- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discus...▲
- 4 Conclusions▲
- References
- Figure
- Fig. 1 XRD patterns of ZnGaNO solid solution and CNIC composites with different proportions
- Fig. 2 FTIR spectra of ZnGaNO solid solution and CNIC composites with different proportions
- Fig. 3 UV-vis diffuse reflectance spectra of ZnGaNO solid solution and CNIC composites with different proportions
- Fig. 4 SEM (a, b), TEM (c) and HRTEM (d) images of 50% CNIC–ZnGaNO composite
- Fig. 5 MO photodegradation over ZnGaNO solid solution and CNIC composites with different proportions
- Fig. 6 Comparison of MO degradation over different photocatalysts under visible light irradiation
- Fig. 7 Schematic illustration for electron-hole separation and transport at visible light-driven CNIC–ZnGaNO composite photocatalyst interface
- Fig. 8 Photoluminescence spectra of CNIC–ZnGaNO composite photocatalysts
J. Cent. South Univ. (2017) 24: 276-283
DOI: 10.1007/s11171-017-3428-1

Synthesis of ZnGaNO solid solution–carbon nitride intercalation compound composite for improved visible light photocatalytic activity
YANG Ming(杨明)1, WAN Li-juan(万丽娟)2, 3, JIN Xiao-qi(金效齐)4
1. School of Transportation, Southeast University, Nanjing 210096, China;
2. Nanjing Vocational Institute of Transport Technology, Nanjing 211188, China;
3. Jiangsu Engineering Technology Research Center for Energy Conservation and
Emission Reduction of Transportation, Nanjing 211188, China;
4. Bio-Chemistry Department, Wenshan University, Wenshan 663000, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract:
Visible-light-driven ZnGaNO solid solution–carbon nitride intercalation compound (CNIC) composite photocatalyst was synthesized via a mixing and heating method. The composite photocatalyst was characterized by X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM), Fourier transform infrared (FT-IR) spectroscopy, UV-vis diffuse reflection spectroscopy, X-ray photoelectron spectroscopy (XPS), photoluminescence (PL) spectroscopy and BET surface area measurements. The activity of ZnGaNO–CNIC composite photocatalyst for photodegradation of methyl orange (MO) is higher than that of either single-phase CNIC or ZnGaNO solid solution. The as-prepared composite photocatalysts exhibit an improved photocatalytic activity due to enhancement for the separation and transport of photo-generated electron–hole pairs.
Key words:
photocatalysis; carbon nitride intercalation compound (CNIC); ZnGaNO; composite;
1 Introduction
In recent years, to remove contamination and realize water splitting for hydrogen or oxygen generation, the photocatalysis technique has been applied by using solar energy; however, highly efficient photocatalysts are still most important to the large-scale industrial applications of photocatalysis technique [1–3]. Usually, in order to improve the photocatalytic activity, to increase the separation efficiency of photogenerated electron–hole pairs is a good method, e.g., the co-catalysts loaded on the surface of catalyst can evidently promote the separation efficiency [4], and to form a composite powder photocatalyst between two kinds of semiconductors is another feasible route. Many oxide composite photocatalysts fabricated by solid state sintering, which offer the driving forces for separation and transfer of photogenerated electron–hole pairs, and the suitably matching band level of conduction and valance bands can induce the improved photocatalytic activity [5]. Graphitic carbon nitride (g-C3N4), a metal- free visible-light responded semiconductor photocatalyst reported by WANG et al [6] for the first time, has attracted vast attention for hydrogen or oxygen generation from photocatalytic water splitting. However, due to the inefficiency in separation and transport of photogenerated carriers and the large optical band gap of 2.7 eV, the photoactivity of g-C3N4 is low [7]. To improve the photocatalytic performance of g-C3N4, in addition to narrow the band gap for more light absorption such as doping with metal and non-metal elements [8–11], another method is to enhance the efficiency in separation and transport of photogenerated holes and electrons to modify the photocatalytic activity.
