
Platinum recovery from dilute platinum solutions using activated carbon
S. AKTAS1, M. H. MORCALI2
1. Institute of Science and Technology, Istanbul Technical University, 34469 Maslak, Istanbul, Turkey;
2. Faculty of Chemistry and Metallurgy, Istanbul Technical University, 34469 Maslak, Istanbul, Turkey
Received 6 January 2011; accepted 14 May 2011
Abstract:
Platinum adsorption on activated carbon, a common adsorbent, from dilute platinum-containing solution of 57.6 mg/L was investigated. The effects of different adsorption parameters on the platinum adsorption were reported in detail. The percentage of platinum adsorption increased with increasing the amount of adsorbent, as well as with increasing contact time and temperature. A platinum adsorption of 75% was obtained using 400 mg of activated carbon at a shaking rate of 200 r/min for 3 h at 25 °C. A platinum adsorption of 88%, however, was obtained at 80 °C using 200 mg of activated carbon within only 1 h, which indicates that temperature plays a vital role in the adsorption process. A platinum adsorption of 99% or above was attained using 400 mg of activated carbon after contact for 3 h at 80 °C.
Key words:
platinum; adsorption; activated carbon; recovery;
1 Introduction
Platinum is one of the most important precious metals because of its widespread use in many industrial applications, including catalytic converters, thermo- couples, jewelry, platinum electroplating solutions, and laboratory equipment [1-2]. The diminishing availability of mineral sources and increasing demand for platinum metal make its recovery from waste solutions and scrap materials important [3]. Platinum recovery from secondary sources, such as catalytic converters, thermocouples, waste electroplating solutions, and leaching solutions stemming from primary resources, therefore represents an important technology.
The literature contains numerous studies focused on the recovery and selective separation of precious metals. The methods capable of removing metal ions from effluent solutions include precipitation, cementation [4-6], ion-exchange [7-11], and adsorption [12-16]. Both precipitation and cementation techniques result in high efficiencies but generally do not ensure a complete purification, that is, a further treatment is inevitably necessary. Ion-exchange method allows the efficient recovery of metallic ions, but the method is not cost effective. The removal of metal ions by adsorption on activated carbon has been intensively investigated for two decades because of its ease of use and ease of regeneration. The literature contains many reports regarding the production and characterization of activated carbon from agricultural wastes [17-19]. Activated carbon or activated charcoal is a useful sorbent with a microporous structure mainly composed of carbonaceous material. As a preferred adsorbent, activated carbon has been widely used to remove organic pollutants from industrial and municipal wastewaters, flue gases, and volatile solvents [20-21]. As a result of the technical and economic advantages of activated carbon, much attention has been paid to the regeneration of adsorbents with respect to the recovery of platinum metal ions.
The objective of the present study is to outline an effective adsorption process using activated carbon as an adsorbent for the high-efficiency recovery of platinum from dilute platinum solutions and to describe the optimum conditions and parameters for this adsorption process. For this purpose, the quantity of activated carbon, shaking rate, reaction time, and temperature are investigated in this work.
2 Experimental
2.1 Materials and chemicals
A waste solution containing 57.6 mg/L platinum with a pH value of 3.5 was procured from a PGM treatment plant in Istanbul, Turkey. The chemical composition of this waste solution is detailed in Table 1. Distilled water was used for the wet chemical analyses. Analytical-grade activated carbon (150-440 kg/m3) was employed for the adsorption experiments. About 90% of the particles exhibited diameters of less than 100 μm, and the surface area of the activated carbon was 760 m2/g.
Table 1 Composition of waste solution (mg·L-1)

2.2 Equipment
The concentration of platinum ions was measured after each adsorption process by inductively coupled plasma optical emission spectrometry (ICP-OES) using a Perkin Elmer Optical Emission Spectrometer Optima 2100 DV, as well as by atomic absorption spectrophotometry (AAS) carried out on a Perkin Elmer 1100B.
2.3 Methods
The study was carried out by varying one adsorption parameter at a time. For each experiment, 10 mL solution containing 57.6 mg/L platinum was contacted with activated carbon in a falcon tube to avoid exposure to air. In this manner, air was not able to diffuse into the system. Experiments were conducted in a temperature-controlled shaking water bath, to ensure uniform heat convection on the surface of the falcon tube.
