复合电极,ORP是利用Pt电极测量的,饱和Ag/AgCl电极作为参比电极。XRD(X-ray diffractometer)在Bruker D8 Advance X-ray 衍射仪上进行(Cu Kα,λ=1.5406 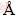 )的。
)的。
2 结果与讨论
2.1 试验结果
2.1.1 Fe2+对黄铜矿生物浸出的影响
图2所示为Fe2+质量浓度对中等嗜热混合微生物浸出黄铜矿的影响。从图2(a)可以看出,在不添加Fe2+的浸出体系中,浸出30 d后,铜浸出率为21%;当添加0.5 g/L的Fe2+时,在浸出的前20 d,能够提高铜的浸出率,之后,铜的浸出基本停止;浸出30 d后,铜的浸出率为18%;当添加1.0 g/L的Fe2+,在浸出的前15 d,能够提高铜的浸出率,之后铜的浸出率低于未添加Fe2+的浸出率,浸出30 d后,铜的浸出率为17%;当添加1.5 g/L的Fe2+时,在浸出的前10 d,对铜的浸出率没有影响,但浸出30 d后,铜的浸出率仅为14%;当添加2.0 g/L的Fe2+时,铜的浸出率一直低于其他试验条件,浸出30 d后,铜的浸出率仅为11%。由此可见,在黄铜矿的中等嗜热混合微生物浸出过程中,铜的浸出率随着添加的Fe2+质量浓度增加而降低。
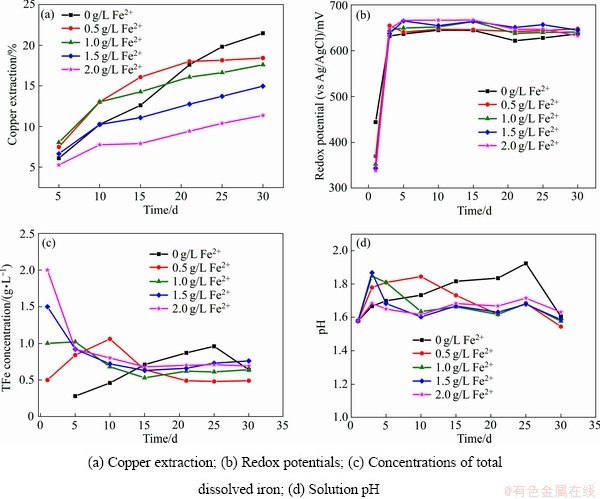
图2 Fe2+对黄铜矿生物浸出的影响
Fig. 2 Impact of Fe2+ on bioleaching of chalcopyrite
从图2(b)可知,即使添加了不同质量浓度的Fe2+,浸出液的电位在浸出5 d后均升高到640 mV左右,说明添加的Fe2+很快就被浸出液中的亚铁氧化微生物氧化成Fe3+(见式(3))。在不添加Fe2+的黄铜矿浸出过程中,在浸出的前25 d,浸出液中总铁质量浓度随着浸出时间的延长而增加,当浸出30 d后,浸出液中的三价铁由于和溶液中的一价阳离子(K+、 、H3O+等)形成黄钾铁矾沉淀而导致总铁质量浓度由浸出25 d时的0.96 g/L降低到0.64 g/L(见式(4))。当添加0.5 g/L Fe2+时,在浸出10 d后,浸出液中总铁质量浓度从刚开始的0.5 g/L增加到1.06 g/L;延长浸出时间至21 d时,浸出液中的三价铁同样由于形成黄钾铁矾沉淀而导致总铁质量浓度降低至0.49 g/L;继续延长浸出时间,浸出液中的总铁质量浓度维持在0.49 g/L,同时,在这段时间内铜的浸出率也维持在一定值。当添加的ρ(Fe2+)≥1 g/L时,浸出液中的总铁质量浓度随着浸出时间的延长而降低(见图2(c))。浸出液的pH变化和总铁质量浓度的变化类似(如图2(d)所示):当添加的ρ(Fe2+)<1 g/L时,浸出液的pH有一个先升高后降低的过程,而当添加的ρ(Fe2+)≥1 g/L的时,浸出液的pH随着浸出时间的延长而降低。
、H3O+等)形成黄钾铁矾沉淀而导致总铁质量浓度由浸出25 d时的0.96 g/L降低到0.64 g/L(见式(4))。当添加0.5 g/L Fe2+时,在浸出10 d后,浸出液中总铁质量浓度从刚开始的0.5 g/L增加到1.06 g/L;延长浸出时间至21 d时,浸出液中的三价铁同样由于形成黄钾铁矾沉淀而导致总铁质量浓度降低至0.49 g/L;继续延长浸出时间,浸出液中的总铁质量浓度维持在0.49 g/L,同时,在这段时间内铜的浸出率也维持在一定值。当添加的ρ(Fe2+)≥1 g/L时,浸出液中的总铁质量浓度随着浸出时间的延长而降低(见图2(c))。浸出液的pH变化和总铁质量浓度的变化类似(如图2(d)所示):当添加的ρ(Fe2+)<1 g/L时,浸出液的pH有一个先升高后降低的过程,而当添加的ρ(Fe2+)≥1 g/L的时,浸出液的pH随着浸出时间的延长而降低。
