
J. Cent. South Univ. (2020) 27: 1373-1385
DOI: https://doi.org/10.1007/s11771-020-4373-3
Comparison of leaching of bornite from different regions mediated by mixed moderately thermophilic bacteria
CAO Li-bo(曹莅波)1, 2, HUANG Zhi-hua(黄志华)2, SUN Xin(孙欣)1, 2, JIN Kai(金凯)1, 2,
CHANG Ke-xin(常可欣)1, 2, QIN Wen-qing(覃文庆)1, 2, QIU Guan-zhou(邱冠周)1, 2,
WANG Jun(王军)1, 2, ZHANG Yan-sheng(张雁生)1, 2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biohydrometallurgy of Ministry of Education, Central South University,Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Bioleaching experiments combined with X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and scanning electron microscopy (SEM) were conducted to investigate three kinds of bornites from different regions leached by moderately thermophilic mixed bacteria of Leptospirillum ferriphilum YSK, Acidithiobacillus caldus D1 and Sulfobacillus thermosulfidooxidans ST. The results of bioleaching experiments showed that the leaching efficiency and the redox potential were significantly increased. The copper extraction efficiencies of three kinds of bornite maintained rapid growth until around the 12th day and no longer increased after the 18th, reaching 83.7%, 96.5% and 86.6%, respectively. The XRD results of the leaching residue indicated that three kinds of bornites all produced jarosite in the late stage of leaching, and the leaching residues from of Daye Museum and Yunnan Geological Museum contained a mass of elemental sulfur. XPS analysis and scanning electron microscopy experiments showed that the surface of mineral particles was jarosite and the copper in the leaching residue was almost dissolved.
Key words:
bornite; bioleaching; thermophilic mixed bacteria; XPS; XRD;
Cite this article as:
CAO Li-bo, HUANG Zhi-hua, SUN Xin, JIN Kai, CHANG Ke-xin, QIN Wen-qing, QIU Guan-zhou, WANG Jun, ZHANG Yan-sheng. Comparison of leaching of bornite from different regions mediated by mixed moderately thermophilic bacteria [J]. Journal of Central South University, 2020, 27(5): 1373-1385.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4373-31 Introduction
As a mineral discovered and used more than 6000 years ago, copper plays a leading role in human development [1-3]. In recent decades, with the development of technology, the consumption of copper concentrate has increased rapidly over time, resulting in the continuous reduction of high-grade copper ore reserves [4-6]. Therefore, it is urgent to recover copper from low-grade copper sulfide ore. Biohydrometallurgy has received wide attention due to its high eco-friendly and low energy consumption compared to the conventional beneficiation-pyrometallurgy process [7-10]. Bornite is often associated with chalcopyrite, and the bornite is second only to chalcopyrite and chalcocite in copper reserves [11-14]. However, chalcopyrite and bornite are limited to laboratory- scale research and small-scale pilot plant tests due to their high lattice energy, easy passivation, and low leaching efficiency [15-18]. There are very few biohydrometallurgical applications in the industry. Thus, the study of bioleaching of bornite has important industrial significance for the industrial recovery of copper in low-grade copper sulfide ore.
At present, the research on the bornite mainly revolves around the intermediate products of the acid leaching, leaching process and the leaching kinetics. BEVILAQUA et al [19] studied the oxidation of bornite (Cu5FeS4) by Acidithiobacillus ferrooxidans by oxygen uptake and shake flasks experiments. After 2 d of reaction in an acid solution, no residual bornite was detected by X-ray diffraction analysis, indicating that the chemical and biological oxidation of the bornite was relatively fast. During acid consumption, the bornite dissolved under chemical and biological action, producing a new solid phase which was identified as the secondary covellite. When the natural chalcopyrite samples were tested in the corresponding experiments, a small amount of chalcopyrite and pyrite were found to indicate that they were more stubborn for acid leaching and bacterial oxidation. WANG et al [13] studied the effects of pyrite on the bioleaching of bornite by bioleaching and electrochemical experiments. The results show that the presence of pyrite significantly increases the copper leaching rate to 95.9%, and the addition of pyrite also reduces acid consumption during the bioleaching process. Electrochemical test results indicate that there is a galvanic effect between the bornite and pyrite, and the bornite is more likely to produce oxidation and reduction. Tafel plot and galvanic corrosion tests manifest that the addition of pyrite accelerates the dissolution of the bornite. Based on this experiment, a mechanical model that how the pyrite accelerated bornite dissolution is proposed. ZHAO et al [15, 20] investigated the difference in leaching of chalcopyrite and bornite by moderately thermophilic. The results present that there is a synergistic effect between chalcopyrite and bornite in the process of bioleaching. The addition of the bornite keeps the potential of the solution in the range of 390-480 mV for a long time, which is beneficial to the dissolution of chalcopyrite. BEVILAQUA et al [21, 22] studied the bioleaching process of the bornite by electrochemical experiments. The results reveal that the Acidithiobacillus ferrooxidans could accelerate the corrosion of the bornite during the leaching process, and the surface of the bornite was found to be blunt by electrochemical impedance spectroscopy (EIS). The passivation film was proven by cyclic voltammetry data.
Biohydrometallurgy technology has environmental and economic advantages over the conventional beneficiation-pyrometallurgy process [23-25]. However, the bioleaching mechanism of bornite is not fully ascertained. Different microbial strains can produce different dissolution efficiencies and dissolution pathways in the bioleaching of sulfide minerals [26-28]. So, whether different types of leaching microorganisms and bornite from different origins would affect the copper leaching process is open question. Therefore, in the present work, the leaching of bornite from different origin by three kinds of moderately thermophilic bacteria was conducted to investigate whether three mixed bacteria and the bornite from different origins influence the bioleaching of bornite. Further research on bornite bioleaching is important and is still required to approach the industrial application.