The first experimental series was performed by varying the amount of activated carbon while keeping the other parameters constant. In the second experimental series, the influence of the shaking rate was studied in the range of 40-200 r/min, where 200 r/min represents the maximum agitation rate of the equipment used in this research. Shaking was performed in a temperature-controlled shaking water bath. The third experimental series were conducted to investigate the effect of the reaction time varying from 1 to 5 h. Later, the influence of temperature was investigated in the range of 25-80 °C.
Solid/liquid separation was performed following each run. For the analyses, the filtered solution was analyzed by ICP-OES following an appropriate dilution when necessary. The efficiency (η) of the adsorption process was calculated using the following equation:
![]() (1)
(1)
where Co is the initial platinum concentration (57.6 mg/L) and Ct represents the concentration at the end of the experiment.
3 Results and discussion
The first experimental series were performed by varying the amount of activated carbon from 40 to 400 mg while keeping the other parameters constant. Figure 1 presents the variation of the platinum adsorption percentage with the amounts of activated carbon. It is apparent from Fig. 1 that the percentage of the recovered platinum increases with increasing the amounts of activated carbon. This increase can be easily explained by the fact that the adsorption reaction are thermodynamically favored by a high ratio of the adsorbent and the metal being adsorbed [21].
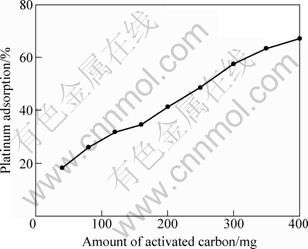
Fig. 1 Effect of amount of activated carbon on platinum adsorption (1 h, room temperature, 200 r/min, 10 mL solution)
When the platinum solution contacts with activated carbon, platinum in the quadrivalent (Pt4+) form is reduced to the metallic form (Pt0). The net reaction takes place as follows [22-27].
The direct reduction of metal ions on carbon surfaces is thermodynamically possible if the electrode potential, ?φ, of the C/[MeLm]h- system is greater than zero, where Me is the metal and Lm is the ligand. This condition is satisfied when the equilibrium potential, φme, of the [MeLm]h–/Me0 pair is more positive than the working potential of the carbon, φc,
?φ=φme-φc>0 (2)
[PtCl6]2-+2e-![]() [PtCl4]2-+2Cl-, φ0=0.684 V (3)
[PtCl4]2-+2Cl-, φ0=0.684 V (3)
[PtCl4]2-+2e-![]() Pt0+4Cl-, φ0=0.755 V (4)
Pt0+4Cl-, φ0=0.755 V (4)
Cs+2H2O→CO+4H++4e-, φ0=0.207 V (5)
The net reaction is
[PtCl6]2-+Cs+2H2O→Pt0+CO+4H++6Cl-
?φ=1.232 V (6)
When the reaction was run for 1 h with large quantities (such as 400 mg) of activated carbon, the adsorption percentage was still below 70%. This result leads to the conclusion that either the reaction time was insufficient or the reaction should be performed at elevated temperatures.
3.1 Effect of shaking rate on platinum adsorption
In this experimental series, the platinum adsorption was investigated as a function of the shaking rate in the range of 40-200 r/min. The duration of the experiments was limited to 1 h. Figure 2 presents the variation of platinum adsorption as a function of the shaking rate. The trend in Fig. 3 establishes that the adsorption percentage increases with increasing the shaking rate. Consequently, the maximum agitation rate of 200 r/min was selected for the rest of the experiments.
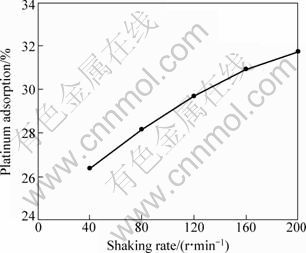
Fig. 2 Platinum adsorption as function of shaking rate (1 h, room temperature, 120 mg, 10 mL solution)
3.2 Effect of time on platinum adsorption
To see the effects of reaction time on the platinum adsorption, experiments were performed in the range of 1-5 h. Figure 3 presents the platinum adsorption with varying reaction time and demonstrates that an increase of the reaction time has a positive effect on the platinum adsorption, that is, the platinum adsorption percentage increases with increasing time. This finding is in accordance with the results of TASDELEN et al [7].
