Trans. Nonferrous Met. Soc. China 24(2014) 3964-3970
Mechanism of exogenous selenium alleviates cadmium induced toxicity in Bechmeria nivea (L.) Gaud (Ramie)
Chun-lin WANG1,2, Yun-guo LIU1,2, Guang-ming ZENG1,2, Xin-jiang HU1,2, Yi-cheng YING1,2, Xi HU1,2, Lu ZHOU1,2, Ya-qin WANG1,2, Hua-ying LI1,2
1. College of Environmental Science and Engineering, Hunan University, Changsha 410082, China;
2. Key Laboratory of Environmental Biology and Pollution Control, Ministry of Education, Hunan University, Changsha 410082, China
Received 18 December 2013; accepted 18 April 2014
Abstract:
The protective role of exogenously supplied selenium (Se6+) on Bechmeria nivea (L.) Gaud (Ramie) subjected to cadmium (Cd) stress was studied in vitro, and the mechanism was discussed by investigating plant growth, malondialdehyde (MDA), activity of antioxidative enzymes and DNA methylation pattern. Plants grown in hydroponic culture were supplied with spraying Se (selenate, 1.2 μmol/L) and cadmium (Cd(NO3)2, 0, 3, 6 and 9 mg/L), individually or simultaneously. At low Se spraying levels, SOD activity was increased by 35.34%, 43.18%, 3.63% under 3, 6 or 9 mg/L cadmium contents, POD was increased by 12.45%, 14.14%, 3.27%, and the level of DNA methylation was decreased by 10.70%, 18.18% and 15.59%, respectively. The results confirmed that spraying low Se on ramie leaves could enhance the activity of SOD and POD, and regulate DNA methylation in ramie leaves.
Key words:
ramie; Se; Cd; DNA methylation; antioxidative enzyme; phytoremediation;
1 Introduction
Heavy metal contamination of soils mainly originates from industry or agriculture, such as smelting industries, residues from metalliferous mines, pesticide, fertilizers, and municipal compost. Since the past century, many regions worldwide suffer from heavy metal pollution due to anthropogenic activities [1]. Cd can be easily assimilated by plant roots and transported to aerial parts, thus entering into the food chain, causing health problems to animals and humans [2]. Cd can result in disorders in biochemical and physiological processes in plants and animals. Several reports showed that Cd caused oxidative stress either by inducing oxygen free radical production and reactive oxygen species (ROS) or by decreasing enzymatic and non-enzymatic antioxidants activity, due to its high affinity towards sulfur containing peptides and protein [3,4]. ROS can seriously affect photosynthesis, respiration, protein metabolism and nutrient uptake [5]. The defense system of plant to ROS was comprised of enzymes including peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathion peroxidase (GPX), glutathione reductase (GR), and other antioxidative compounds, such as glutathione (GSH) and carotenoids [6]. Moreover, previous studies showed the alteration in cytosine methylation throughout the genome, especial at specific loci induced by abiotic stress [7]. BOB et al [8] also reported that ROS may cause DNA injury. DNA methylation has been fully studied in animals, while there are little articles studying the effect of Cd on plant antioxidant enzymes and DNA methylation.
Phytoremediation is an effective solution which refers to the use of green plants for the immobilization and removal of contaminants or rendering them harmlessness. However, the key to phytoremediation is that plant can grow under the condition of high concentration of heavy metals and have efficient ability to absorb heavy metals [9]. Bechmeria nivea (L.) Gaud (Ramie) is a fibre crop and wide spread plant in China. Ramie plant has advantages of easy to survive, large biomass and developed root system. Moreover, it has strong ability to resist Cd stress [10]. In our previous researches, the uptake, distribution pattern and tolerance mechanisms of Cd in ramie were investigated. Besides, the present experiments demonstrated that spermine and Se could enhance the antioxidative ability of ramie under Cd stress, via improving chlorophyll and vigour of AsA-GSH cycle [11].