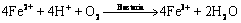 (3)
(3)
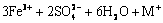 →
→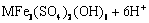 (4)
(4)
式中:M+= K+、Na+、H3O+、 。
。
2.1.2 Fe3+对黄铜矿生物浸出的影响
图3所示为Fe3+质量浓度对中等嗜热混合微生物浸出黄铜矿的影响。从图3(a)可以看出,Fe3+的添加对黄铜矿的浸出影响较小,浸出30 d后,添加0、0.5、1.0、1.5和2.0 g/L的Fe3+相对应的铜浸出率分别为21%、21%、20%、19%和18%。在所有浸出体系中,浸出液中的总铁质量浓度和pH均呈现出先升高后降低的过程(见图3(b)~(c))。在不添加Fe3+的浸出体系中,浸出液的电位在浸出5 d后升高到640 mV左右,而在添加不同质量浓度的Fe3+浸出体系中,浸出液的电位在浸出5 d后升高到660 mV左右。同时,添加不同Fe3+质量浓度的浸出试验结果表明,虽然在浸出过程中形成了黄钾铁矾,但是并没有明显阻碍黄铜矿的浸出。
2.1.3 Cu2+对黄铜矿生物浸出的影响
图4所示为Cu2+质量浓度对中等嗜热混合微生物浸出黄铜矿的影响。从图4(a)可以看出,Cu2+的添加对黄铜矿的浸出并没有明显的影响:浸出30 d后,添加0、0.5、1.0、1.5和2.0 g/L的Cu2+相对应的铜浸出率分别为21%、22%、19%、20%和19%。图4(b)显示,在浸出的前21 d,浸出液中总铁质量浓度和溶液pH均随着浸出时间的延长而增加;浸出21 d后,浸出液中的三价铁由于形成黄钾铁矾沉淀而导致浸出液中的总铁质量浓度降低,同时也导致溶液pH的降低(见图4(b)~(c))。在所有浸出体系中,浸出液的电位在浸出5 d后升高到630~640 mV(见图4(d))。
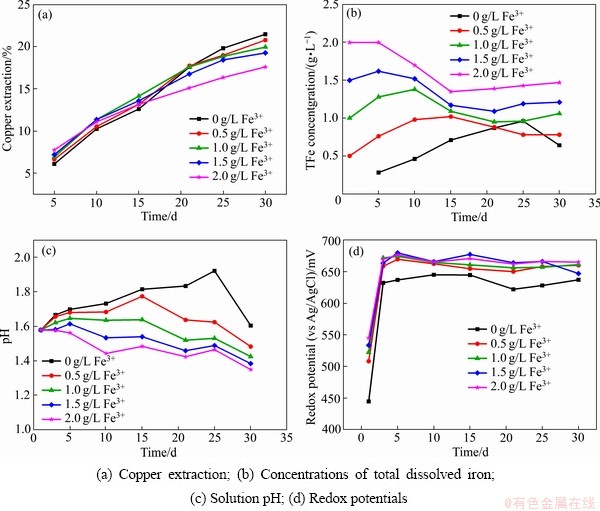
图3 Fe3+对黄铜矿生物浸出的影响
Fig. 3 Effect of Fe3+ on bioleaching of chalcopyrite
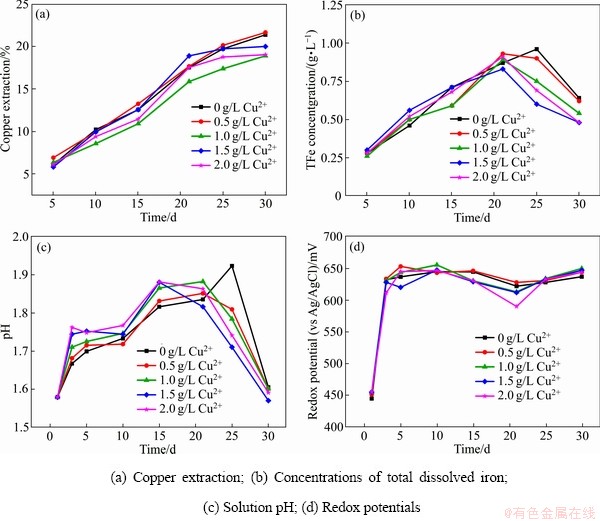
图4 Cu2+对黄铜矿生物浸出的影响
Fig. 4 Effect of Cu2+ on bioleaching of chalcopyrite
2.1.4 Fe2+和Cu2+对黄铜矿生物浸出的影响
图5所示为Fe2+和Cu2+质量浓度对中等嗜热混合微生物浸出黄铜矿的影响。浸出30 d后,与同时添加0、0.5、1.0、1.5和2.0 g/L的Fe2+和Cu2+相对应的铜浸出率分别为21%、19%、16%、11%和11%,可见,同时添加Fe2+和Cu2+与单独添加Fe2+对黄铜矿浸出的影响是相似的,即铜的最终浸出率随着添加Fe2+和Cu2+质量浓度的增大而降低。浸出过程中的总铁质量浓度、溶液电位和pH的变化也和图2的试验结果是相似的。
2.2 分析与讨论