3.3 Effect of temperature on platinum adsorption
The effect of temperature on platinum adsorption was studied in the temperature range of 25-80 °C. Figure 4 presents the platinum adsorption as a function of temperatures and shows that increasing temperature results in an increase in the platinum adsorption percentage. This result is also in accordance with the result of Ref. [7].
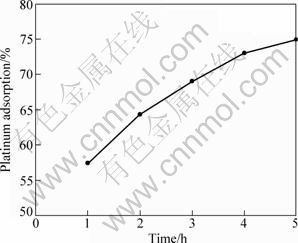
Fig. 3 Platinum adsorption as function of reaction time (room temperature, 200 r/min, 300 mg activated carbon, 10 mL solution)
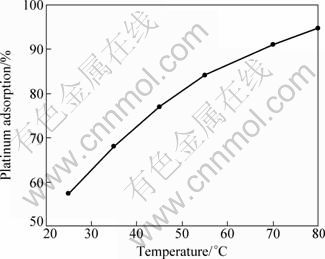
Fig. 4 Platinum adsorption as function of temperature (1 h, 200 r/min, 300 mg activated carbon, 10 mL solution)
Figures 5 and 6 display the variation in the platinum adsorption percentage as a function of time at various temperatures. As evidenced from Fig. 5, an increase in temperature from 25 °C to 80 °C resulted in an increase of 40% in the platinum adsorption. The effect of time is more noticeable between 25 °C and 55 °C. Careful comparison of Fig. 5 and Fig. 6 indicates that increasing the amount of activated carbon results in the increment of the adsorption percentage. The figures also underscore the role that temperature plays an important role in the adsorption process. Thus, platinum adsorption should not be carried out at room temperature if a higher degree of recovery is sought, even though increasing reaction temperature is not always easy and may require additional investment.
After the adsorption process, the activated carbon was treated at 600 °C for 1 h. Figure 7 shows the XRD pattern of the activated carbon before and after the adsorption. The XRD pattern indicates that the adsorbed platinum was in the metallic form.
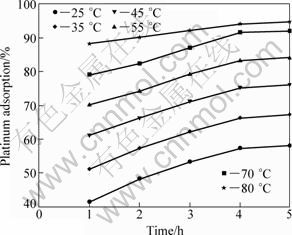
Fig. 5 Platinum adsorption as function of both temperature and time (200 r/min, 200 mg activated carbon, 10 mL solution)
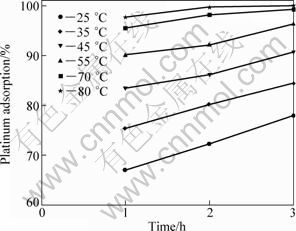
Fig. 6 Platinum adsorption as function of both temperature and time (200 r/min, 400 mg activated carbon, 10 mL solution)
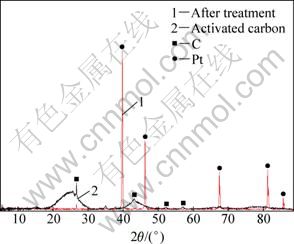
Fig. 7 XRD patterns of activated carbon before and after platinum adsorption
4 Conclusions
The platinum adsorption using activated carbon is effective for recovering platinum metal from dilute platinum solutions. With excessive amount of activated carbon, such as 400 mg, a platinum adsorption percentage greater than 99% was easily attained at shaking rate of 200 r/min after contacting for 3 h at 70 °C. Decreasing the temperature from 70 °C to room temperature, however, decreased the adsorption percentage to 77%, which indicates that temperature plays a vital role in this process. Consequently, platinum adsorption should not be performed at room temperature because lower temperatures will result in platinum loss.
The loading capacity was found to be 2.7 mg Pt per gram of activated carbon. Increasing the shaking rate and contact time led to an increase in the platinum adsorption percentage. Increasing the shaking rate from 40 to 200 r/min increased the platinum adsorption percentage by 5%, which indicates that shaking is necessary for platinum adsorption from dilute platinum solutions. Increasing contact time from 1 to 5 h increased the platinum adsorption percentage by 10% when the reaction was run at room temperature. Among the parameters investigated in this study, temperature was the most effective in terms of adsorption percentage.