Se, an essential trace element, is often applied to phytoremediation by strengthening the anti-stress capability of plant. During last two decades, some plant species grown in Se-enriched media have shown enhanced resistance to certain abiotic stresses, e.g., drought, chilling, salinity and heavy metals stresses [12-16]. HASANUZZMAN et al [17] and SINGH et al [18] also reported that low concentration of Se exerted a positive effect on growth and stress tolerance of crop and Indian mustard. Moreover, the influence of Se on fatty acid composition, the antioxidative enzymes activity and DNA methylation in plants were also studied in some articles, which demonstrated that Se played an important role in relieving physiological toxicity induced by heavy metal stress [7,8,19]. While the protective effects of Se on plant have become a hotspot, and the protective mechanism has not been fully investigated. The protective mechanisms of Se have been studied in some papers, which reported that the possible mechanisms were: 1) Se reduced the absorption and accumulation of Cd, or removed Cd from metabolically active cellular sites, 2) the addition of Se had reduced the oxygen radicals by antioxidant enzymes and inhibited the changes of DNA methylation [16,20,21].
The focus of this study is to explore the protective mechanism of Se to Cd stress. The possible protective mechanisms of Se on ramie are discussed in this paper. The following indicators are detected: 1) the concentration of Cd in leaves, shoots and roots and the concentration of Se in leaves, 2) the content of chlorophyll and carotenoid in leaves, 3) the content of MDA in leaves and roots, and 4) the activity of SOD, POD and CAT and the DNA methylation in leaves.
2 Experimental
2.1 Plant materials and growth conditions
One-month-old ramie plants were collected from Ramie Research Institute, Hunan Agricultural University, China. Plants were acclimatized in a hydroponic system with 25% Hoagland nutrient solution. Media with 0, 3, 6 and 9 mg/L of Cd(NO3)2 were chosen to investigate the protective effect of Se (Na2SeO4) at the concentration of 0, 0.6, 1.2 and 1.8 μmol/L. Selenate was sprayed on leaves every two days [17]. Roots and leaves were harvested after four weeks, then roots were washed with 10 mmol/L CaCl2 for about 10 min to displace extra cellular Cd, and leaves were washed with deionized water. The liquid medium was aerated and renewed after every 48 h. The growth chamber conditions were: temperature 25 °C/20 °C (day/night), light intensity PAR 300 μmol/(m2·s) and relative humidity (60±5)%.
2.2 Determination of Cd and Se contents
Plant tissues were dried at 105 °C for 30 min, and then at 80 °C till constant mass. Oven dried plant samples (0.5 g) were digested with 3 mL HNO3 and 1 mL HClO4 solutions in conical flask by heating (100 °C) until it turned into transparent. Digested liquid was transferred into 100 mL flask and diluted by de-ionized water to volume for measurement. Cd was analyzed by atomic absorption spectrometry with a hollow-cathode lamp (TAS-990F, Beijing Persee, China). Se was analyzed by fluorescence atomy spectrometer (Fluoromax 4, HORIBA jobin Yvon, France).
2.3 Determination of chlorophyll and carotenoid contents
Frozen leaf tissues were homogenized in 80% ice-cold acetone in the dark, and then centrifuged at 2000 g for 10 min. Then, chlorophyll and carotenoid contents were determined spectrophotometrically on the supernatant at 646, 663 and 470 nm using a UV spectrophotometer (UV2550, Shimadzu, Japan), as described by LIU [10].
2.4 Estimation of lipid peroxidation
The MDA contents of leaves and roots were determined by using the thiobarbituric acid method [11]. Plant tissues (0.5 g), including roots and leaves, were homogenized in 10 mL 10% (w/v) TCA. The homogenate was centrifuged at 10000 r/min for 10 min. Then, 2 mL of 10% TCA containing 0.5% thiobarbituric acid (TBA) was added to 2 mL of the aliquot of the supernatant. The mixture was heated in a water bath at 95 °C for 30 min, and then cooled at room temperature and centrifuged twice at 15000 r/min at 4 °C for 15 min. The specific absorbance (at 532 nm) of the extract and the non-specific background absorbance (at 600 nm) were measured with the UV spectrophotometer (UV2550, Shimadzu, Japan). The MDA content was calculated as nmol/g fresh mass.
2.5 Analyses of antioxidant enzyme activity
To extract SOD and POD, frozen leaf samples (0.5 g in fresh mass) were homogenized with mortar and pestle under chilled condition in the buffer: 50 mmol/L, pH 7.8. Phosphate-buffered saline containing 0.1% (v/v) Triton X-100, 1% (w/v) polyvinylpyrollidone (PVP) and 0.9% (w/v) NaCl was used as extraction buffer for CAT. Afterwards, the homogenate was centrifuged at 15000 r/min for 20 min at 4 °C and the supernatant was used for determining enzyme activity, which was processed at 25 °C. The contents of POD, SOD and CAT in leaves were determined using assay kit produced in Jiancheng Biotech, Nanjing, China. These experiments were carried out strictly followed the instructions of manufactory. And the samples were measured with ELIASA (Thermo Scientific Multiskan GO, Thermo Electron, American).