在FeSO4-Fe2(SO4)3浸出体系中,其溶液电位主要由Fe3+和Fe2+的比值决定[34-35]。从式(5)可知,增加溶液氧化还原电位或者Fe3+浓度能够提高铜的浸出率。许多研究结果也表明,当浸出液中Fe3+摩尔浓度低于0.1 mol/L时,增加Fe3+浓度能够提高铜的浸出率[36-38],而当浸出液中Fe3+浓度高于0.1 mol/L时,增加Fe3+浓度并不能明显提高铜的浸出率[38]。在浸出过程中,由于在黄铜矿表面形成钝化膜从而导致铜的浸出率较低,通常元素硫(S0)[39-42]、二硫化物(S22-)[39, 43]、多硫化物 ( )[39, 44-45]和铁的氧化物或氢氧化物[41, 46-49](包括黄钾铁矾)等都被认为可能导致黄铜矿表面钝化。前期的研究结果表明,黄铜矿在Fe3+浸出过程中,会在其表面形成了
)[39, 44-45]和铁的氧化物或氢氧化物[41, 46-49](包括黄钾铁矾)等都被认为可能导致黄铜矿表面钝化。前期的研究结果表明,黄铜矿在Fe3+浸出过程中,会在其表面形成了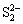 和
和 从而导致黄铜矿表面钝化,同时Fe3+的氧化性不足以氧化
从而导致黄铜矿表面钝化,同时Fe3+的氧化性不足以氧化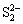 和
和 ,因此黄铜矿的浸出率较低[34-35]。
,因此黄铜矿的浸出率较低[34-35]。
CuFeS2+2Fe2(SO4)3→CuSO4+5FeSO4+2S0 (5)
黄铜矿在Fe3+浸出体系或者高溶液电位(>500 mV (vs. SCE))浸出环境中并不能获得令人满意的结果[9, 29, 50-51];相反,越来越多的研究表明,当溶液电位维<450 mV (vs. SCE)时,黄铜矿更容易被浸出[9, 26, 50, 52-53]。CORDOBA等[50]在68 ℃条件下考察了溶液电位对黄铜矿浸出的影响。当浸出液的起始电位为300和400 mV(vs. Ag/AgCl)时,浸出前5 d铜的浸出率分别大于80%和90%。继续延长浸出时间,溶液电位增加到500 mV(vs. Ag/AgCl),此时铜的浸出基本上已停止。当浸出液的起始电位≥500 mV(vs. Ag/AgCl)时,浸出13 d后,铜的浸出率仍然低于40%。VILCAEZ等[53]认为,当溶液电位>450 mV(vs. Ag/AgCl),黄铜矿是被溶液中的Fe3+直接氧化,当溶液电位<450 mV(vs. Ag/AgCl),黄铜矿首先形成中间产物,中间产物再进一步被氧化释放铜离子。VILCAEZ等[54]认为黄铜矿的溶解包含阴极还原和阳极氧化两个过程,而阴极还原和阳极氧化又由溶液中的初始Fe3+和Fe2+质量浓度决定。当溶液中初始Fe3+质量浓度较高时,黄铜矿的氧化速率大于还原速率;相反,溶液中初始Fe2+质量浓度较高时,黄铜矿的还原速率大于氧化速率。因此,他们认为在低电位溶液条件下,黄铜矿的浸出不是受Fe3+质量浓度控制,而是受Fe2+质量浓度控制,由于Fe2+对于辉铜矿的形成是非常重要的。当增加[Fe3+]/[Fe2+]时,溶液电位将超过辉铜矿形成的关键电位,此时仅存在黄铜矿的直接氧化[53-54]。同时,HIROYOSHI[25-27, 29]通过研究Fe2+和Cu2+对黄铜矿浸出的影响发现,当溶液电位在310~400 mV (vs. Ag/AgCl)范围内,Cu2+的添加能够促进黄铜矿的溶解。HIROYOSHI等[26]根据试验结果假设了一个黄铜矿在低电位条件下的两步浸出模型,黄铜矿首先被还原成更容易氧化分解的中间产物-辉铜矿(见式(1)),然后辉铜矿再被溶液中的Fe3+氧化释放铜离子(见式(2))。因此,溶液电位需要维持在黄铜矿向辉铜矿转化的电位区间,同时又需要保证辉铜矿的氧化分解电位。