2 Materials and methods
2.1 Materials
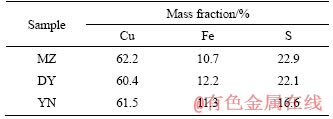
Three types of bornites are from Meizhou Geological Museum, Daye Museum, and Yunnan Geological Museum, respectively, which are named MZ, DY, and YN. Three kinds of mineral samples were crushed and manually selected, and after grinding and mineral sieving, pure minerals of 200 mesh (0.74 mm) bornite were obtained. The results of X-ray diffraction analysis (XRD) (Figure 1) indicate that the main phases in three samples are all bornite with no obvious impurities. The chemical analysis of three main elements of Cu, Fe, and S in three kinds of bornites is conducted by X-ray fluorescence as shown in Table 1. According to the theoretical grade (63.3%) of the Cu element of bornite, the purities of three bornites are calculated as 98.26%, 95.42% and 97.16%, respectively, which are all of high purity. All can be used as a pure mineral for experiments.
Three moderately thermophilic microorganisms, including Leptospirillum ferriphilum YSK, Acidithiobacillus caldus D1 and Sulfobacillus thermosulfidooxidans ST were obtained from the Key Laboratory of Biohydrometallurgy of Ministry of Education,China. All of them were cultured in 9K medium, which consisted of (NH4)2SO4 (3.0 g/L), K2HPO4 (0.5 g/L), MgSO4·7H2O (0.5 g/L), KCl (0.1g/L) and Ca(NO3)2 (0.01 g/L) [29]. Microorganisms were cultured in a constant temperature shaking incubator at 45°C, 170 r/min and the pH was maintained at about 1.7 with 10% sulfuric acid solution during the cultivation. The cell concentration of moderately thermophilic mixed bacteria was observed with a microscope every two days. The resulting culture which was filtered, centrifuged and concentrated when the microorganism grew to the logarithmic phase and the cell concentration exceeded 1.0×107 cells/mL, was used as inoculum for the bioleaching experiments.
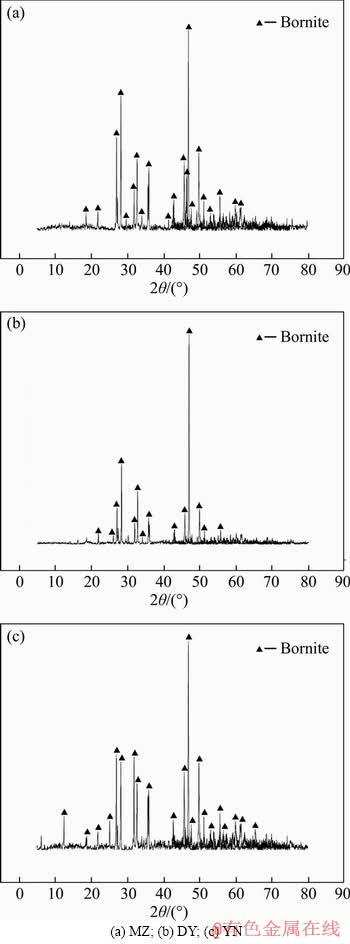
Figure 1 X-ray powder diffraction analyses of three kinds of bornite samples:
Table 1 Chemical element analysis of three chalcopyrite samples used in the experiment
2.2 Methods
250-mL Erlenmeyer flasks were used as culture vessels for bioleaching experiments. After autoclaving the 9K medium under pressure of 0.1 MPa, 121 °C for 30 min and sterilizing the mineral sample by ultraviolet separately, 90 mL of 9K medium, 10 mL of bacterial cultures and 2 g of bornite were added to the Erlenmeyer flask. The slurry concentration was 2% (W/V). The initial pH of the solution was adjusted to 1.7 with 10% sulfuric acid. All flasks were placed in a constant temperature shaking incubator at 45 °C and rotation speed of 170 r/min.
During the leaching process, the pH and ORP of the leaching solution were measured by a pH meter (PHSJ-4A) and a potentiometer (BPP922). The concentration of Fe2+ and Cu2+were measured by inductively coupled plasma-atomic emission spectrometer (ICP-AES) and the bacterial numbers were counted with an Olympus microscope (BX43). The water evaporated during leaching was supplemented with the same amount of distilled periodically, and the solution consumed by sampling analysis was compensated by sterile 9K medium. When the leaching experiment was done, all the solutions were filtered through a neutral filter paper, and the slag was washed 3 times with the adjusted solution having a pH of 1.70 and dried in a vacuum drying oven at room temperature for 24 h for the corresponding detection.
The XRD analysis of mineral samples was examined by Bruker D8 Advance X-ray diffractometer (Cu Kα, λ=1.5406 A), setting an operating voltage of 40 kV, current of 40 mA, steps of 0.004°, a step time of 28.5 s and temperature of 25 °C. A Thermo Fisher Scientific ESCALAB 250Xi photoelectron spectrometer was used to analyze the surface of the bornite samples.
3 Results and discussion
3.1 Leaching of sterile system
3.1.1 Parameter changes of sterile system
Figure 2 shows the copper extraction rate and the ORP of three different origin bornites in the sterile leaching process at pH=1.7. It can be seen from Figure 2(a) that under the acid leaching conditions, the copper extraction rate of three kinds of bornite has experienced two similar periods: the steady rise period (day 1 to day 8) and the stationary period (day 9 to the end of the leaching). After analyzing the ORP curve of bornite (Figure 2(b)), it can be seen that the ORP changes of bornites during the acid leaching process are not obvious and have been maintained at around 360 mV. The total amount of supplemental sulfuric acid is similar during the whole bioleaching process (Figure 2(c)), which indicate that the leaching process of bornite was an acid-consuming reaction. Therefore, the pH of solution increases (Figure 2(d)). At the end of leaching, the change of pH decreases. Analyzing the physicochemical parameters of leaching, it was obviously found that three kinds of bornites exhibited consistent leaching behavior and leachability under sterile acid leaching conditions. The copper extraction rates of bornite MZ, DY and YN were 28.7%, 31.8% and 29.7%, respectively.
3.1.2 Analysis of leaching residue
To further explore the composition of the sterile leaching residue, SEM analysis was utilized to analyze the surface of three kinds of bornites. Figure 3 shows the surface morphology results of bornites from three different regions. It can be seen that after the aseptic acid leaching, the surface characteristics of three kinds of bornite leaching slags are similar, but the surface of YN leaching slag is the smoothest and the edges are clear; the corrosion of MZ and DY porphyrite is more serious. After magnifying observation multiple, it is easy to find out that MZ and DY bornites have scaly materials covering the mineral surface.