Recently, a carbon nitride intercalation compound (CNIC) exhibiting the alluring prospect for solar energy conversion, has been formed through coordinating alkali metals into the C–N plane of carbon nitride by a simple molten salt route, and thus significantly improved the electrical conductivity of g-C3N4, which is beneficial to improve the separation and transport efficiency of the photogenerated electron-hole pairs and exhibiting such high quantum yield for solar hydrogen production [12]. Furthermore, it has been well demonstrated that to build g-C3N4-based hybrids photocatalysts with various materials, such as TiO2 [13], SnS2 [14], TaON [15], Bi2WO6 [16], SrTiO3:Rh [17], BiOBr [18], AgX/g-C3N4 (X=Br, I) [19], graphene [20] and MWNTs [21], can efficiently promote the separation and transfer of the photogenerated carriers. Therefore, to make an efficient photocatalyst by combining a proper material with CNIC is expected to improve the photocatalytic activity for photocatalytic degradation of organic pollutants over carbon nitride. Carbon black/carbon nitride intercalation compound (CNIC) composite and Cr-doped SrTiO3– carbon nitride intercalation compound (CNIC) composite photocatalyst have been synthesized [7, 22]; however, the composite photocatalyst composed of CNIC and oxynitride photocatalyst has not been studied.
Oxynitride photocatalysts with moderate band gap and usually absorbing more visible light than many oxide photocatalysts, have been recognized as very important semiconductor photocatalysts in photocatalysis fields, e.g. several typical visible-light-driven oxynitride photocatalysts including N-doped-TiO2 [23], GaN:ZnO solid solution [24] and TaON [25], have been developed to induce the photocatalytic reaction.
In the present study, considering the synergic effect between ZnGaNO and carbon nitride intercalation compound (CNIC), the novel heterostructured ZnGaNO– CNIC photocatalyst was synthesized via the mixing and heating method. The characteristics of the organic-inorganic heterogeneous composite powders with various amounts of CNIC were analyzed. The amount of CNIC was optimized in terms of maximizing the photocatalytic activity for enhancing the methyl orange (MO) photodegradation under visible light irradiation. The MO photodegradation experiments indicated that the composite structure could improve the photocatalytic activity of ZnGaNO–CNIC composite due to the improvement of separation efficiency of photogenerated electron–hole pairs.
2 Experimental
2.1 Materials
The starting materials utilized were zinc oxide (ZnO), gallium oxide (Ga2O3), LiCl·H2O, KCl, NaCl and melamine (C3H6N6) (analysis purity grade, Sinopharm Chemical Reagent Co. Ltd., China).
2.2 Synthesis of ZnGaNO solid solution–CNIC composite
Firstly, ZnGa2O4 was synthesized by a solid-state reaction of ZnO and Ga2O3 at 1100 °C for 12 h. And then, its further nitridation was performed at 850 °C for 15 h under a flow of NH3 (250 mL/min) into a ZnGaNO solid solution [26].
The carbon nitride intercalation compound (CNIC) was synthesized by a simple molten salt route [12]. Melamine was used as a precursor. The eutectic mixture of LiCl·H2O–KCl–NaCl (1:1:1 mass ratio) was selected as a solvent. The mixture of eutectic salts (LiCl·H2O– KCl–NaCl with 1:1:1 mass ratio) and melamine with a mass ratio of 15:1 for eutectic salts to melamine was prepared and finely ground in a mortar. The mixture was then transferred into a quartz glass beaker and heated to 500 °C for 1 h with a ramp rate of 5 °C/min under semiclosed environment. After it was cooled to room temperature, the obtained yellow powder products were washed thoroughly with deionized water several times and then collected by centrifugation and dried at 60 °C for 10 h.
Then, the mixture of ZnGaNO and CNIC powder was put together with 30%, 50% and 70% CNIC (mass fraction) in the mortar and ground till thoroughly mixed (denoted as 30%, 50% and 70% ZnGaNO–CNIC respectively). Then, the mixture was calcined at 300 °C for 1 h in a tubular furnace under N2 atmosphere. A mixture of CNIC and ZnGaNO with 50% CNIC prepared by the physical mixed method without heat treatment was also prepared as a reference (denoted as 50% ZnGaNO/CNIC). The photocatalyst of g-C3N4 was prepared as a reference by directly heating melamine at 500 °C (heating rate: 20 °C/min) for 2 h, and the further deammonation treatment was set at 520 °C for 2 h [27] in the semiclosed system to prevent sublimation of melamine.