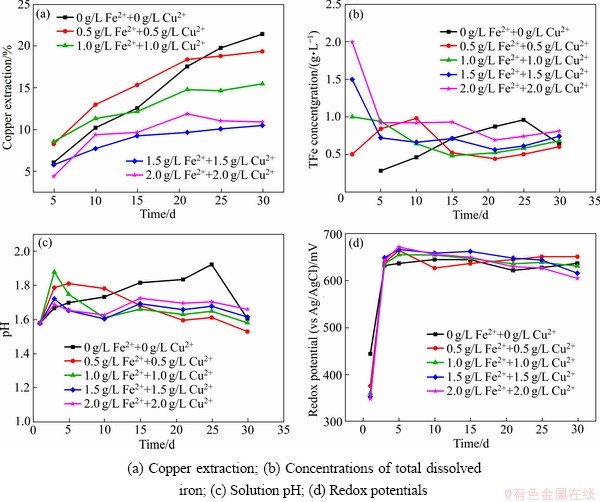
图5 Fe2+和Cu2+对黄铜矿浸出的影响
Fig. 5 Effect of Fe2+ and Cu2+ on bioleaching of chalcopyrite
在本研究中,当向浸出液中添加低质量浓度的Fe2+(无论是否有Cu2+存在)时,在浸出的前期能够促进黄铜矿的浸出,而添加较高质量浓度的Fe2+时,铜的浸出率反而降低。其可能的原因主要为:首先,在生物浸出过程中,添加的Fe2+很快就被浸出液中高活性的亚铁氧化微生物氧化成Fe3+,然而黄铜矿并不能被Fe3+有效浸出。因此,在有高活性的亚铁氧化微生物存在的黄铜矿生物浸出体系中,添加Fe2+和Cu2+并不能有效地促进黄铜矿浸出。其次,黄铜矿的生物浸出机理有直接作用和间接作用,通常是在两种机制共同作用的结果[55-56]。添加少量的Fe2+可以使浸矿微生物快速生长繁殖,从而促进黄铜矿的浸出;然而当溶液中初始Fe2+质量浓度较高时,亚铁氧化微生物优先氧化溶液中的Fe2+而较少作用于黄铜矿,因此,铜的浸出率较低。
通过以上分析可知,在生物浸出过程中,可以通过调节浸矿微生物组成降低微生物的亚铁氧化活性,提高硫氧化能力来控制溶液电位,从而强化黄铜矿的生物浸出。
3 结论
1) 浸出液中添加低质量浓度的Fe2+在浸出前期能够促进黄铜矿的浸出,而添加较高质量浓度的Fe2+时,铜的浸出率反而降低。
2) 在浸出液中添加不同质量浓度的Fe3+时,由于形成黄钾铁矾从而导致总铁质量浓度降低,但铜的浸出率并没有明显变化;同时,添加不同质量浓度的Cu2+对黄铜矿的浸出没有明显的影响。
3) 在浸出液中添加Fe2+和Cu2+与单独添加Fe2+对黄铜矿浸出的影响是相似的,即铜的最终浸出率随着添加Fe2+和Cu2+质量浓度的增大而降低。
4) 在生物浸出过程中,可以通过调节浸矿微生物组成降低微生物的亚铁氧化活性,提高硫氧化能力来控制溶液电位,从而强化黄铜矿的生物浸出。
REFERENCES
[1] LI Y, KAWASHIMA N, LI J, et al. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite[J]. Advances in Colloid and Interface Science, 2013, 197: 1-32.
[2] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, et al. Leaching of chalcopyrite with ferric ion. Part Ⅰ: General aspects[J]. Hydrometallurgy, 2008, 93(3/4): 81-87..
[3] WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphides — A review[J]. Hydrometallurgy, 2006, 84(1): 81-108.