2.6 DNA extraction and hydrolysis using improved CTAB method extraction
DNA extraction and digestion were slightly adjusted in accordance with the method of PARRA et al [22]. 50 μL (100 °C, 1 h) 70% perchloric acid was added to 100 μL of DNA solution (containing about 30 μg of DNA) and hydrolyzed, the pH value was adjusted to 3-5 with KOH (1 mol/L), centrifuged 5 min at 9800 r/min after KClO4 precipitate formed. The content of 5-cytosine (5-MeC) was measured by liquid chromatograph (Agient 1100, America). The supernatant was injected to the Hypersil BDS C18 column at room temperature. The column temperature was 40 °C. The mobile phase was compounded with sodium pentanesulfonate (5 mmol/L), triethylamine (0.2%), methanol (5%) and ultrapure water. The pH value was adjusted to 5.5 using 10% phosphoric acid. The flow rate was set to 0.5 mL/min, and the wavelength of the UV detector was set to 273 nm. The contents of Cytosine (C) and 5-methylcytosine (5-MeC) were determined by comparing the peak areas of sample and standard sample. The level of DNA methylation was detected by calculating the 5-MeC/(5-MeC+C). The Cytosine and 5-methylcytosine standard sample were purchased from Sigama [23].
2.7 Statistical analyses
Data were processed preliminary using Microsoft Excel, further analyzed through analysis of variance using Origin Pro 8.0 statistical package and SPSS Ver. 13.0 (Statistical Package for Social Science for Windows, SPSS, Inc., Chicago, IL, USA). Statistical analyses were carried out by analysis of variance (ANOVA) tests. Significant differences among mean values were determined by the SPSS (Statistical Product and Service Solutions) at P<0.05.
3 Result and discussion
3.1 Cd and Se accumulation
The important quantitative indicators such as accumulation of Cd and Se in roots and leaves of ramie plants are shown in Table 1. The accumulation of Cd in leaves, shoots and roots increased obviously with the increase of Cd in nutrient solution, but reduced by supplying with Se together. Besides, the absorption of Se in leaves showed obvious downward trend with the increase of Cd concentration. The changes of Se and Cd accumulation in ramie may be ascribed to the antagonism effect between Se and Cd. Table 1 also shows that the migration percentage of Cd (the accumulation of Cd in aerial parts/the accumulation of Cd in roots) had a decrease in the group with the addition of Se. The results seem to be in good agreement with the previous study, which implied a lower translocation of Cd within the plant. It demonstrated an effective way to defend Cd stress, because the roots showed greater resistance to stress ability relative to the aerial parts of plants [24,25]. In this study, reducing the absorption of Cd and making most of Cd accumulated in the roots may be assumed as the first way of Se to defense against heavy metal stress.
Table 1 Effect of different concentrations of Cd and low concentration of Se (1.2 μmol/L) on uptake and accumulation of Cd and Se in ramie

3.2 Carotenoid and chlorophyll levels
Photosynthesis is the basic way for the synthesis of organic compounds and the source of energy. Chlorophyll, an important factor of photosynthesis, can affect the growth of plants. Carotenoids are both photosynthetic pigments and important endogenous antioxidant compounds. They play a protective role on the chlorophyll and the elimination of ROS [26]. Figure 1(a) shows that an increase of carotenoids concentration was observed, when either supplied with Cd or Se, individually or simultaneously. The results indicate that Se greatly improved the carotenoid content in the plant leaves, which may be one of the factors that Se can promote the growth of chlorophyll. As seen from Fig. 1(b), chlorophyll content in the leaves increased under 3 mg/L Cd, but decreased significantly under high concentration of Cd content. This response can be attributed to the fact that low concentration of Cd is a nutrient rather than a poison. Cd exhibits toxicity when the concentration exceeds a certain value. It was reported that Cd can affect the development of chloroplast and inhibit the photosynthesis [27]. The damage of chlorophyll may be due to the fact that Cd damages the lipid membrane of chloroplast or causes a higher oxidative [28]. However, the chlorophyll concentration had a significant (P<0.05) increase when Cd and Se were simultaneously supplied, especially at 3 and 6 mg/L Cd stresses, increased by 29.56% and 34.85%, respectively. The results may be because Cd can combine with —SH group of antioxidative enzyme which plays an important role in removing ROS [28,29]. Moreover, it also may be contributed to the fact that Cd impedes the absorption of some micronutrients which are the necessities of chlorophyll synthesis [10,30].