2.3 Characterization
The samples were characterized by X-ray diffraction (XRD) for phase identification on a Rigaku Ultima III diffractometer with Cu Kα radiation (λ= 0.154 nm, 40 kV, 40 mA) and a scan rate of 10 (°)/min (Rigaku corp., Japan). The specific surface area was obtained on a Micromeritics TriStar 3000 instrument (Micromeritics Instrument Corp., USA) at 77 K and calculated from the linear part of the Brunauer– Emmett–Teller (BET) plot ranging from P/P0= 0.05 to P/P0=0.15 based on the BET equation. The infrared optical properties were measured on a Nicolet Nexus 870 FTIR spectrometer using KBr pellet technique (Thermo Nicolet Corp., USA). Ultraviolet visible (UV-vis) diffuse reflection spectra were measured using a UV-vis spectrophotometer (UV-2550, Shimadzu Corp., Japan) and converted from reflection to absorbance by the Kubelka-Munk method. Their band gaps are calculated by equation of αhv=A(hv–Eg)n/2 (α, v, A, and Eg signify the absorption coefficient, light frequency, proportionality constant, and band gap respectively, and n equals 1 or 4, depending on whether the transition is direct or indirect, here n=1 because these materials are direct-gap semiconductors.). X-ray photoelectron spectroscopy (XPS) and energy dispersed spectrometry (EDS) data were collected on a THERMO FISHER SCIENTIFIC K-Alpha instrument (Thermo Fisher Scientific Corp., USA) and an EDAX genesis spectrometer respectively (EDAX Inc., USA). The microstructures of the products were observed by a field emission scanning electron microscope (FE-SEM; NOVA230, with accelerating voltage 15 kV, FEI Ltd., USA), and a high resolution transmission electron microscope (HR-TEM; JEM-2100, 200 kV, JEOL Ltd., Japan). The photoluminescence (PL) spectroscopy was detected with a spectrofluorometer (Cary Eclipse, VARIAN Corp., USA) at room temperature.
2.4 Photocatalytic activity tests
The methyl orange (MO) dye was used to test the photocatalytic activities of the samples and the photocatalytic reaction was preformed in a Pyrex reactor. 0.1 g catalyst was dispersed in 100 mL MO aqueous solution (4 mg/L). The light irradiation system was composed of a 300 W Xe lamp with cut-off filter L42 for visible light and a water filter for removing heating effects. For all experiments, the reaction solutions for all experiments of the MO photodegradation were first stirred in the dark for 1 h to reach the adsorption– desorption equilibrium of MO on catalysts. The photocatalytic degradation efficiency of MO was evaluated using the UV-vis absorption spectra to measure the peak value of a maximum absorption of MO solution at wavelength of 463 nm.
3 Results and discussion
The powder XRD patterns of CNIC, ZnGaNO and ZnGaNO–CNIC composites are shown in Fig. 1. From Fig. 1, it can be seen that for the sample with 100% CNIC, there are the series of diffraction peaks found at 8.3°, 12.1°, 21.5°, 27.8°, 32.6° and 36.41°, which can be assigned to the (00l) reflections by analogy to graphite intercalation compounds [28], suggesting that a carbon nitride intercalation compound was successfully prepared. The XRD pattern (Fig. 1) indicates that the 0% CNIC product has a wurtzite ZnGaNO structure, similar to that of GaN and ZnO, which is consistent with the XRD pattern of the previously reported results [29]. The ZnGaNO–CNIC composite samples present two compositions: CNIC and ZnGaNO, and with the increase of the content of CNIC, the intensity of the (007) peak of CNIC becomes stronger. For the ZnGaNO solid solution, the Zn-to-Ga ratio was determined by XPS and EDS to check the composition. The O-to-N ratio determined by XPS was 0.94. The Zn/Ga ratio determined by XPS was 0.03, while it was 0.11 determined by EDS. XPS is a surface chemical analysis technique that can obtain the surface composition; however, EDS can obtain the bulk composition of a material [30]. The starting Zn-to-Ga ratio is usually higher than 1 for the ZnGaNO solid solutions prepared through solid-state reaction, however, due to the Zn volatilization at the high temperature, the Zn-to-Ga ratio in the nitridation product is lower [29]. The volatilization of Zn atoms on the surface is easier than in the bulk under nitridation, thus a low Zn-to-Ga ratio was detected on the surface of the as-prepared ZnGaNO sample.