Acknowledgements
Special thanks are due to TUBITAK for financial support and Mr. Tayfun AKTAS for making possible analyses conducted outside the university.
References
[1] FUJIWARA K, RAMESH A, MAKI T, HASEGAWA H, UEDA K. Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto L-lysine modified crosslinked chitosan resin [J]. Journal of Hazardous Materials, 2007, 146: 39-50.
[2] RAMESH A, HASEGAWA H, SUGIMOTO W, MAKI T, UEDA K. Adsorption of gold(III), platinum(IV) and palladium(II) onto glycine modified crosslinked chitosan resin [J]. Bioresource Technology, 2008, 99: 3801-3809.
[3] BROOKS C S. Metal recovery from industrial wastes [M]. Chelsea: Lewis Publishers, 1991: 267.
[4] AKTAS S. Silver recovery from spent silver oxide button cells [J]. Hydrometallurgy, 2010, 104: 106-111.
[5] AKTAS S, MORCALI M H, YUCEL O. Silver recovery from waste radiographic films by cementation and reduction [J]. Canadian Metallurgical Quarterly, 2010, 49: 147-154.
[6] AKTAS S. Silver recovery from silver-rich photographic processing solutions by copper [J]. Canadian Metallurgical Quarterly, 2008, 47: 37-44.
[7] TASDELEN C, AKTAS S, ACMA E, GUVENILIR Y. Gold recovery from dilute gold solutions using DEAE-cellulose [J]. Hydrometallurgy, 2009, 96: 253-257.
[8] MO J J, SHI L M, GU Y B, YAN G B. Adsorption of platinum (IV) onto D301R resin [J]. Rare Metals, 2008, 27(3): 233-237.
[9] GAITA R, AL-BAZI S J. An ion-exchange method for selective separation of palladium, platinum and rhodium from solutions obtained by leaching automotive catalytic converters [J]. Talanta, 1995, 42(2): 249-255.
[10] ALGUACIL F J, COBO A, COEDO A G, DORADO M T, SASTRE A. Extraction of platinum(IV) from hydrochloric acid solutions by amine alamine 304 in xylene: Estimation of the interaction coefficient between PtCl62- and H+ [J]. Hydrometallurgy, 1997, 44(1-2): 203-212.
[11] GUIBAL E, VINCENT T, LARKIN A, TOBIN J M. Chitosan sorbents for platinum recovery from dilute solutions [J]. Industrial & Engineering Chemistry Research, 1999, 38(10): 4011-4022.
[12] PESAVENTO M, PROFUMO A, ALBERTI G, CONTI F. Adsorption of lead(II) and copper(II) on activated carbon by complexation with surface functional groups [J]. Analytica Chimica Acta, 2003, 480(1): 171-180.
[13] CHEN J P, WANG X Y. Removing copper, zinc, and lead ion by granular activated carbon in pretreated fixed-bed columns [J]. Separation and Purification Technology, 2000, 19(3): 157-167.
[14] KONONOVA O N, LUKIANOV A N, DEREVYASHKIN M V, KHOLMOGOROV A G, KACHIN S V, GORYAEVA N G. Sorption of palladium on carbon adsorbents from nitric acid solutions [J]. Journal of Porous Materials, 2008, 15(1): 61-66.
[15] DAVIDSON R J. Mechanism of gold adsorption on activated charcoal [J]. Journal of the South African Institute of Mining and Metallurgy, 1974: 67-75.
[16] DAVIDSON R J, VERONESE V, NKOSI M V. The use of activated carbon for the recovery of gold and silver from gold-plant solutions [J]. The South African Institute of Mining and Metallurgy, 1979: 281-297.
[17] KADIRVELU K, NAMASIVAYAM C. Agricutural by-product as metal adsorbent: Sorption of lead (II) from aqueous solution onto coirpith carbon [J]. Enviromental Technology, 2000, 21(10): 1091-1097.
