
Electrochemical preparation and characterization of gold-polyaniline core-shell nanocomposites on highly oriented pyrolytic graphite
LI Nian-feng(李年丰)1, 2, LEI Ting(雷 霆)2, LIU Yong(刘 咏)2,
HE Yue-hui(贺跃辉)2, ZHANG Yang-de(张阳德)1
1. Xiangya Hospital, Central South University, Changsha 410008, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 27 November 2009; accepted 6 April 2010
Abstract:
A simple electrochemical method for the in situ preparation of homogeneously dispersed gold-polyaniline core/shell nanocomposite particles with controlled size on the highly oriented pyrolytic graphite (HOPG) was demonstrated. The HOPG surface was modified preferentially by covalent bonding of a two-dimensional 4-aminophenyl monolayer employing diazonium chemistry. AuCl4- ions were attached to the Ar-NH2 termination and reduced electrochemically. This results in the formation of Au nuclei that could be further grown into gold nanoparticles. The formation of polyaniline as the shell wrap of Au nanoparticle was established by localized electro-polymerization. These core–shell nanocomposites prepared were characterized by AFM and cyclic voltammetry. The results show that the gold-polyaniline core-shell composites on HOPG have a mean particle size of 100 nm in diameter and the polyaniline shell thickness is about 15 nm.
Key words:
core-shell structure; nanocomposites; polyaniline; gold nanoparticles; highly oriented pyrolytic graphite (HOPG);
1 Introduction
In recent years, the development of inorganic particle and polymer composites, particularly, composites of metal particles and conducting polymers with core-shell structure, has attracted considerable interest due to their interesting properties and a wide range of the promising potential applications in technological fields[1-2]. Polyaniline (PANI) is the mostly studied conducting polymer and some polyaniline-based core-shell nanocomposites such as Ag/ polyaniline[3-4], Ni/polyaniline[5] and silica- polyaniline[6] have been well demonstrated.
Highly oriented pyrolytic graphite (HOPG) is an ideal single crystal carbon material and well-oriented with good conductivity and resistance to chemicals, and suitable as a substrate for particle deposition[7-9]. In order to prepare uniform nanoparticles with narrow size distribution on HOPG, pre-activating treatments of HOPG are usually exploited[10-11], which often lead to severe surface disruption and formation of blisters[12-13]. Alternatively, TANG et al[14] reported a new method of electrochemical fabricating Ag nanoparticles on 4-aminophenyl monolayer modified HOPG surface. On the almost defect-free surface of HOPG electrode, Ag+ cations are preadsorbed and followed by electrochemical reduction, resulting in the formation of Ag nuclei that could be further grown. The covalent bonding of a two-dimensional 4-aminophenyl monolayer on the HOPG surface was thought to provide a uniform functional surface by compact packing of amino groups, which could in turn effectively prevent the preferred nucleation process on the substrate surface and benefit for considerably narrower size distributions of particles than in the case of random nucleation[15]. This strategy of coordinating a metal ion to a functionalized surface with —NH2 or —SO3H terminal groups followed by its subsequent electrochemical or chemical reduction provides a means of preparation of a two-dimensional metallic overlayer[16-17].
Previously, we successfully fabricated gold- polyaniline nanocomposites with core-shell structure on ITO glass substrate through a facile electrochemical synthesis[18]. In the present study, we report the in situ electrochemical preparation of homogeneously dispersed gold-polyaniline composites with core-shell nanostructure on 4-aminophenyl monolayer modified HOPG, and characterization of the resulting nanocomposites is also investigated by AFM and cyclic voltammetry.
2 Experimental
2.1 Materials
4-nitrophenyl diazonium fluoroborate and terabutylammonium terafluoroborate (n-Bu4NBF4), 4-aminothiophenol, and HAuCl4 salt were purchased from Shanghai Reagent Ltd., Co., China. Aniline was vacuum distilled and stored in the dark at 0 oC before being used. Fresh surface of HOPG was obtained by peeling off the top layer with sticky tape before being used[19] and was used as working electrode by sealing in a Teflon jacket with an opening in the bottom, in which an O-ring was pressure fitted against the HOPG, and a 0.5 cm2 geometric area of the graphite surface was exposed. Pure water was obtained by passing it through Millipore Milli-Q water purification. All other chemicals were of analytical grade and used as-received unless otherwise noted.