Fig. 1 XRD patterns of ZnGaNO solid solution and CNIC composites with different proportions
Figure 2 shows the Fourier transform infrared (FTIR) spectra of CNIC, ZnGaNO and ZnGaNO–CNIC composites. The FTIR spectrum of the pure CNIC sample shows the features very similar to those of the published results [12]. As shown in Fig. 2, a band at 2177 cm–1 corresponds to a cyano group stretch, which can be attributed to loss of ammonia. The FTIR spectroscopy (Fig. 2) shows that several stretching peaks of CN heterocycles of g-C3N4 at 1589, 1408 and 1238 cm-1 [31] shift to 1560, 1378 and 1278 cm-1 after forming CNIC, respectively. The position shift for CNIC would indicate the strong interaction between insertions and the s-triazine ring [12]. The strong hydroxyl stretching at 3380 cm–1 in the FTIR spectrum indicates that the CNIC has a hydrophilic surface, which isbeneficial for a highly dispersed state and thus a sufficient surface area for photocatalytic reaction. The FTIR spectra of ZnGaNO–CNIC composites show that the characteristic bands for CNIC still remain. For the pure ZnGaNO, the broad absorption band near 560 cm-1 corresponds to Ga–N stretching vibration in hexagonal- type GaN crystal [32, 33], while for the composite, with the increase of the content of CNIC, the decreasing intensity of the band near 560 cm-1 may be due to the existence of the organic component.

Fig. 2 FTIR spectra of ZnGaNO solid solution and CNIC composites with different proportions
UV-vis diffuse reflectance spectroscopy was used to investigate the optical absorption properties of the samples. Figure 3 shows the UV-vis absorption spectra of CNIC, ZnGaNO and ZnGaNO–CNIC composites. It can be observed from Fig. 3 that the absorption edge of the ZnGaNO sample occurs at ca. 466 nm and the band gap energy is calculated at 2.77 eV. After being coupled with CNIC, the absorption edge shifts to the lower energy region. It can be seen that the absorption edges of the composite samples shift to longer wavelengths with increasing the amount of CNIC. The decrease in band gaps of the samples is from 2.77 eV of ZnGaNO to 2.71 eV of CNIC when the content of CNIC is increased from 0 to 100%.
The morphology of 50% ZnGaNO–CNIC composite material was investigated using SEM, TEM and high-resolution TEM (HRTEM) respectively, as shown in Fig. 4. Scanning electron microscopy (SEM) observations (Figs. 4(a) and (b)) show that the CNIC has tubular morphology (several hundreds of nanometers in width and several micrometers in length). The specific surface area of CNIC reaches 118.4 m2/g, much higher than 10 m2/g for bulk g-C3N4 [6, 15, 27], owing to the walls of CNIC nanotubes formed by agglomeration of the small nanoplates (corresponding to seven repeating identity periods) and the porous structure [12]. The TEM image (Fig. 4(c)) shows that the particle size of the ZnGaNO was estimated to be about 200 nm and the HRTEM image (Fig. 4(d)) shows that the lattice fringe with a spacing of 0.26 nm corresponds to interplanar spacing of (002) plane of wurtzite ZnGaNO, which is consistent with the XRD result.