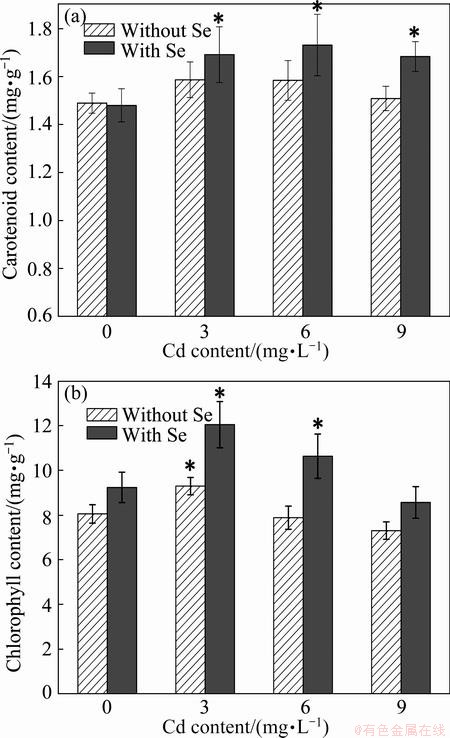
Fig. 1 Chlorophyll and carotenoid levels in leaf tissues of ramie subjected to Cd or simultaneously sprayed Se (Data are means ± SE of 3 replicates; (*), P<0.05)
3.3 Analyses of antioxidative enzyme activity
Both biotic and abiotic stresses cause oxidative damage to plants by triggering the increase of ROS. In order to alleviate the oxidative damage of important sites and maintain a homeostasis in cell, plant will start antioxidant defense system to remove ROS and relieve oxidative stress. POD, SOD and CAT are three common and important antioxidant enzymes, which play an important role of antioxidant defense system. At low Se addition level, SOD activity was increased by 35.34%, 43.18%, 3.63% under 3, 6, 9 mg/L Cd contents, POD was increased by 12.45%, 14.14%, 3.27%, respectively (Fig. 2).

Fig. 2 Antioxidant enzyme (POD, SOD, CAT) activities in leaf tissues of ramie subjected to Cd or simultaneously sprayed Se (Data are means ± SE of 3 replicates; (*), P<0.05)
A Cd-induced increase of the SOD activity was observed in Phaseolus vulgaris [31] and Juncus effusus L. [32], while a decrease was reported in Alyssum [33]. Increase in the SOD activity was due to the increase of ROS or the improvement of expression of genes encoding SOD [6]. Reduction in SOD activity was attributed to a damage of enzyme protein induced by free radicals and peroxides [19], or an inactivation of enzyme by H2O2 [34]. A Cd-induced increase in POD activity was observed in perennial ryegrass [19] and B. Napus [35], but a decrease was observed in Indian mustard [36]. Different responses of POD and SOD in different plants mean the property of antioxidant enzyme depending on the plant species. It can be seen that the activities of POD and SOD changed slightly at 9 mg/L Cd content, which may be due to the fact that the damage from Cd-stress was so serious that the plant lost the ability to repair the damaged plants. CAT is one of the major ROS scavenger, which can decompose H2O2 to water and molecular oxygen, and can enhance the removal of toxic peroxides [37], while CAT activity showed different phenomena in Fig. 2. This phenomenon may be because Se could replace Cd to combine with the SOD and POD zymoprotein (—SH or other activating groups), but this effect had not happened in CAT [38,39]. Moreover, others showed different results. They found a decrease of CAT in Phaseolus vulgaris [40], and an increase in pea plants [41]. This phenomenon may be attributed to the photo-inactivation of enzyme or may be associated with the degradation caused by induced peroxisomal proteases [34].