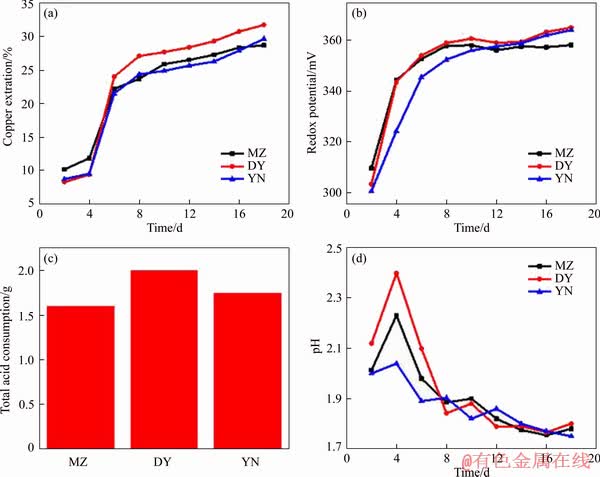
Figure 2 Variations of copper extraction (a), redox potential (b), total acid consumption (c) and pH (d) during sterile leaching of three kinds of bornites
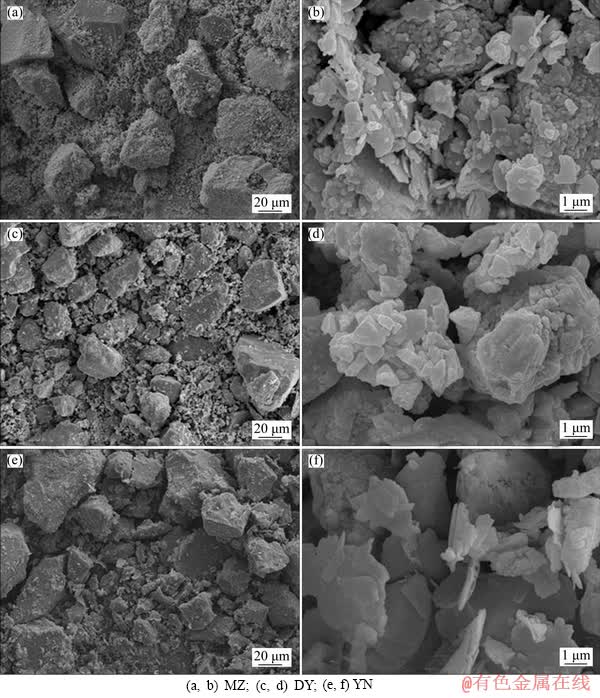
Figure 3 Surface morphology of acid leaching residues of three kinds of bornite samples:
In order to further analyze the composition of the surface material of the leaching slag, X-ray photoelectron spectroscopy (XPS) analysis was carried out on the leaching slag of three kinds of bornite. Figure 4 shows the full spectra of three kinds of bornite leaching slag. The corresponding peaks are shown in Table 2. The Cu 2p3/2, Cu 2p1/2 and Cu LMM peaks of three kinds of bornite leaching slags are obvious and the binding energies of the respective peaks are similar. According to the binding energy of Cu+ in bornite is 932.0-932.9 eV, the main compounds are Cu2O (932.4 eV), CuS (932.0-932.4 eV) and Cu2S (932.9 eV). The binding energy range of Cu2+ is 933.0-933.8 eV [30], and studies have shown that the Cu LMM binding energy of CuS is 568.5 eV [31]. According to the binding energy analysis, the main copper- containing material on the surface of the leaching slag after acid leaching of three kinds of bornite is CuS [32]. The S 2p XPS spectra of MZ, DY, YN are shown in Figure 5. The binding energy and full width at half maximum of each peak are listed in Table 3. The results show that there are three peaks in the leaching slag of three kinds of bornites. By analyzing the binding energy and the full width at half maximum of each peak, the peak near 161.3 eV corresponds to monosulfide (S2-) [33, 34]; the peak near 163.4 eV corresponds to polysulfide (Sn2-) [35, 36]; the peak near 168.5 eV corresponds to the sulfate substance (SO42-) [37, 38]. By fitting the composition ratio of sulfur-containing substances on the surface of three kinds of bornite leaching slag, the results are shown in Figure 6. The results show that the surface materials of three kinds of bornite leaching slag are monosulfide (S2-) and polysulfide (Sn2-) and SO42-, and the proportion of polysulfide (Sn2-) is slightly higher than that of monosulfide (S2-).