[18] KIM J W, SOHN M H, KIM D S, SOHN S M, KWON Y S. Production of granular activated carbon from waste walnut shell and its adsorption characteristics for Cu2+ ion [J]. Journal of Hazardous Materials, 2001, 85(3): 301-315.
[19] TATY-COSTODES V C, FAUDUET H, PORTE C, DELACROIX A. Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris [J]. Journal of Hazardous Materials, 2003, 105(1-3): 121-142.
[20] ZHANG H P. Regeneration of exhausted activated carbon by electrochemical method [J]. Chemical Engineering Journal, 2002, 85(1): 81-85.
[21] BANSAL R C, GOYAL M. Activated carbon adsorption [M]. Boca Raton: Taylor & Francis Group CRC Press, 2005: 40.
[22] LIN C C. Kinetics and mechanisms of adsorption of heavy metal ions on activated carbon [D]. Texas: Texas Tech University, 1979.
[23] CHEN S X, XU R M, HUANG H X, YI F Y, ZHOU X, ZENG H M. Reduction–adsorption behavior of platinum ions on activated carbon fibers [J]. Journal of Materials Science, 2007, 42: 9572-9581.
[24] SIMANOVA S A, SHUKAREV A V, LYSENKO A A, GREBENNIKOV S F, ASTASHKINA O V. Adsorption of palladium, platinum, and gold chloride complexes by carbon fibers with various structures [J]. Fibre Chemistry, 2008, 40(4): 365-375.
[25] SAIPANYA S, SARAKONSRI T. Preparation and applications of precious metals adsorbed activated carbon cloth [J]. Advanced Materials, 2010, 93-94: 296-299.
[26] AGEEVA L D, KOLPAKOVA N A, KOVYRKINA T V, POTSYAPUN N P, BUINOVSKII A S. Mechanism and kinetics of the sorption of platinum, palladium, and gold on activated carbon from UV-illuminated chloride solutions [J]. Journal of Analytical Chemistry, 2001, 56 (2): 137-139.
[27] QADEER R. Adsorption of ruthenium ions on activated charcoal: Influence of temperature on the kinetics of the adsorption process [J]. Journal of Zhejiang University Science B, 2005, 6 (5): 353-356.
活性炭从含铂稀溶液中回收铂
S. AKTAS1, M. H. MORCALI2
1. Institute of Science and Technology, Istanbul Technical University, 34469 Maslak, Istanbul, Turkey;
2. Faculty of Chemistry and Metallurgy, Istanbul Technical University, 34469 Maslak, Istanbul, Turkey
摘 要:采用活性炭从含57.6 g/L Pt的稀溶液中吸附回收Pt。研究不同吸附工艺参数对Pt吸附回收率的影响。结果表明,随着活性炭用量的增加、吸附时间的延长和温度的升高,Pt的吸附回收率也提高。在振荡速率200 r/min,吸附时间3 h和25 °C的条件下,使用400 mg活性炭,Pt的回收率可以达到70%。然而,在80 °C下吸附1 h,使用200 mg活性炭,Pt的吸附率就可达到88%,表明温度对吸附的影响是显著的。在80 °C下吸附3 h,使用400 mg活性炭,Pt的吸附率可达到99%以上。
关键词:Pt;吸附;活性炭;回收
(Edited by YUAN Sai-qian)
Corresponding author: S. AKTAS; Tel: +90-5337230576; E-mail: serdaraktash@yahoo.com
DOI: 10.1016/S1003-6326(11)61091-1
Abstract: Platinum adsorption on activated carbon, a common adsorbent, from dilute platinum-containing solution of 57.6 mg/L was investigated. The effects of different adsorption parameters on the platinum adsorption were reported in detail. The percentage of platinum adsorption increased with increasing the amount of adsorbent, as well as with increasing contact time and temperature. A platinum adsorption of 75% was obtained using 400 mg of activated carbon at a shaking rate of 200 r/min for 3 h at 25 °C. A platinum adsorption of 88%, however, was obtained at 80 °C using 200 mg of activated carbon within only 1 h, which indicates that temperature plays a vital role in the adsorption process. A platinum adsorption of 99% or above was attained using 400 mg of activated carbon after contact for 3 h at 80 °C.