3.4 Lipid peroxidation analysis
Plant produces ROS through enzymatic and non-enzymatic system, which can attack the biofilm polyunsaturated fatty acids, causing lipid peroxidation, and thus forming lipid peroxides. Lipid peroxidation not only transforms active oxygen into the active chemical substance, but also amplifies the role of ROS [42]. ROS induces cell damage through the peroxidation of polyunsaturated fatty acid in biofilm, and causes cell damage by decomposing products of lipid peroxide. Therefore, the amount of MDA often reflects the extent of lipid peroxidation, which indirectly reflects the degree of cell damage in plant [43].
It can be seen that MDA content gradually increased with the increase of Cd content in the nutrition solution. With the addition of Se, MDA content declined by 11.96%, 27.55%, 11.03% in roots and declined by 13.61%, 17.73%, 13.76% in leaves (Fig. 3) respectively under 3, 6 and 9 mg/L Cd contents. The results indicated that, when ramie plants were exposed to heavy metals, plant cells accelerated the generation of ROS. And the decrease of MDA in leaf and root might be contributed to the increase of POD and SOD activities due to Se addition, which was an effective way to remove ROS [12,39]. CHEN et al [44] also reported that Se decreased MDA content. They found that the pattern appeared to be counteracted by increases in SOD activities and carotenoids contents. Figure 3 also shows that MDA content in leaves was significantly higher than that in roots, which provided an evidence that roots presented more ability to resist Cd stress.

Fig. 3 MDA contents in leaf and root tissues of ramie subjected to Cd or simultaneously sprayed Se (Data are means ± SE of 3 replicates; (*), P<0.05)

Fig. 4 DNA methylation level in leaf tissues of ramie subjected to Cd or simultaneously sprayed Se (Data are means ± SE of 3 replicates; (*), P<0.05)
3.5 Analysis of DNA methylation pattern
DNA methylation is the form of chemical modification of DNA, which can alter genetic expression without changing the DNA sequence. The effect of Se on DNA methylation in ramie leaves under Cd stress was studied from the ratio 5-MeC/(C + 5-MeC) (Fig. 4). The demethylation and methylation of DNA cytosines in specific regions of the genes or in their promoters played a crucial role in regulating gene expression during plant development [45]. Our study showed an unusual increase in DNA methylation under Cd stress, while the level of DNA methylation was decreased by 10.70%, 18.18% and 15.59% when ramie plants were sprayed simultaneously with low content of Se. In control samples, the content of 5-MeC in ramie leaves was 22.4%-22.8%. The results accorded with the previous experimental study, which reported that the contents of 5-MeC in wheat, tomato and peas were 22.4%, 23% and 23.2%, respectively [46].
The mechanisms of DNA methylation in animal have been studied thoroughly, while only little studies were performed in plant. LEE et al [47] reported that Ni declined the activity of 5-methylcytosine methyltransferase in Chinese hamster cells, but the 5-MeC content in the genome rapidly increased. HIX et al found a high share of the methyl radical and a high degree of DNA methylation [48]. A hypothesis has been proposed that the increase of DNA methylation level in leaves may be due to the accumulation of free radicals produced from the methyl stress, and methyl radicals directly attack DNA cytosine, resulting in a 5-MeC level improved [22]. A similar trend was observed in wheat young spike cells under Cu, Cd and Hg treatments at toxic concentrations [48]. However, Se addition in combination with Cd appeared to decrease the level of DNA methylation, which kept the plants away from the alternation in the abnormal methylation. The protective role of Se to resist the changes in DNA methylation pattern might be attributed to the removal of ROS or an elimination of Cd from enzymes [7].
4 Conclusions
1) The content of chlorophyll and MDA and the activity of SOD and POD in ramie increased under low concentration of Cd, but decreased significantly under high concentration.
2) Under the condition of 3, 6 and 9 mg/L Cd content, when ramie plants were sprayed simultaneously with low content of Se, the activity of SOD and POD increased by 35.34%, 43.18%, 3.63% and 12.45%, 14.14%, 3.27%, respectively. While the level of DNA methylation was decreased by 10.70%, 18.18% and 15.59%.
3) The alleviation mechanism of exogenous Se on ramie plants exposed to Cd stress may include: reduction of the absorption and accumulation of Cd, and removal of Cd from metabolically active cellular sites; reduction of oxygen radicals by antioxidant enzymes; and effect of maintaining the stability of DNA methylation.