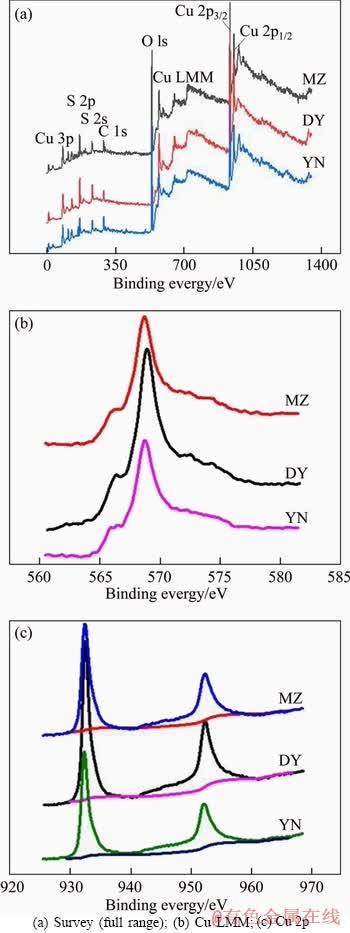
Figure 4 XPS spectra of acid leaching residues of three kinds of bornite:
Table 2 Binding energy of Cu 2p and Cu LMM peaks of acid leaching residues of three kinds of bornites
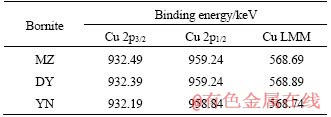
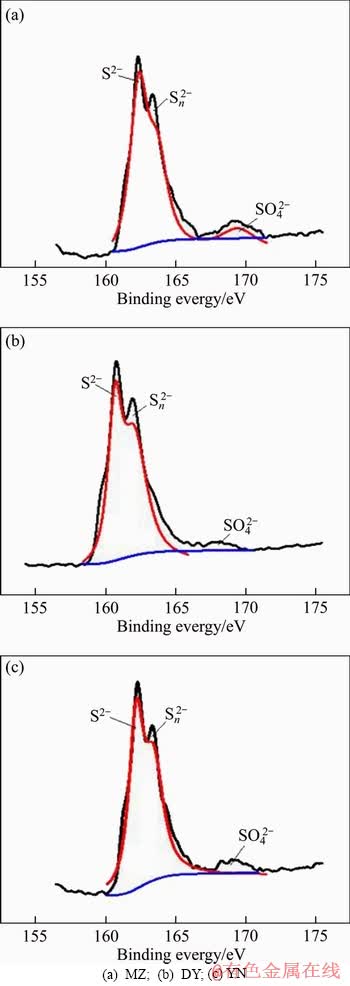
Figure 5 XPS spectra of S 2p peak of acid leaching residues of three kinds of bornites:
Table 3 Binding energy and FWHM of S 2p3/2 peaks of acid leaching residues of three kinds of bornite
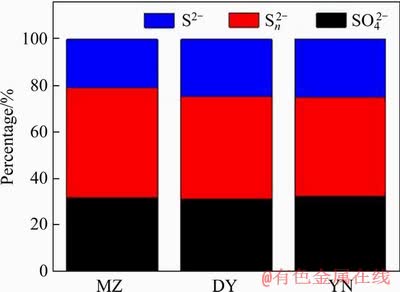
Figure 6 Ratio of composition of sulfur-containing substances on surface of leaching slag
3.2 Leaching of biological system
3.2.1 Parameter changes of biological system
Figure 7 shows the variation of copper extraction rate and the ORP of three bornites from different origins during bioleaching by moderately thermophilic mixed bacteria. In the first 4 days of bioleaching, the copper leaching rate of three kinds of bornite increased slowly. Leaching mainly depends on the original Fe3+ oxidation and acid leaching in the leaching solution. The bacteria grew slowly in the lag phase, and the concentration of the bacteria and the concentration of Fe3+ in the solution are low, resulting in low redox potential in the leaching system. From day 4 to day 12, the moderately thermophilic mixed bacteria began to grow rapidly into the logarithmic phase (Figure 7(d)), and the bacterial cell concentration increased. The bacterial metabolic activity increased rapidly, and the ability to oxidize Fe2+ increased, so the rate of Fe3+ generated is much higher than consumption of Fe3+ in the biological system and the total iron concentration in the bioleaching system began to increase gradually (Figure 7(c)). The redox potential of bornite also began to increase rapidly, and the ORP was in 350-470 mV (Figure 7(b)). The bornites from three different regions experienced a process that the leaching rate was rapidly increased (Figure 7(a)). Therefore, the potential of 350-470 mV is favourable for leaching. It is consistent with previous studies on the effect of potential on the leaching rate of bornite that the suitable potential range for leaching of bornite is 370-470 mV [39, 40].
In the later stage of bioleaching (day 12 to day 18), the leaching rates of MZ and YN were stable, 83.7% and 86.6%, respectively. The leaching rate of DY increased to 96.7% slowly, and the leaching rate of DY was slightly higher than MZ or YN bornite. The energy substance that can be used by bacteria in the late stage of bioleaching was consumed. Therefore, the concentration of bacteria in the leaching solutions began to decrease after reaching the stable period (Figure 7(d)); in the later stage of leaching, the biological leaching system began to form precipitates, such as jarosite, and the consumption of Fe in the solution is more than the production of Fe, resulting in a decrease in the total iron concentration (Figure 7(c)).
3.2.2 Analysis of bioleaching residue
Figure 8 shows the XRD analysis of three kinds of bornite leached residues by moderately thermophilic mixed bacteria. The results show that the leaching slag phase of three kinds of bornite all produced jarosite and sulfur. Besides, the presence of quartz was detected in the leaching residue, and quartz was not detected in the ore. It may be due to the detection limit of quartz. After the leaching, the content of the bornite is reduced, and the percentage of quartz is increased indirectly to the detection limit, so it can be detected. This is also consistent with the elemental analysis results that the bornite contains 0.026 Si.
Scanning electron microscopy analysis of the surface morphology of the bornite bioleaching slag is shown in Figure 9. The analysis shows that after the leaching by the moderately thermophilic mixed bacteria, three kinds of leaching slags have obvious surface characteristics: Three kinds of bornite are severely corroded; the surface is not flat; the edges and corners are not obvious. After magnifying observation multiples, it is found that the scaly-like substances are closely and regularly covered on the mineral surface and should be jarosite. The strong oxidation of bacteria and the low pH of the solution (pH<2.5) inhibit the formation of substances, such as thiosulfate [81-83]. So, during the bioleaching process by moderately thermophilic mixed bacteria, jarosite was formed in three kinds of bornite, and the reaction process is as follows [41].
3Fe2(SO4)3+12H2O+A2SO4→2AFe3(SO4)2(OH)6↓+6H2SO4 (1)
where A represents a monovalent ion such as K+, Na+, NH4+.