2.2 Instrumentation
AFM characterization of electrodeposited gold and gold-polyaniline nanostructures was performed in air on Nanoscaping-IIIA atomic force microscope. Instrument was operated in AC mode. The tips used were commercially available with a diameter of 10 nm. The resonant frequency of the tips was consistently around 70 kHz and the drive amplitude was varied as necessary to obtain high signal–to–noise images. Electrochemical measurements were performed on CHI-660C electrochemical workstation with a three-electrode system comprising a HOPG electrode as working electrode, a platinum wire as auxiliary electrode, and a saturated calomel electrode (SCE) as reference electrode. All measurements were performed at room temperature. Electrolyte solutions were deaerated by purging with high purity nitrogen prior to and blanked with nitrogen during electrochemical experiments.
2.3 HOPG surface modification with 4-aminophenyl monolayer
The modification of HOPG by 4-aminophenyl monolayer aimed at obtaining an Ar-NH2 terminated surface, and was carried out mainly as previously described by TANG et al[14] and PINSON and PODVORICA[17]. Briefly, the freshly cleaved HOPG electrode was firstly immersed into a 0.1 mol/L terabutylammonium terafluoroborate (n-Bu4NBF4) in acetonitrile (ACN) solution containing 5 mmol/L 4-nitrophenyl diazonium fluoroborate and cycled between -0.75 V and 0.75 V (vs SCE) at a scan rate of 0.200 V/s for 20 scans. This operation resulted in grafting 4-nitrophenyl radical group on HOPG surface. After rinsing and ultrasonically washing with water, the modified electrode was transferred to the protonic solution (90% H2O+10% EtOH (volume fraction)+ 0.1 mol/L KCl), and the reduction of —NO2 group to —NH2 group on the electrode surface was performed by applying a potential of -1.4 V for 15 min.
2.4 Preparation of Au nanoparticles on 4-aminophenyl monolayer-grafted HOPG substrate
The in situ preparation of Au nanoparticles was carried out by attachment of AuCl4- onto the —NH2 surface and functionalized by its electrochemical reduction. Briefly, the 4-aminophenyl-grafted HOPG electrode was immersed in 5 mmol/L HAuCl4 solution for 3 min. After rinsing thoroughly with water, the Au3+ cations adsorbed with HOPG substrate were transferred to 0.1 mol/L KCl blank solution and a pulsed potential stepped from 0 V to -0.8 V was applied for 200 ms to grow Au nanoparticles on the 4-aminophenyl monolayer-grafted HOPG electrode. Upon successively repeated immersion in plating solution for different time and potential stepping, Au nanoparticles with various sizes and number densities were obtained. Finally, the as-prepared Au/HOPG electrode was removed from the solution and rinsed with water and dried by N2 stream.
2.5 Preparation of gold-polyaniline core-shell nanoparticles on HOPG
The preparation of core-shell nanoparticles on HOPG substrate was carried out in Ref.[18]. Briefly, 4-aminothiophenol (4-ATP) monolayer assembly at gold nanoparticles was firstly achieved by immersion of the as-prepared Au-HOPG electrode in ethanol solution containing 1 mmol/L 4-ATP overnight, and the succeeding deposition of aniline around gold nanoparticle surfaces was carried out by cycling from -0.10 V to 0.78 V in 5 mmol/L aniline + 0.5 mol/L H2SO4 solution. This led to the preparation of polyaniline-covered gold nanoparticles on HOPG substrate. Finally, the as-obtained core-shell nanoparticles on HOPG substrate were removed from the solution and rinsed with ultrapure water and subsequently dried in a stream of N2 before AFM experiments were performed.
3 Results and discussion
3.1 Micrograph of Au and Au-PANI core-shell particles on HOPG
Fig.1 describes the procedure for the preparation of gold-polyaniline core-shell nanocomposites on HOPG surface. Briefly, there are three steps involved in the procedure: 1) formation of 4-aminophenyl monolayer covalently attached to HOPG surface; 2) adsorption of Au3+ cations on the 4-aminophenyl monolayer grafted HOPG surface through coordination of amino– group, and subsequent growth of gold particles by a pulsed potentiostatic method; 3) formation of 4-aminothiophenol (4-ATP) capped Au nanoparticles on modified HOPG and succeeding polymerization of aniline.