References
[1] NABULO G, YOUNG S D, BLACK C R. Assessing risk to human health from tropical leafy vegetables grown on contaminated urban soils [J]. Science of the Total Environment, 2010, 408(22): 5338-5351.
[2] GUO Z H, WANG F Y, SONG J, XIAO X Y, MIAO X F. Leaching and transferring characteristics of arsenic, cadmium, lead and zinc in contaminated soil-giant reed-water system [J]. Journal of Central South University: Science and Technology, 2011, 42(8): 2184-2192. (in Chinese)
[3] GILL S S, HASANUZZAMAN M, NAHAR H, MACOVEI A, TUTEJA N. Importance of nitric oxide in cadmium stress tolerance in crop plants [J]. Plant Physiol Biochern, 2013, 63: 254-261.
[4] BENAVIDES M P, GALLEGO S M, TOMARO M L. Cadmium toxicity in plants [J]. Braz J Plant Physiol, 2005, 17: 21-34.
[5] SHUILLA U C, SINGH J, JOSHI P C, ILAILILAR P. Effect of bioaccumulation of cadmium on biomass productivity, essential trace elements, chlorophyll biosynthesis, and macromolecules of wheat seedlings [J]. Biol Trace Elem Res, 2003, 92: 257-273.
[6] LARSON R A. The antioxidants of higher plants [J]. Phytochemistry, 1988, 27: 969-978.
[7] LUILENS L N, ZHAN S H. The plant genome's methylation status and response tostress, unplications for plant unprovernent [J]. Curr Opin Plant Biol, 2007, 10: 317-322.
[8] BOB B, WILHELM G, RUSSELL J. Biochemistry and molecular biology of plants [M]. American Society of Plant Physiologists, Rockville, 2000: 1158-1203.
[9] YANG M, XIAO X Y, MIAO X F, GUO Z H, WANG F Y. Effect of amendments on growth and metal uptake of giant reed (Arundo donax L.) grown on soil contaminated by arsenic, cadmium and lead [J]. Transaction of Nonferrous Metals Society of China, 2012, 22: 1462-1469.
[10] LIU Y G, YE F, ZENG G M, CHAI L Y, SONG X C, MIN Z Y, XIAO X. Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud [J]. Environ Exp Bot, 2008, 62: 389-395.
[11] LIU Y G, WANG X, ZENG G M, QU D, GU J J, ZHOU M, CHAT L Y. Cadmium-induced oxidative stress and response of the ascorbate-glutathione cycle in Bechmeria nivea (L.) Gaud [J]. Chemosphere, 2007, 69: 99-107.
[12] YAO X Q, CHU J Z, WANG G Y. Effects of selenium on wheat seedlings under drought stress [J]. Biol Trace Elem Res, 2009, 130: 283-290.
[13] HAWRYLAIL N B. Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress [J]. Biol Trace Elem Res, 2009, 132: 259-269.
[14] CHU J Z, YAO X Q, ZHANG Z N. Responses of wheat seedlings to exogenous selenium supply under cold stress [J]. Biol Trace Elem Res, 2010, 136: 355-363.
[15] HSETYLAIL B, MATRASZED R, SZYMA N M. Response of lettuce Lactuca sativa L. to selenium in nutrient solution contaminated with nickel [J]. Veg Crops Res Bull, 2007, 67: 63-70.
[16] CARTES P, JARA A A, PINILLA L, ROSASA, MORA M L. Selenium improves the antioxidant ability against aluminium- induced oxidative stress in ryegrass roots [J]. Ann Appl Biol, 2010, 156: 297-307.
[17] HASANUZZMAN M, HOSSAIN M A, FUJITA M. Selenium in higher plants: Physiological role, antioxidant metabolism and biotic stress tolerance [J]. J Plant Physiol, 2010, 5: 354-375.
[18] SINGH M, SINGH H, BHANDARI D K. Interaction of selenium and sulfur on the growth and chemical composition of raya [J]. Soil Sci, 1980, 129: 238-244.
[19] LUO H J, LI H Y, ZHANG X T, FU J M. Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress [J]. Ecotoxicology, 2011, 20: 770-778.
[20] FILEK M, ILESILINEN R, HARTIILAINEN H, SZAREJILO I, JANIAIL A, MISZALSILI Z, GOLDA A. The protective role of selenium in rape seedlings subjected to cadmium stress [J]. J Plant Physiol, 2008, 165: 833-844.