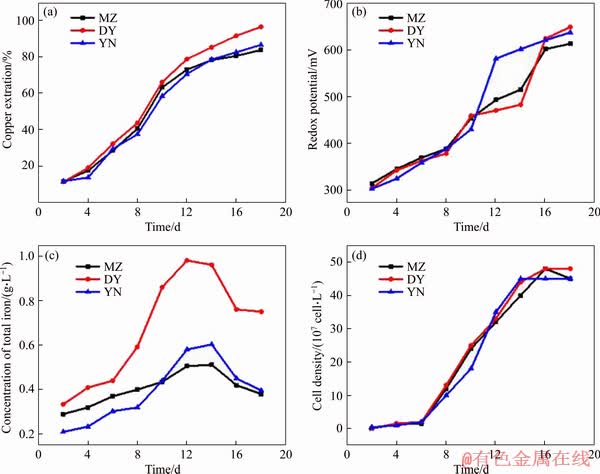
Figure 7 Variations of copper extraction (a), redox potential (b), concentration of total iron (c) and cell density (d) during bioleaching of three kinds of bornite
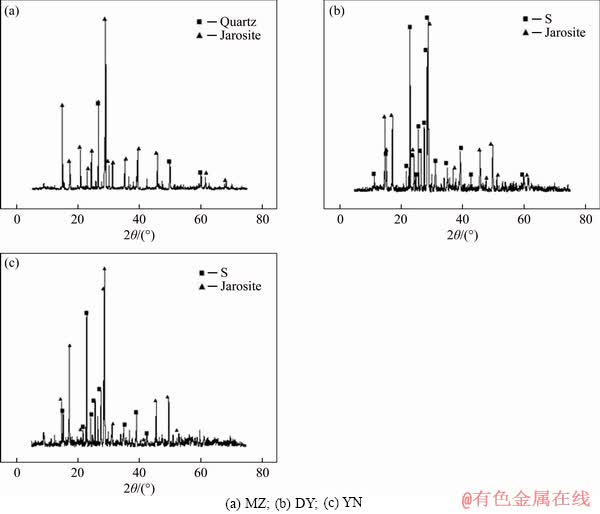
Figure 8 XRD analyses of three kinds of bornite bioleaching residue:
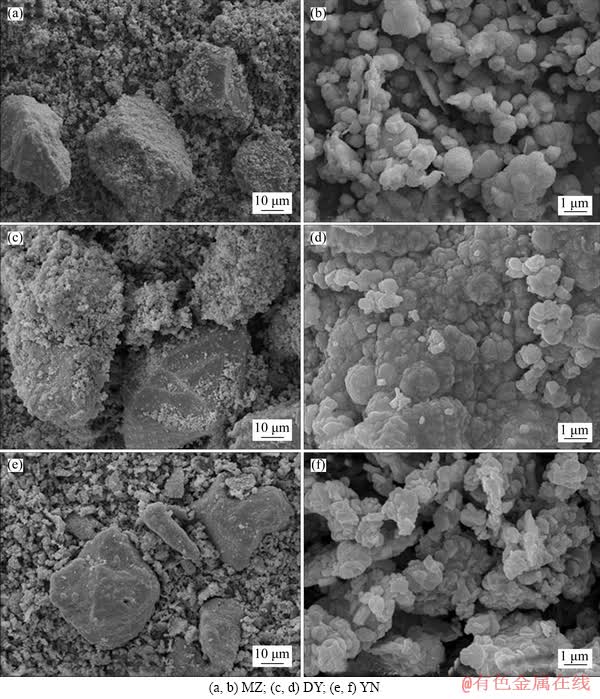
Figure 9 Surface morphology of bioleaching residues of three kinds of bornite samples:
Figure 10 shows the XPS spectra of Cu 2p and Cu LMM peaks in the leaching slag after bioleaching. The bioleaching residue of three kinds of bornite showed only a weak Cu peak and without obvious peaks. The reason for this phenomenon may be that three kinds of bornite are completely leached, and the copper in the bioleaching slag has substantially dissolved.
The S 2p3/2 XPS spectra of bioleaching residue are shown in Figure 11. The results show that there are three peaks in three kinds of bornite bioleaching residue. By analyzing the binding energy of each peak, it is known that the peak near 160.1 eV corresponds to monosulfide (S2-), and the peak near 163 eV corresponds to polysulfide (Sn2-). The peak near 168.3 eV corresponds to a sulfate substance (SO42-).
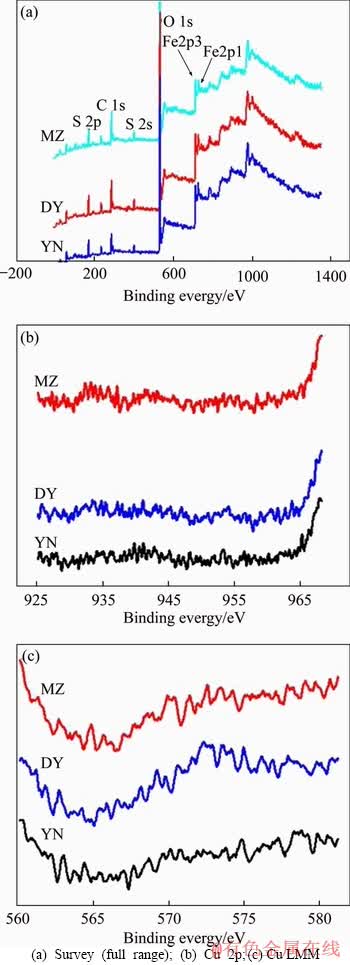
Figure 10 XPS spectra of bioleaching residues of three kinds of bornite:
The composition ratio of the sulfur-containing substances on the surface of three kinds of bornite bioleaching residue is shown in Figure 12. The results show that the surface materials of three kinds of bornite bioleaching residues are mainly S2-, Sn2- and SO42- and the sulphate substances (SO42-) on the surface of residue account for the majority. This is related to jarosite covering the surface of mineral particles. Because XPS analysis can only analyze elements with surface nanometer thickness, jarosite masked the detection of sulfur in the inner layer [42]. The composition ratios of the sulfur content of three kinds of bornite are similar. They contain similar proportions of polysulfide (Sn2-) and the proportion of polysulfide (Sn2-) is slightly more than that of monosulfide (S2-).