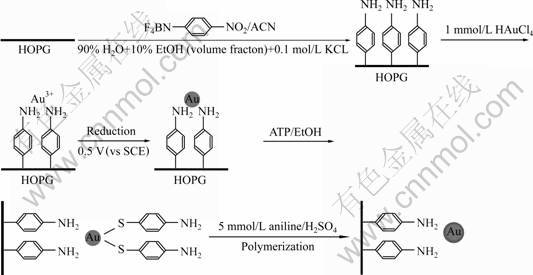
Fig.1 Procedure for preparation of core-shell gold-polyaniline nanocomposites on HOPG surface through electrochemical method
The modification process and characterization of the 4-aminophenyl monolayer on HOPG surface were discussed in detail by LIU et al[20]. Herein, we focus on studying the surface morphology and distribution of gold nanoparticles on HOPG surface by AFM. Fig.2 shows a typical AFM image of the 4-aminophenyl monolayer-modified HOPG surfaces after adsorption of Au3+ cations by one period of immersion in plating solution for 3 min. It is noticed that there exist no domain structures or aggregates on the surface, instead, a relatively rougher surface was observed in comparison with a freshly bare HOPG surface (not shown here), which suggests that the Au3+ cations are homogeneously adsorbed on the 4-aminophenyl monolayer-grafted HOPG surface through coordination of amino (—NH2) group.

Fig.2 AFM image of 4-aminophenyl monolayer-modified HOPG surfaces after adsorption of Au3+ cations by one period of immersion in 5 mmol/L HAuCl4 plating solution for 3 min
Fig.3 shows the AFM images of gold particles with a uniform distribution on HOPG, prepared under different experimental conditions following the procedure described in section 2.4. A typical AFM image of gold nanoparticles prepared by one period of immersion in plating solution and electroreduction is shown in Fig.3(a), which suggests that the gold nanoparticles exhibit a spherical structure with a mean diameter of ca. 25 nm. When higher concentration of plating solution is used, larger gold particle size of 100 nm in diameter could be formed as seen in Fig.3(b). It is noted that gold nanoparticles with different density and size could be generated easily by regulating the immersion time and concentration of plating solution.
A typical topographic non-contact AFM image of the Au-polyaniline core-shell composites is shown in Fig.4. As can be seen, the nanocomposites are almost in spherical shape of about 100 nm in diameter with a uniform size distribution and high density. These structures are taken to be gold-polyaniline core-shell particles, in which the Au nanoparticle, prepared by immersion in 10 mmol/L HAuCl4 plating solution for 5 min, is the core of the nanocomposite, and the polyaniline, electropolymerised by 30 cycles, is the shell wrap of the Au nanoparticle. The polyaniline film thickness is estimated to be around 15 nm based on the integrated anodic oxidation peak charge of cyclic voltammetric curve.

Fig.3 AFM images of gold particles on 4-aminophenyl monolayer-modified HOPG, with different particle size and density: (a) 25 nm, immersion in 5 mmol/L HAuCl4 plating solution for 3 min; (b) 100 nm, immersion in 8 mmol/L HAuCl4 plating solution for 8 min

Fig.4 AFM image of gold-polyaniline core-shell composites on HOPG (prepared by immersion in 10 mmol/L HAuCl4 plating solution for 5 min and electropolymerisation by 30 cycles in polyaniline solution) with mean particle size of 100 nm in diameter (Polyaniline shell thickness is about 15 nm.)