[4] BRIERLEY J A, BRIERLEY C L. Present and future commercial applications of biohydrometallurgy[J]. Hydrometallurgy, 2001, 59(2/3): 233-239.
[5] 孙建之, 陈勃伟, 温建康, 等. 镍矿湿法冶金技术应用进展及研究展望[J]. 中国有色金属学报, 2018, 28(2): 356-364.
SUN Jian-zhi, CHEN Bo-wei, WEN Jian-kang, et al. Application and research progresses of hydrometallurgy technology for nickel ore[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(2): 356-364.
[6] 文旭祥, 孙占学, 周义朋, 等. 微生物浸铀研究进展[J]. 中国有色金属学报, 2020, 30(2): 411-420.
WEN Xu-xiang, SUN Zhan-xue, ZHOU Yi-peng, et al. Advances in research on microbial leaching of uranium[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(2): 411-420.
[7] 邱冠周, 刘学端. 用生物技术的钥匙开启矿产资源利用的大门[J]. 中国有色金属学报, 2019, 29(9): 1848-1858.
QIU Guan-zhou, LIU Xue-duan. Biotech key to unlock mineral resources value[J]. The Chinese Journal of Nonferrous Metals, 2019, 29(9): 1848-1858.
[8] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, et al. Leaching of chalcopyrite with ferric ion. Part Ⅳ: The role of redox potential in the presence of mesophilic and thermophilic bacteria[J]. Hydrometallurgy, 2008, 93(3/4): 106-115.
[9] QIN W, YANG C, LAI S, et al. Bioleaching of chalcopyrite by moderately thermophilic microorganisms[J]. Bioresource Technology, 2013, 129: 200-208.
[10] VILCAEZ J, SUTO K, INOUE C. Bioleaching of chalcopyrite with thermophiles: Temperature-pH-ORP dependence[J]. International Journal of Mineral Processing, 2008, 88(1/2): 37-44.
[11] ZHU W, XIA J, YANG Y, et al. Sulfur oxidation activities of pure and mixed thermophiles and sulfur speciation in bioleaching of chalcopyrite[J]. Bioresource Technology, 2011, 102(4): 3877-3882
[12] LI S, ZHONG H, HU Y, et al. Bioleaching of a low-grade nickel-copper sulfide by mixture of four thermophiles[J]. Bioresource Technology, 2014, 153: 300-306.
[13] 王军, 李旖旎, 庄 田, 等. 黄铜矿精矿中等嗜热微生物浸出过程及其优化[J]. 中国有色金属学报, 2016, 26(5): 1120-1128.
WANG Jun, LI Yi-ni, ZHUANG Tian, et al. Bioleaching of chalcopyrite concentrate with moderate thermophilic bacteria and its optimization[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(5): 1120-1128.
[14] RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M L, et al. New information on the chalcopyrite bioleaching mechanism at low and high temperature[J]. Hydrometallurgy, 2003, 71(1/2): 47-56.
[15] SAND W, ROHDE K, SOBOTKE B, et al. Evaluation of Leptospirillum ferrooxidans for Leaching.[J]. Applied and Environmental Microbiology, 1992, 58(1): 85-92.
[16] BRIERLEY J A. Thermophilic iron-oxidizing bacteria found in copper leaching dumps.[J]. Applied and Environmental Microbiology, 1978, 36(3): 523-525.
[17] BRIERLEY J A, LOCKWOOD S J. The occurrence of thermophilic iron-oxidizing bacteria in a copper leaching system[J]. Fems Microbiology Letters, 1977, 2(3): 163-165.
[18] 姚国成, 温建康, 高焕芝, 等. 中等嗜热菌浸出黄铜矿及其表面钝化的研究[J]. 中南大学学报(自然科学版), 2010, 41(4): 1234-1239.
YAO Guo-cheng, WEN Jian-kang, GAO Huan-zhi, et al. Chalcopyrite bioleaching by moderate thermophilic bacteria and surface passivation[J]. Journal of Central South University (Science and Technology), 2010, 41(4): 1234-1239.
[19] ZHOU H, LIU X, FU B, et al. Isolation and characterization of Acidithiobacillus caldus from several typical environments in China[J]. Journal of Central South University of Technology, 2007, 14(2): 163-169.
[20] FU B, ZHOU H, ZHANG R, et al. Bioleaching of chalcopyrite by pure and mixed cultures of Acidithiobacillus spp and Leptospirillum ferriphilum[J]. International Biodeterioration & Biodegradation, 2008, 62(2): 109-115.
[21] KINNUNEN P, PUHAKKA J A. Characterization of iron-and sulphide mineral-oxidizing moderately thermophilic acidophilic bacteria from an Indonesian auto-heating copper mine waste heap and a deep South African gold mine[J]. Journal of Industrial Microbiology & Biotechnology, 2004, 31(9): 409-414.