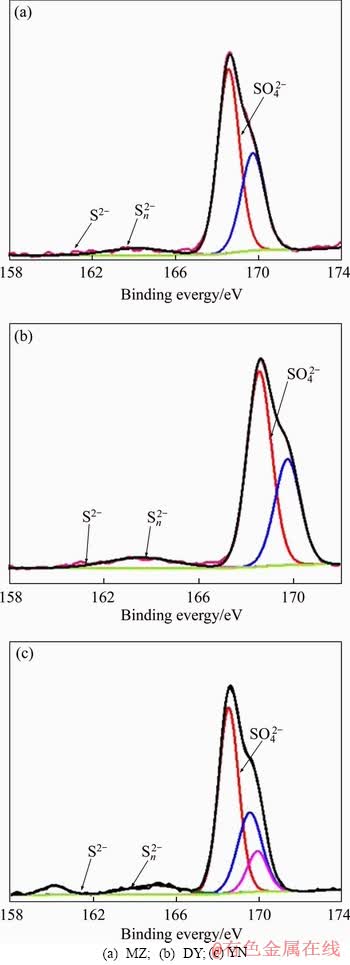
Figure 11 Fitting analyses of S 2p peaks of bioleaching residues of three kinds of bornite:
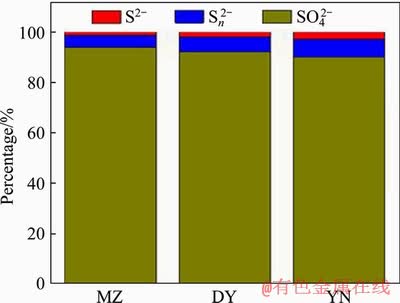
Figure 12 Sulfur species on surface of bioleaching residues of three kinds of bornite
4 Conclusions
1) Under the conditions of sterile acid leaching, three bornites from different origins showed consistent leaching behavior and leachability during the 18-day leaching process. The copper extraction efficiencies of bornite were 28.7%, 31.8%, and 29.7%, respectively. There was no significant difference. The leaching rates of three kinds of bornite MZ, DY and YN bioleaching by moderately thermophilic mixed bacteria were 83.7%, 96.5%, and 86.6%, respectively. The leaching rate of DY bornite was slightly higher than that of MZ and YN bornite. Compared with sterile control, the presence of moderately thermophilic mixed bacteria significantly accelerated the leaching of bornite.
2) During the bioleaching process by moderately thermophilic mixed bacteria, the leaching rate of bornite MZ, DY and YN all experienced a process that was stable first and then rapidly increased and finally stabilized. From the 12th day, the leaching rate of three kinds of bornite began to stabilize. The potential of the solution was maintained in the favorable leaching interval (370-470 mV) for a long time, and then copper continued to dissolve. Three kinds of bornites MZ, DY and YN showed higher leaching rate.
3) After bioleaching by moderately thermophilic mixed bacteria, the leaching residues of MZ, DY and YN of bornite were severely corroded, the surface was uneven, and the scaly material tightly and regularly covered the mineral surface which was jarosite. And the leaching residues of three kinds of bornite mainly contain sulfur, monosulfide (S2-) and polysulfide (Sn2-) was less.
References
[1] PRADHAN N, NATHSARMA K C, RAO K S, SUKLA L B, MISHRA B K. Heap bioleaching of chalcopyrite: A review [J]. Minerals Engineering, 2008, 21(5): 355-365. DOI: 10.1016/j.mineng.2007.10.018.
[2] HONG Mao-xin, WANG Xing-xing, WU Ling-bo, FANG Chao-jun, HUANG Xiao-tao, LIAO Rui, ZHAO Hong-bo, QIU Guan-zhou, WANG Jun. Intermediates transformation of bornite bioleaching by Leptospirillum ferriphilum and Acidithiobacillus caldus [J]. Minerals, 2019, 9(3): 159. DOI: 10.3390/min9030159.
[3] YANG Cong-ren, QIN Wen-qing, ZHAO Hong-bo, WANG Jun, WANG Xing-jie. Mixed potential plays a key role in leaching of chalcopyrite: Experimental and theoretical analysis [J]. Industrial & Engineering Chemistry Research, 2018, 57(5): 1733-1744. DOI: 10.1021/acs.iecr.7b02051.
[4] NORGATE T, JAHANSHAHI S. Low grade ores-Smelt, leach or concentrate? [J]. Minerals Engineering, 2010, 23(2): 65-73. DOI: 10.1016/j.mineng.2009.10.002.
[5] WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review [J]. Hydrometallurgy, 2006, 84: 81-108. DOI: 10.1016/ j.hydromet.2006.05.001.
[6] ZHAO Chun-xiao, YANG Bao-jun, WANG Xing-xing. Catalytic effect of visible light and Cd2+ on chalcopyrite bioleaching [J]. Transactions of Nonferrous Metals Society of China, 2020, 30(4): 1078-1090. DOI: 10.1016/S1003- 6326(20)65279-7.
[7] KHOSHKHOO M, DOPSON M, SHCHUKAREV A, SANDSTROM A. Chalcopyrite leaching and bioleaching: An X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution [J]. Hydrometallurgy, 2014, 149: 220-227. DOI: 10.1016/j.hydromet.2014.08.012.
[8] ZHANG Yan-sheng, QIN Wen-qing, WANG Jun, ZHEN Shi-jie, YANG Cong-ren, ZHANG Jian-wen, NAI Shao-shi, QIU Guan-zhou. Bioleaching of chalcopyrite by pure and mixed culture [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(6): 1491-1496. DOI: 10.1016/s1003- 6326(09)60031-5.
[9] WU Ling-bo, YANG Bao-jun, WANG Xing-xing, WU Bai-qiang, HE Wan-li, GAN Min, QIU Guan-zhou, WANG Jun. Effects of single and mixed energy sources on Intracellular nanoparticles synthesized by Acidithiobacillus ferrooxidans [J]. Minerals, 2019, 9(3): 163. DOI: 10.3390/ min9030163.
[10] FANG Chao-jun, YU Shi-chao, WANG Xing-xing, ZHAO Hong-bo, QIN Wen-qing, QIU Guan-zhou, WANG Jun. Synchrotron radiation XRD investigation of the fine phase transformation during synthetic Chalcocite acidic ferric sulfate leaching [J]. Minerals, 2018, 8(10): 461. DOI: 10.3390/ min8100461.
[11] PESIC B, OLSON F A. Leaching of bornite in acidified ferric chloride solutions [J]. Metallurgical Transactions B, 1983, 14(4): 577-588. DOI: 10.1007/BF02653944.
[12] KOTO K, MORIMOTO N. Superstructure investigation of bornite, Cu5FeS4, by the modified partial Patterson function [J]. Acta Crystallographica, 2010, 31(9): 2268-2273. DOI: 10.1107/ S0567740875007376.