3.2 Electrochemical behavior of Au and Au-PANI particles on HOPG
The electrochemical behavior of the Au particles on HOPG was studied in 0.5 mol/L H2SO4 solution by cyclic voltammetry, and the results are shown in Fig.5. The gold nanoparticles are prepared following description in section 2.4 by two periods of immersion and electroreduction. For comparison, a control cyclic voltammetry of freshly cleaved HOPG was also performed under the same condition. Fig.5 shows the corresponding CV curves of the Au-HOPG and bare HOPG electrodes. There is no observable Faradaic current on the bare HOPG electrode shown by curve d in Fig.5. The cyclic voltammogram of the first scan for an Au-HOPG electrode (curve a in Fig.5(a)) shows a single oxidation peak at 0.9 V along with the reduction peak at 0.55 V, which attributes to the reduction of gold surface oxide. This cyclic voltammetric response is in good agreement with values reported previously for Au electrodeposition onto HOPG[19, 21-22], in which the first anodic peak was assigned to a 3e oxidation. Therefore, this gives the confirmation that gold particles are successful formed on the grafted HOPG electrode surface, and the pair of redox peaks is ascribed to be Au(III)/Au(0) couple. It has been reported the standard reduction potential for the Au(III)/Au(0) couple, φ0, is 0.804 V vs Ag/AgCl[21],namely, 0.783V vs SCE. Fig.5 shows that the reduction of Au on HOPG centers at 0.55 V, approximately 0.25 V negative of φ0 for formal potential of gold reduction. It was considered that this hysteresis was characteristic of slow nucleation followed by diffusion controlled growth of the Au deposits[21-22].
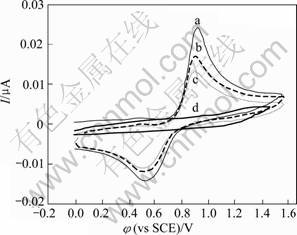
Fig.5 Cyclic voltammogram in 0.5 mol/L H2SO4 at scan rate of 0.100 V/s for Au nanoparticles on 4-aminophenyl group- modified HOPG at the first scan (a), second scan (b), third scan (c) and freshly bare HOPG (d) under same condition
A close inspection of the reoxidation–reduction behavior of gold nanoparticles on the subsequent second and third cycling scan (curves b and c in Fig.5, respectively) in unstirred solution reveals that both anodic and cathodic peaks decrease gradually. This is likely due to the flux of a significant portion of Au3+ cation from reoxidation of Au(0) at the electrode to solution and less diffusion back to electrode surface, and thus less Au3+ is re-reduced. Moreover, it is worthy to note that there is no characteristic oxidation and reduction of Au observed for a bare HOPG surface after Au3+ cations preadsorption, which implies that Au3+ cations cannot be adsorbed and reduced on the bare HOPG electrode, as the same case as an Ag+ cation preadsorption on HOPG [14].
The cyclic voltammetry of Au-PANI core-shell nanocomposites on HOPG in H2SO4 solution is shown in Fig.6. Fig6(b) shows the second scan image of CV in the 0-0.8 V potential range. As can be seen, the characteristic features of gold are no longer visible anymore; instead, two peaks are observed at +0.45 V and +0.35 V, which appear to result from the coverage of the gold particles by the formed polyaniline. This pair of peaks is commonly assumed to correspond to the first redox couple of conventional PANI [23-24]. This suggests that the growth of polyaniline does occur and form a coverage on gold particle surface. The PANI film thickness was estimated by integration of the anodic oxidation peak charge using the published conversion factor of 500 C/cm3[24]. The integrated charge value of 0.75 mC/cm2 was obtained, which was corresponding to 15 nm-thick PANI layer. The process for the polyaniline layer growth on gold surfaces is described as follows. It is reported that the onset oxidation of 4-ATP occurred at 730 mV against SCE, about 260 mV earlier than that of aniline in solution phase, which was approximately at 1.05 V[25-27]. Thus, with the potential being controlled at the onset oxidation potential of 4-ATP, 4-ATP radical cations were initially electrogenerated on the gold particle surface in aqueous media, which acted as a point of nucleation to initiate the polymerization reaction of aniline monomer. Therefore, during the electro- polymerisation, PANI was formed within the 4-ATP sites, and then it preferentially grew around the gold particles rather than on the underlying electrode surface because there were no radical cations in solution nor on the bare electrode surface while holding the electrode potential at the oxidation potential of 4-ATP. The formed PANI could continue the in situ electropolymerisation due to its conduction. As a result, a localized electropolymerization of aniline on ATP molecular sites, which are covalently attached on gold, resulted in the formation of polyaniline layer wrapped on the gold particle surface, leading finally to the fabrication of core-shell composites. Obviously, little polymerization appeared to take place at the underlying HOPG electrode surface, and the control of polyaniline film thickness becomes convenient and easy by the control of cycling numbers, in other words, the amount of PANI formed on gold particle surface is determined by the electro- polymerisation time.