[21] SUN H W, HA J, LIANG S X, KANG J. Protective role of selenium on garlic growth under cadmium stress [J]. Commun Soil Sci P1antAnal, 2010, 41: 1195-1204.
[22] PARRA R, PASTOR M T, PEREZ P E, AMO M J B. Effect of in vitro shoot multiplication and somatic embryogenesis on 5-methylcytosine content in DNA of Myrtus communis L. [J]. Plant Growth Regal, 2001, 33: 131-136.
[23] GE C L, LIU X Y, SUN J H, LUO S S, WANG Z G. The effect of heavy metal stress on the level of DNA methylation of rice and wheat [J]. Journal of Plant Physiology and Molecular Biology, 2002, 28: 363-368.(in Chinese)
[24] KRUPA Z, OQUIST G, HUNER N P A. The effects of cadmium on photosynthesis of Phaseolus wlgaris-A fluorescence analysis [J]. Physiol Plant, 1993, 88: 626-30.
[25] PEDRERO Z, MADRID Y, HARTIILAINEN H, CAMARA C. Protective effect of selenium in broccoli (Brassica oleracea) plants subjected to cadmium exposure [J]. J Agric Food Chern, 2008, 56: 266-271.
[26] HSN R M, ZHANG J P, SILIBSTED L H. Reaction dynamics of flavonoids and carotenoids as antioxidants [J]. Molecules, 2012, 17: 2140-2160.
[27] SUN Y B, ZHOU Q X, DIAO C Y. Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. [J]. Bioresour Technol, 2008, 99: 1103-1110.
[28] SINGH S, ESPEN S, D’SOUZA S F. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant [J]. Chemosphere, 2006, 62: 233-246.
[29] MISHRA S, SRIVASTAVA S, TRIPATHI R D, GOVINDARAJAN R, ILURAILOSE S V, PRASAD M N V. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L [J]. Plant Physiol Biochem, 2006, 44: 25-37.
[30] GALLEGO S M, BENAVIDES M P, TOMARO M L. Effect heavy metal ion excess on sunflower leaves: Evidence for involvement of oxidative stress [J]. Plant Sci, 1996, 121: 151-159.
[31] SOMASHEILARAIAH B V, PADMAJA K, PRASAD A R K. Phytotoxicity of cadmium ions on germinating seedlings of mungbean (Phaseolus vulgaris): Involvement of lipid peroxides in chlorophyll degradation [J]. Physiol Plant, 1992, 85: 85-89.
[32] NAJEEB U, JILANI G, ALI S, SARWAR M, XU L, ZHOU W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid [J]. J Hazard Mater, 2011, 186: 565-574.
[33] SCHICILLER H, CASPI H. Response of antioxidant enzymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum [J]. Physiol Plant, 1999, 105: 39-44.
[34] DIXIT V, PANDEY V, SHYAM R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad) [J]. J Exp Bot, 2001, 52: 1101-1109.
[35] THOMAS H, OUGHAM H, HORTENSTEINER S. Recent advances in the cell biology of chlorophyll catabolism [J]. Adv Bot Res, 2001, 35: 1-52.
[36] MARILOVSKA Y K, GORINOVA N I, NEDILOVSILA M P, MITEVA K M. Cadmium induced oxidative damage and antioxidative responses in Brassica juncea plants [J]. Biol Plant, 2009, 53: 151-154.
[37] MITTLER R. Oxidative stress, antioxidants and stress tolerance [J]. Trends Plant Sci, 2002, 7: 405-410.
[38] LIU Y, JIANG G X. The effect of Se on rape under Cd and Pb compound contamination [J]. Journal of Anhui Agn Sci, 2010, 38: 11096-11098. (in Chinese)
[39] KUMAR M, BUO A J, BAGHEL R S, REDDY C R h, JHA B. Selenium and spermine alleviate cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidants and DNA methylation [J]. Environ Exp Bot, 2012, 51: 129-138.
[40] SANDALIO L M, DALURZO H C, GOMEZ M, ROMERO P M C, DELRIO L A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants [J]. J Exp Bot, 2001, 52: 2115-2126.
[41] PARILHEY S, NAITHANI S C, ILESHAVILANT S. ROS production and lipid catabolism in desiccating Shorea robusta seeds during aging [J]. Plant Physiol Biochem, 2012, 57: 261-267.