[13] WANG Xing-xing, LIAO Rui, ZHAO Hong-bo, HONG Mao-xing, HUANG Xiao-tao, PENG Hong, WEN Wen, QIN Wen-qing, QIU Guan-zhou, HUANG Cao-ming. Synergetic effect of pyrite on strengthening bornite bioleaching by Leptos-pirillum ferriphilum [J]. Hydrometallurgy, 2018, 176: 9-16. DOI: 10.1016/j.hydromet.2017.12.003.
[14] YANG Bao-jun, LIN Mo, FANG Jing-hua, ZHANG Rui-yong, LUO Wen, WANG Xing-xing, LIAO Rui, WU Bai-qiang, WANG Jun, GAN Min. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans [J]. Science of the Total Environment, 2020, 698: 134175. DOI: 10.1016/ j.scitotenv.2019.134175.
[15] ZHAO Hong-bo, WANG Jun, GAN Xiao-wen, QIN Wen-qing, HU Ming-hao, QIU Guan-zhou. Bioleaching of chalcopyrite and bornite by moderately thermophilic bacteria: An emphasis on their interactions [J]. International Journal of Minerals, Metallurgy, and Materials, 2015, 22(8): 777-787. DOI: 10.1007/s12613-015-1134-7.
[16] WANG Jun, TAO Lang, ZHAO Hong-bo, HU Ming-hao, ZHENG Xi-hua, PENG Hong, GAN Xiao-wen, XIAO Wei, CAO Pan, QIN Wen-qing. Cooperative effect of chalcopyrite and bornite interactions during bioleaching by mixed moderately thermophilic culture [J]. Minerals Engineering, 2016, 95: 116-123. DOI: 10.1016/j.mineng.2016.06.006.
[17] CHANG Ke-xin, ZHANG Yan-sheng, ZHANG Jia-ming, LI Teng-fei, WANG Jun, QIN Wen-qing. Effect of temperature- induced phase transitions on bioleaching of chalcopyrite [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(10): 2183-2191. DOI: 10.1016/S1003-6326(19)65124-1.
[18] HUANG Xiao-tao, ZHAO Hong-bo, ZHANG Yan-sheng, LIAO Rui, WANG Jun, QIN Wen-qing, QIU Guan-zhou. A strategy to accelerate the bioleaching of chalcopyrite through the goethite process [J]. Mining, Metallurgy & Exploration, 2018, 35(4): 171-175. DOI: 10.19150/mmp.8593.
[19] BEVILAQUA D, GARCIA O, TUOVINEN O. Oxidative dissolution of bornite by Acidithiobacillus ferrooxidans [J]. Process Biochemistry, 2010, 45(1): 101-106. DOI: 10.1016/ j.procbio.2009.08.013.
[20] ZHAO Hong-bo, WANG Jun, TAO Lang, CAO Pan, YANG Cong-ren, QIN Wen-qing, QIU Guan-zhou. Roles of oxidants and reductants in bio-leaching system of chalcopyrite at normal atmospheric pressure and 45 °C [J]. International Journal of Mineral Processing, 2017, 162: 81-91. DOI: 10.1016/j.minpro.2017.04.002.
[21] BEVILAQUA D, ACCIARI H A, BENEDETTI A V, FUGIVARA C S, TREMILIOSI FILHO G, GARCIA O Jr. Electrochemical noise analysis of bioleaching of bornite (Cu5FeS4) by Acidithi-obacillus ferrooxidans [J]. Hydrometallurgy, 2006, 83: 50-54. DOI: 10.1016/ j.hydromet.2006.03.037.
[22] BEVILAQUA D, ACCIARI H, ARENA F, BENEDETTI A, FUGIVARA C, TREMILIOSI FILHO G, GARCIA JUNIOR O. Utilization of electrochemical impedance spectroscopy for monitoring bornite (Cu5FeS4) oxidation by Acidithiobacillus ferrooxidans [J]. Minerals Engineering, 2009, 22: 254-262. DOI: 10.1016/j.mineng.2008.07.010.
[23] ZHAO Hong-bo, ZHANG Yi-sheng, ZHANG Xian, QIAN Lu, SUN Meng-lin, YANG Yu, ZHANG Yan-sheng, WANG Jun, KIM Hyunjung, QIU Guan-zhou. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview [J]. Minerals Engineering, 2019, 136-140: 154. DOI: 10.1016/j.mineng.2019. 03.014.
[24] NGUYEN K A, BORJA D, YOU J, HONG G, JUNG H, KIM H. Chalcopyrite bioleaching using adapted mesophilic microorganisms: Effects of temperature, pulp density, and initial ferrous concentrations [J]. Materials Transactions, 2018, 59(11): 1860-1866. DOI: 10.2320/matertrans. M2018247.
[25] ZHAO Hong-bo, HUANG Xiao-tao, HU Ming-hao, ZHANG Chen-yang, ZHANG Yi-sheng, WANG Jun, QIN Wen-qing, QIU Guan-zhou. Insights into the surface trans formation and electrochemical dissolution process of bornite in bioleaching [J]. Minerals, 2018, 8(4): 173. DOI: 10.3390/ min8040173.
[26] GERICKE M, PINCHES A, ROOYEN J. Bioleaching of a chalcopyrite concentrate using an extremely thermophilic culture [J]. International Journal of Mineral Processing, 2001, 62: 243-255. DOI: 10.1016/S0301-7516(00) 00056-9.
[27] CLARK D A, NORRIS P. Oxidation of mineral sulphides by thermophilic microorganisms [J]. Minerals Engineering, 1996, 9: 1119-1125. DOI: 10.1016/0892-6875(96)00106-9.
[28] YANG Bao-jun, ZHAO Chun-xiao, LUO Wen, LIAO Rui, GAN Min, WANG Jun, LIU Xue-duan, QIU Guan-zhou. Catalytic effect of silver on copper release from chalcopyrite mediated by Acidithiobacillus ferrooxidans [J]. Journal of Hazardous Materials, 2020, 392: 122290. DOI: 10.1016/ j.jhazmat.2020.122290.