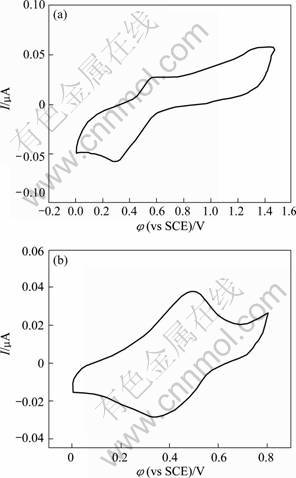
Fig.6 Cyclic voltammetry of gold-polyaniline core-shell composite particles on HOPG in 0.5 mol/L H2SO4 solution at scan rate of 0.100 V/s (a) and second scan CV between 0 V and 0.8 V (b) (Polyaniline film thickness is calculated to be around 15 nm.)
4 Conclusions
1) Au-polyaniline composite particles with core-shell structure were synthesized in situ on the surface of amino groups (—NH2) functionalized HOPG substrate through molecular design by the electrochemical reduction of diazonium compounds.
2) The as-prepared Au-polyaniline core-shell
particles disperse homogeneously on HOPG surface with uniform particle sizes. Moreover, the size and density of the resulting nanoparticles can be easily regulated by selecting appropriate electroplating conditions.
3) Undoubtedly, electrochemical method remains an effective, facile and controllable method for making particles and importantly, the polyaniline shell component could be further modified by chemical reactions and thus functionalized for technological applications.
References
[1] HAN Xiao, WANG Yuan-sheng. Microwave absorbing prediction of Fe3O4/conductive polyaniline nanocomposites with core-shell structure [J]. Journal of Aeronautical Materials, 2008, 28(4): 82-87. (in Chinese)
[2] GRANOT E, KATZ E, BADNAR B, WILLNER I. Enhanced bioelectrocatalysis using Au-nanoparticle/polyaniline hybrid systems in thin films and microstructured rods assembled on electrodes [J]. Chem Mater, 2005, 17(18): 4600-4609.
[3] SHENG Y J, SHUANG X X, LIAN X Y, WU Y, ZHAO C. Synthesis and characterization of Ag/polyaniline core-shell nanocomposites based on silver nanoparticles colloid [J]. Mater Lett, 2007, 61(13): 2794-2797.
[4] KHANNAP K, SINGHN S C, VISWANATH A K. Synthesis of Ag/polyaniline nanocomposite via an in situ photo-redox mechanism [J]. Mater Chem Phys, 2005, 92(1): 214-219.
[5] DONG Xing-long, ZUO Fang, ZHONG Wu-bo, LI Zhe-nan, CHEN Ping. Preparation and characteristics of nano-Ni/polyaniline nanocomposite particles [J]. Journal of Functional Materials, 2005, 36(10): 1558-1560. (in Chinese)
[6] LUO X L, KILLARD A J, MORRIN A, SMYTH M R. In situ elctropolymerised silica-polyaniline core-shell structures: Electrode modification and enzyme biosensor enhancement [J]. Electrochimica Acta, 2007, 52(5): 1865-1870.
[7] WANG Li-li, MA Xu-cun, QI Yun, JIANG Peng, JIAO Jian, BAO Xin-he, JIA Jin-feng, XUE Qi-kun. Controlled growth of uniform silver clusters on HOPG [J]. Journal of Chinese Electron Microscopy Society, 2005, 24(1): 1-5. (in Chinese)
[8] ZHANG Hui, ZHANG Zhen, YAO Yun-xi, TAN Da-li, BAO Xin-he. Growth and characterization of platinum nanoclusters on modified HOPG surfaces [J]. Journal of Chinese Electron Microscopy Society, 2006, 25(4): 287-292. (in Chinese)
[9] IGNACIO L S, DONG C L, YOUNG D K. Ag nanoparticles on highly ordered pyrolytic graphite (HOPG) surfaces studied using STM and XPS [J]. Surface Science, 2005, 588(1/3): 6-18.
[10] ZHANG J D, WANG E K. STM investigation of HOPG superperiodic features caused by electrochemical pretreatment [J]. J Electroanal Chem, 1995, 399(1/2): 83-89.