[42] AKBULUT M, CAILIR S. The effects of Se phytotoxicity on the antioxidant systems of leaf tissues in barley (Hordeum vulgare L.) seedlings [J]. Plant Physiol Biochem, 2010, 48: 160-166.
[43] MOHAMED A A. CASTAGNA A, RANIERI A, TOPPI L S. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis [J]. Plant Physiol Biochem, 2012, 57: 15-22.
[44] CHEN T F, ZHENG W J, WONG Y S, YANG F. Selenium-induced changes in activities of antioxidant enzymes and content of photosynthetic pigments in spirulina platensis [J]. J Integr Plant Biol, 2008, 50: 40-48.
[45] CAUSEVIC A, DELAUNAY A, OUNNAR S, RIGHEZZA M, DELMOTTE F, BRIGNOLAS F, HAGEGE D, MAURY S. DNA methylating and dernethylating treatments modify phenotype and cell wall differentiation state in sugarbeet cell lines [J]. Plant Physiol Biochem, 2005, 43: 681-691.
[46] XU Q, BAO Y. Plant DNA methylation and its biological significance [J]. Journal of Qufu Normal University: Natural Science, 2011, 37: 3.(in Chinese)
[47] LEE Y W, BRODAY L, COSTA M. Effects of nickel on DNA methyltransfeerase activity and genomic DNA methylation levels [J]. Mutat Res, 1998, 415: 213-218.
[48] HIX S, AUGUSTO O. DNA methylation by tent- butylhydroperoxide-iron (II): A role for the transition metal ion in the production of DNA base adducts [J]. Chem-Biol Interact, 1999, 118: 141-149.
外源硒对苎麻的镉胁迫毒性的缓解机制
王春林1,2,刘云国1,2,曾光明1,2,胡新将1,2,尹怡诚1,2,胡 熙1,2,周 璐1,2,王亚琴1,2,李华英1,2
1. 湖南大学 环境科学与工程学院,长沙 410082;2. 湖南大学 环境生物与控制教育部重点实验室,长沙 410082
摘 要:研究施加外源硒(Se6+)对苎麻镉胁迫毒性的保护机制。主要讨论硒对苎麻生长、MDA、抗氧化酶活性以及DNA甲基化水平的影响。苎麻分别在含镉浓度为0、3、6、9 mg/L条件下培养,并对苎麻叶面喷洒硒酸钠(1.2 μmol/L)。结果表明,镉浓度为3、6、9 mg/L营养液中培养的苎麻,经过向叶片喷洒低浓度的硒酸钠(1.2 μmol/L)处理后,苎麻叶片中的SOD活性增加了35.34%、43.18%、 3.63%,POD活性增加了12.45%、14.14%、3.27%,DNA甲基化下降了10.70%、18.18%、15.59%。结果证实了低浓度硒能强化苎麻SOD、POD活性以及平衡苎麻体内DNA甲基化。
关键词:苎麻;硒;镉;DNA甲基化;抗氧化酶;植物修复
(Edited by Sai-qian YUAN)
Foundation item: Project (41271332) supported by the National Natural Science Foundation of China; Project (11JJ2031) supported by the Natural Science Foundation of Hunan Province, China
Corresponding author: Yun-guo LIU; Tel: +86-731-88649208; Fax: +86-731-88822829; E-mail: liuyunguo@hnu.edu.cn
DOI: 10.1016/S1003-6326(14)63557-3
Abstract: The protective role of exogenously supplied selenium (Se6+) on Bechmeria nivea (L.) Gaud (Ramie) subjected to cadmium (Cd) stress was studied in vitro, and the mechanism was discussed by investigating plant growth, malondialdehyde (MDA), activity of antioxidative enzymes and DNA methylation pattern. Plants grown in hydroponic culture were supplied with spraying Se (selenate, 1.2 μmol/L) and cadmium (Cd(NO3)2, 0, 3, 6 and 9 mg/L), individually or simultaneously. At low Se spraying levels, SOD activity was increased by 35.34%, 43.18%, 3.63% under 3, 6 or 9 mg/L cadmium contents, POD was increased by 12.45%, 14.14%, 3.27%, and the level of DNA methylation was decreased by 10.70%, 18.18% and 15.59%, respectively. The results confirmed that spraying low Se on ramie leaves could enhance the activity of SOD and POD, and regulate DNA methylation in ramie leaves.