[22] WU C, ZENG W, ZHOU H, et al. Bioleaching of chalcopyrite by mixed culture of moderately thermophilic microorganisms[J]. Journal of Central South University of Technology, 2007, 14(4): 474-478.
[23] FENG S, YANG H, XIN Y, et al. A novel and highly efficient system for chalcopyrite bioleaching by mixed strains of Acidithiobacillus[J]. Bioresource Technology, 2013, 129: 456-462.
[24] ZHU W, XIA J, PENG A, et al. Characterization of apparent sulfur oxidation activity of thermophilic archaea in bioleaching of chalcopyrite[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(8): 2383-2388.
[25] HIROYOSHI N, MIKI H, HIRAJIMA T, et al. A model for ferrous-promoted chalcopyrite leaching[J]. Hydrometallurgy, 2000, 57(1): 31-38.
[26] HIROYOSHI N, MIKI H, HIRAJIMA T, et al. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions[J]. Hydrometallurgy, 2001, 60(3): 185-197.
[27] HIROYOSHI N, KUROIWA S, MIKI H, et al. Synergistic effect of cupric and ferrous ions on active-passive behavior in anodic dissolution of chalcopyrite in sulfuric acid solutions[J]. Hydrometallurgy, 2004, 74(1/2): 103-116.
[28] HIROYOSHI N, KITAGAWA H, TSUNEKAWA M. Effect of solution composition on the optimum redox potential for chalcopyrite leaching in sulfuric acid solutions[J]. Hydrometallurgy, 2008, 91(1/4): 144-149.
[29] HIROYOSHI N, HIROTA M, HIRAJIMA T, et al. A case of ferrous sulfate addition enhancing chalcopyrite leaching[J]. Hydrometallurgy, 1997, 47(1): 37-45.
[30] HOLMES P R, CRUNDWELL F K. Kinetic aspects of galvanic interactions between minerals during dissolution[J]. Hydrometallurgy, 1995, 39(1): 353-375.
[31] JIANG L, WEI D, LIU W, et al. Effects of Fe3+ and Ag+ on column bioleaching of a low-grade sulfide copper ore[J]. International Journal of Electrochemical Science, 2019: 6303-6314.
[32] 张卫民, 谷士飞. Fe3+对低品位原生硫化铜矿细菌浸出影响的研究[J]. 中国有色冶金, 2008(1): 35-37.
ZHANG Wei-min, GU Shi-fei. Study on the effect of ferric ion on bacterial leaching of low-grade primary copper sulfide ores[J]. China Nonferrous Metallurgy, 2008(1): 35-37.
[33] NICOL M, MIKI H, VELASQUEZ-YEVENES L. The dissolution of chalcopyrite in chloride solutions Part 3. Mechanisms[J]. Hydrometallurgy, 2010, 103(1/4): 86-95.
[34] YANG C, QIN W, ZHAO H, et al. Mixed potential plays a key role in leaching of chalcopyrite: Experimental and theoretical analysis[J]. Industrial & Engineering Chemistry Research, 2018, 57(5): 1733-1744.
[35] WU S, YANG C, QIN W, et al. Sulfur composition on surface of chalcopyrite during its bioleaching at 50 ℃[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4110-4118.
[36] LI J, KAWASHIMA N, KAPLUN K, et al. Chalcopyrite leaching: The rate controlling factors[J]. Geochimica et Cosmochimica Acta, 2010, 74(10): 2881-2893.
[37] KAPLUN K, LI J, KAWASHIMA N, et al. Cu and Fe chalcopyrite leach activation energies and the effect of added Fe3+[J]. Geochimica et Cosmochimica Acta, 2011, 75(20): 5865-5878.
[38] HIRATO T, MAJIMA H, AWAKURA Y. The leaching of chalcopyrite with ferric sulfate[J]. Metallurgical Transactions B, 1987, 18(3): 489-496.
[39] KLAUBER C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution[J]. International Journal of Mineral Processing, 2008, 86(1): 1-17.
[40] SASAKI K, TAKATSUGI K, TUOVINEN O H. Spectroscopic analysis of the bioleaching of chalcopyrite by Acidithiobacillus caldus[J]. Hydrometallurgy, 2012, 127/128: 116-120.
[41] HARMER S L, THOMAS J E, FORNASIERO D, et al. The evolution of surface layers formed during chalcopyrite leaching[J]. Geochimica et Cosmochimica Acta, 2006, 70(17): 4392-4402.