[11] MOHAMED S E D, TADASHI S, TAKEO O. Oxygen reduction at Au nanoparticles electrodeposited on different carbon substrates [J]. Electrochimica Acta, 2006, 52(4): 1792-1798.
[12] HATHCOCK K W, BRUMFIELD J C, GOSS C A, IRENE E A, MURRAY R W. Incipient electrochemical oxidation of highly oriented pyrolytic graphite: Correlation between surface blistering and electrolyte anion intercalation [J]. Anal Chem, 1995, 67(13): 2201-2206.
[13] GOSS C A, BRUMFIEDL J C, IRENE E A, MURRAY R W. Imaging the incipient electrochemical oxidation of highly oriented pyrolytic graphite [J]. Anal Chem, 1993, 65(10): 1378-1389.
[14] TANG Z Y, LIU S H, DONG S J, WANG E K. Electrochemical synthesis of Ag nanoparticles on functional carbon surfaces [J]. J Electroanal Chem, 2001, 502(1/2): 146-151.
[15] GUNTER P, NIEMANTSVERDRIET J W, RIBEIRO F H, SOMORJAI G A. Surface science approach to modeling supported catalysts [J]. Catal Rev Sci Eng, 1997, 39(1/2): 77-168.
[16] DOWNARD A J. Electrochemically assisted covalent modification of carbon electrodes [J]. Electroanalysis, 2000, 12(14): 1085-1096.
[17] PINSON J, PODVORICA F. Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts [J]. Chem Soc Rev, 2005, 34(5): 429-439.
[18] LEI Ting. Preparation of novel core-shell nanoparticles by electrochemical synthesis [J]. Trans Nonferrous Met Soc China, 2007, 17(6): 1343-1346.
[19] BOXLEY C J,WHITE H S, LISTER T E,PINHERO P J. Electrochemical deposition and reoxidation of Au at highly oriented pyrolytic graphite: Stabilization of Au nanoparticles on the upper plane of step edges [J]. J Phys Chem B, 2003, 107(2): 451-458.
[20] LIU S, TANG Z, SHI Z, NIU L, WANG E K,DONG S J. Electrochemical preparation and characterization of silicotungstic heteropolyanion monolayer electrostatically linked aminophenyl on carbon electrode surface [J]. Langmuir, 1999, 15(21): 7268-7275.
[21] SCHMIDT U, DONTEN M, OSTERYOUNG J G. Gold electrocrystallization on carbon and highly oriented pyrolytic graphite from concentrated solutions of LiCl [J]. J Electrochem Soc, 1997, 144(6): 2013-2021.
[22] DIAZ M A, KELSALL G H, WELHAM N J. Electrowinning coupled to gold leaching by electrogenerated chlorine: I. Au(III)-Au(I)/Au kinetics in aqueous Cl2/Cl- electrolytes [J]. J Electroanal Chem, 1993, 361(1/2): 25-38.
[23] INZELT G. Simultaneous chronoamperometric and quartz crystal microbalance studies of redox transformations of polyaniline films [J]. Electrochim Acta, 2000, 45(22/23): 3865-3876.
[24] DINH H N, BIRSS V I. Characteristics of the polyaniline anodic pre-peak[J]. Electrochimica Acta, 1999, 44(26): 4763-4771.
[25] JIAO L S, WANG Z J, NIU L, SHEN J, YOU T Y, DONG S J, IVASKA A. In situ electrochemical SERS studies on electrodeposition of aniline on 4-ATP/Au surface [J]. Journal of Solid State Electrochemistry, 2006, 10(11): 886-893.
[26] JUKKA L, KARI K, MINNA M, TAPIO O, JOUKO K. Electrochemical post self-assembly transformation of 4-aminothiophenol monolayers on gold electrodes [J]. Langmuir, 1998, 14(7): 1705-1715.
[27] HAYES W A, SHANNON C. Electrochemistry of surface-confined mixed monolayers of 4-aminothiophenol and thiophenol on Au [J]. Langmuir, 1996, 12(5): 3688-3694.
Foundation item: Project(50721003) supported by the Creative Research Group of National Natural Science Foundation of China; Project(50825102) supported by the National Science Fund for Distinguished Young Scholars, China
Corresponding author: LEI Ting; Tel: +86-13203176590; E-mail: tlei@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(10)60647-4