[29] SILVERMAN M, LUNDGREN D. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. II. Manometric studies [J]. Journal of Bacteriology, 1959, 78(3): 326-331. https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC2904 34/.
[30] LI Y, KAWASHIMA N, LI J, CHANDRA A, GERSON A. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite [J]. Advances in Colloid and Interface Science, 2013, 197-198: 1-32. DOI: 10.1016/j.cis.2013.03.004.
[31] PLATZMAN I, BRENER R, HAICK H, TANNENBAUM R. Oxidation of polycrystalline copper thin films at ambient conditions [J]. The Journal of Physical Chemistry C, 2008, 112: 1101-1108. DOI: 10.1021/jp076981k.
[32] GOH S, BUCKLEY A, ROBERT N. Copper (II) sulfide? [J]. Minerals Engineering, 2006, 19: 204-208. DOI: 10.1016/ j.mineng.2005.09.003.
[33] GOH S, BUCKLEY A, LAMB R, ROSENBERG R, MORAN D. The oxidation states of copper and iron in mineral sulfides, and the oxides formed on initial exposure of chalcopyrite and bornite to air [J]. Geochimicaet Cosmochimica Acta, 2006, 70: 2210-2228. DOI: 10.1016/ j.gca.2006.02.007.
[34] GHAHREMANINEZHAD A, DIXON D G, ASSELIN E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution [J]. Electrochimica Acta, 2013, 87: 97-112. DOI: 10.1016/j.electacta.2012.07.119.
[35] ACRES R, HARMER S, BEATTIE D. Synchrotron XPS studies of solution exposed chalcopyrite, bornite, and heterogeneous chalcopyrite with bornite [J]. International Journal of Mineral Processing, 2010, 94: 43-51. DOI: 10.1016/ j.minpro.2009.11.006.
[36] HARMER S, THOMAS J, FORNASIERO D, GERSO A. The evolution of surface layers formed during chalcopyrite leaching [J]. Geochimica et Cosmochimica Acta, 2006, 70: 4392-4402. DOI: 10.1016/j.gca.2006.06.1555.
[37] KLAUBER C, PARKER A, BRONSWIJK W, WATLING H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy [J]. International Journal of Mineral Processing, 2001, 62: 65-94. DOI: 10.1016/S0301- 7516(00)00045-4.
[38] KLAUBER C. Fracture-induced reconstruction of a chalcopyrite (CuFeS2) surface [J]. Surface & Interface Analysis, 2003, 35: 415-428. DOI: 10.1002/sia.1539.
[39] CANCHO L, BLAZQUEZ M, BALLESTER A,GONZALEZ F, MUNOZ J. Bioleaching of a chalcopyrite concentrate with moderate thermophilic microorganisms in a continuous reactor system [J]. Hydrometallurgy, 2007, 87: 100-111. DOI: 10.1016/j.hydromet.2007.02.007.
[40] AHMADI A, SCHAFFIE M, PETERSEN J, SCHIPPERS A, RANJBAR M. Conventional and electrochemical bioleaching of chalcopyrite concentrates by moderately thermophilic bacteria at high pulp density [J]. Hydrometallurgy, 2011, 106: 84-92. DOI: 10.1016/j.hydromet.2010.12.007.
[41] JENSEN A, WEBB C. Ferrous sulphate oxidation using thiobacillus-ferrooxidans: A review [J]. Process Biochemistry, 1995, 30: 225-236. DOI: 10.1016/0032- 9592(95)85003-1.
[42] PEAK D, FORD R, SPARKS D. An in situ ATR-FTIR investigation of sulfate bonding mechanisms on goethite [J]. Journal of Colloid & Interface Science, 1999, 218: 289-299. DOI: 10.1006/jcis.1999.6405.
(Edited by YANG Hua)
中文导读
中等嗜热混合菌介导的不同产地斑铜矿浸出差异的比较
摘要:本文通过嗜铁钩端螺旋菌、嗜酸喜温硫杆菌以及嗜热硫氧化硫化杆菌三种中等嗜热混合菌对三种不同产地的斑铜矿进行生物浸出,结合X射线光电子能谱(XPS)、X射线衍射(XRD)和扫描电子显微镜(SEM)研究了不同产地斑铜矿浸出行为的差异性。浸出实验结果表明,与空白组相比,生物浸出组的浸出效率和氧化还原电位有显著提高。三种斑铜矿的铜浸出率在前12天一直快速增长,18天后不再增长,此时浸出率分别83.7%,96.5%,86.6%。浸出渣的XRD结果表明,三种斑铜矿在浸出后期均产生黄钾铁矾,其中大冶和云南的浸出渣中含有大量的硫元素。XPS分析和扫描电子显微镜实验结果表明,矿渣表面是黄钾铁矾,浸出渣中几乎没有铜。
关键词:斑铜矿;生物浸出;中等嗜热混合菌;XPS;XRD
Foundation item: Project(51974363) supported by the National Natural Science Foundation of China
Received date: 2019-06-30; Accepted date: 2020-04-15
Corresponding author: ZHANG Yan-sheng, PhD; Tel: +86-13808473404; E-mail: Zhangyansheng405@126.com; ORCID: 0000-0002- 4230-1640
Abstract: Bioleaching experiments combined with X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and scanning electron microscopy (SEM) were conducted to investigate three kinds of bornites from different regions leached by moderately thermophilic mixed bacteria of Leptospirillum ferriphilum YSK, Acidithiobacillus caldus D1 and Sulfobacillus thermosulfidooxidans ST. The results of bioleaching experiments showed that the leaching efficiency and the redox potential were significantly increased. The copper extraction efficiencies of three kinds of bornite maintained rapid growth until around the 12th day and no longer increased after the 18th, reaching 83.7%, 96.5% and 86.6%, respectively. The XRD results of the leaching residue indicated that three kinds of bornites all produced jarosite in the late stage of leaching, and the leaching residues from of Daye Museum and Yunnan Geological Museum contained a mass of elemental sulfur. XPS analysis and scanning electron microscopy experiments showed that the surface of mineral particles was jarosite and the copper in the leaching residue was almost dissolved.