[42] 张雁生, 覃文庆, 王 军, 等. 中温嗜酸硫杆菌浸出低品位硫化铜矿[J]. 矿冶工程, 2007(4): 25-30.
ZHANG Yan-sheng, QIN Wen-qing, WANG Jun, et al. Bioleaching of copper sulfide ore by Acidithiobacillus Ferrooxidans and Acidithiobacillus Thiooxidans[J]. Mining and Metallurgical Engineering, 2007(4): 25-30.
[43] KLAUBER C, PARKER A, BRONSWIJK W V, et al. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy[J]. International Journal of Mineral Processing, 2001, 62(1): 65-94.
[44] GHAHREMANINEZHAD A, DIXON D G, ASSELIN E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution[J]. Electrochimica Acta, 2013, 87: 97-112.
[45] ACRES R G, HARMER S L, BEATTIE D A. Synchrotron XPS studies of solution exposed chalcopyrite, bornite, and heterogeneous chalcopyrite with bornite[J]. International Journal of Mineral Processing, 2010, 94(1/2): 43-51.
[46] ZHU W, XIA J L, YANG Y, et al. Sulfur oxidation activities of pure and mixed thermophiles and sulfur speciation in bioleaching of chalcopyrite[J]. Bioresource Technology, 2011,102(4): 3877-3882.
[47] 马鹏程, 杨洪英, 王路平, 等. pH对黄铜矿细菌浸铜的影响[J]. 有色金属(冶炼部分), 2015(3): 1-4.
MA Pen-cheng, YANG Hong-ying, WANG Lu-ping, et al. Effect of pH value on bioleaching of chalcopyrite[J]. Nonferrous Metals, 2015(3): 1-4.
[48] 杨洪英, 潘颢丹, 佟琳琳, 等. 黄铜矿表面生物氧化膜的形成过程[J]. 金属学报, 2012, 48(9): 1145-1152.
YANG Hong-ying, PAN Hao-dan, TONG Lin-lin, et al. Formation process of biological oxide film on chalcopyrite crystal surface[J]. Acta Metallurgica Sinica, 2012, 48(9): 1145-1152.
[49] 孟春瑜, 刘文彦, 刘兴宇, 等. 生物浸出中黄铜矿与铁离子间的相互影响[J]. 中南大学学报(自然科学版), 2015, 46(9): 3176-3182.
MENG Chun-yu, LIU Wen-yan, LIU Xing-yu, et al. Interaction between iron ion and chalcopyrite in bioleaching[J]. Journal of Central South University (Science and Technology), 2015, 46(9): 3176-3182
[50] CORDOBA E M, MUNOZ J A, BLAZQUEZ M L, et al. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential[J]. Hydrometallurgy, 2008, 93(3/4): 88-96.
[51] GU G, HU K, ZHANG X, et al. The stepwise dissolution of chalcopyrite bioleached by Leptospirillum ferriphilum[J]. Electrochimica Acta, 2013, 103: 50-57.
[52] SANDSTRoM  , SHCHUKAREV A, PAUL J. XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential[J]. Minerals Engineering, 2005, 18(5): 505-515.
, SHCHUKAREV A, PAUL J. XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential[J]. Minerals Engineering, 2005, 18(5): 505-515.
[53] VILCAEZ J, YAMADA R, INOUE C. Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures[J]. Hydrometallurgy, 2009, 96(1/2): 62-71
[54] VILCAEZ J, INOUE C. Mathematical modeling of thermophilic bioleaching of chalcopyrite[J]. Minerals Engineering, 2009, 22 (11): 951-960.
[55] 张雁生. 低品位原生硫化铜矿的细菌浸出研究[D]. 长沙: 中南大学, 2007.
ZHANG Yan-shen. The bioleaching of low grade copper sulphide ore[D]. Changsha: Central South University, 2007.
[56] 张英杰, 杨显万. 硫化矿细菌浸出机理[J]. 有色金属, 1997, 49(4): 39-44.
ZHANG Ying-jie, YANG Xian-wan. Mechanism in bioleaching of sulfide minerals[J]. Nonferrou Smetals,1997, 49(4): 39-44.
Effects of Cu2+, Fe2+, and Fe3+ on bioleaching of chalcopyrite by moderate thermophilic mixed bacteria
TIAN Zu-yuan1, LI Hao-dong1, WEI Qian1, JIAO Fen1, 2, QIN Wen-qing1, 2, YANG Cong-ren1, 2
(1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Hunan Province for Clean and Efficient Utilization of Strategic Calcium-containing Mineral Resources, Central South University, Changsha 410083, China)
Abstract: The effects of Fe2+, Fe3+, and Cu2+ on bioleaching of chalcopyrite by moderate thermophilic mixed bacteria were studied at 50 ℃ and pH 1.6. The experimental results show that addition of lower concentration of Fe2+ can enhance of the bioleaching of chalcopyrite in the early stage, while addition of higher concentration of Fe2+ will case the reduce the copper extraction percentage. When different concentrations of Fe3+ are added, the total Fe concentration will decrease owing to the formation of jarosite, but the copper extraction does not change significantly. The addition of Cu2+ at different concentrations has no obvious effect on the bioleaching of chalcopyrite. The effect of adding Fe2+ and Cu2+ on chalcopyrite leaching is similar to that of adding Fe2+ alone. Therefore, during the bioleaching process, the solution potential can be controlled by adjusting the composition of the microorganisms-decreasing the ability of ferrous oxidation and increasing the ability of sulfur oxidation-to enhance the bioleaching of chalcopyrite.
Key words: chalcopyrite; moderate thermophilic bacterial; Cu2+; Fe2+; Fe3+
Foundation item: Projects(51904339, 51974364) supported by the National Natural Science Foundation of China; Project(2018TP1002) supported by Key Laboratory of Hunan Province for Clean and Efficient Utilization of Strategic Calcium-containing Mineral Resources, China
Received date: 2020-03-18; Accepted date: 2020-09-17
Corresponding author: YANG Cong-ren; Tel: +86-13874962836; E-mail: yangcongren@csu.edu.cn
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51904339,51974364);战略含钙矿物资源清洁高效利用湖南省重点实验室(2018TP1002)
收稿日期:2020-03-18;修订日期:2020-09-17
通信作者:杨聪仁,副教授,博士;电话:13874962836;E-mail:yangcongren@csu.edu.cn
摘 要:在50 ℃、pH 1.6的条件下,研究了Fe2+、Fe3+和Cu2+对中等嗜热混合菌浸出黄铜矿的影响。结果表明:添加低质量浓度Fe2+时,在浸出前期能够促进黄铜矿的浸出;而添加较高质量浓度Fe2+时,铜的浸出率反而降低;当添加不同质量浓度Fe3+时,由于形成黄钾铁矾而导致总铁质量浓度降低,但铜的浸出率并没有明显变化;添加不同质量浓度Cu2+对黄铜矿的浸出没有显著的影响。同时,添加Fe2+和Cu2+与单独添加Fe2+对黄铜矿的浸出影响是相似的。因此,在生物浸出过程中,可以通过调节浸矿微生物组成降低微生物的亚铁氧化活性,提高硫氧化能力来控制溶液电位,从而强化黄铜矿的生物浸出。
[5] 孙建之, 陈勃伟, 温建康, 等. 镍矿湿法冶金技术应用进展及研究展望[J]. 中国有色金属学报, 2018, 28(2): 356-364.
[6] 文旭祥, 孙占学, 周义朋, 等. 微生物浸铀研究进展[J]. 中国有色金属学报, 2020, 30(2): 411-420.
[7] 邱冠周, 刘学端. 用生物技术的钥匙开启矿产资源利用的大门[J]. 中国有色金属学报, 2019, 29(9): 1848-1858.
[13] 王军, 李旖旎, 庄 田, 等. 黄铜矿精矿中等嗜热微生物浸出过程及其优化[J]. 中国有色金属学报, 2016, 26(5): 1120-1128.
[18] 姚国成, 温建康, 高焕芝, 等. 中等嗜热菌浸出黄铜矿及其表面钝化的研究[J]. 中南大学学报(自然科学版), 2010, 41(4): 1234-1239.
[32] 张卫民, 谷士飞. Fe3+对低品位原生硫化铜矿细菌浸出影响的研究[J]. 中国有色冶金, 2008(1): 35-37.
[42] 张雁生, 覃文庆, 王 军, 等. 中温嗜酸硫杆菌浸出低品位硫化铜矿[J]. 矿冶工程, 2007(4): 25-30.
[47] 马鹏程, 杨洪英, 王路平, 等. pH对黄铜矿细菌浸铜的影响[J]. 有色金属(冶炼部分), 2015(3): 1-4.
[48] 杨洪英, 潘颢丹, 佟琳琳, 等. 黄铜矿表面生物氧化膜的形成过程[J]. 金属学报, 2012, 48(9): 1145-1152.
[49] 孟春瑜, 刘文彦, 刘兴宇, 等. 生物浸出中黄铜矿与铁离子间的相互影响[J]. 中南大学学报(自然科学版), 2015, 46(9): 3176-3182.
[55] 张雁生. 低品位原生硫化铜矿的细菌浸出研究[D]. 长沙: 中南大学, 2007.