Fig. 3 UV-vis diffuse reflectance spectra of ZnGaNO solid solution and CNIC composites with different proportions

Fig. 4 SEM (a, b), TEM (c) and HRTEM (d) images of 50% CNIC–ZnGaNO composite
The photodegradation activities for the investigated samples are evaluated using MO and the results are shown in Fig. 5. In our experiments, MO photolysis is not observable in the absence of catalyst, which indicates that MO is stable under visible light irradiation. From Fig. 5, it can be seen that, after 6 h of visible light illumination, MO removal over ZnGaNO is 88.7% and that CNIC shows a photocatalytic performance with a degradation ratio of 94.3%. The as-prepared composite photocatalysts show better activity than single-phase CNIC or ZnGaNO solid solution. Significantly, the 50% ZnGaNO–CNIC composite presents a sharp increase in the catalytic activity for MO decomposition, which induced 100% degradation within 4 h light irradiation. 11.4%, 12.4%, 12.9%, 13.2% and 13.5% MO before the photoreaction was adsorbed on the surface of CNIC, ZnGaNO and 30%, 50%, 70% ZnGaNO–CNIC composites, respectively. As shown in Fig. 5, after 4 h light irradiation, 79.5%, 71.8%, 76.5%, 100%, 85.4% was photocatalytically degraded over CNIC, ZnGaNO and 30%, 50%, 70% ZnGaNO–CNIC composites, respectively. For the ZnGaNO–CNIC composite, the CNIC content was important to achieve the high photocatalytic activity. The suitable content of CNIC caused its well dispersion on the ZnGaNO surface, which promoted the transfer and separation of photogenerated electrons and holes. However, at contents higher than 50%, the heterojunction structures and interfaces between the CNIC and ZnGaNO particles decreased. The interfacial charge transfer was suppressed and thus the photocatalytic activity for MO degradation was reduced.
For comparison, the mixed material of CNIC and ZnGaNO without heat treatment (50% ZnGaNO/CNIC), pure g-C3N4, and the commercial nitrogen-doped-TiO2 with a specific surface area about 65 m2/g (TPS201,Sumitomo Corp. Japan) were also used as photocatalysts in degradation of MO. Figure 6 shows the degradation activities of MO over different photocatalysts under visible light irradiation. From Fig. 6, the 50% ZnGaNO–CNIC composite shows much higher activity than pure g-C3N4, pure CNIC, nitrogen-doped TiO2 or 50% ZnGaNO/CNIC photocatalyst. This clearly indicates that the advantage of heating treatment is the formation of chemically bonded interfaces between the two materials, and the ZnGaNO–CNIC composite was determined as an efficient visible-light-driven photocatalyst for the degradation of MO.

Fig. 5 MO photodegradation over ZnGaNO solid solution and CNIC composites with different proportions

Fig. 6 Comparison of MO degradation over different photocatalysts under visible light irradiation
Generally, hydroxyl radicals (·OH) and superoxide (O2· or HOO·) reactive oxidation species, are formed during the photocatalytic reaction [9]. Hydroxyl radical may be the active species for composite photocatalytic reaction system [34]. Hydroxyl radicals (·OH) is generated via the photogenerated hole oxidation [35] or multistep reduction of O2 induced by photogenerated electron (O2+e→O2·-,O2·-+e+2H+→H2O2, H2O2+e→·OH+OH-) [36]. The photodegradation activity of photocatalysts is generally associated with reduction ability of photogenerated electrons in conduction band and oxidation ability of photogenerated holes in valence band [9]. The oxidation potential of CNIC is 1.53 V [12], which means that the photogenerated holes are incapable of directly oxidizing adsorbed hydroxyl groups to generate hydroxyl radicals (·OH) (2.7 V vs NHE) [9]. Therefore, a reliable speculation may be that the ·OH in the composite photocatalytic reaction system is formed from the multistep reduction of O2.
The BET specific surface area of the samples was investigated by nitrogen adsorption. The BET specific surface areas of the CNIC, ZnGaNO, 30%, 50% and 70% ZnGaNO–CNIC samples are 118.4, 14.5, 33.1, 72.2, 85.1 m2/g, respectively. Thus, the change in photocatalytic performance is not linked with the difference in specific surface area. The enhancement of photocatalytic activity of the composite materials may be attributed to the more effective separation of the photogenerated electron–hole pairs. Based on the band gap positions, the CB and VB edge potentials of CNIC were determined at -1.17 and 1.53 eV at pH 7 (versus the normal hydrogen electrode) [12], respectively. It has been reported that p–d repulsion (e.g., O 2p–Zn 3d) for type II–VI semiconductors shifts the valence band maximum upwards while with the conduction band minimum unchanged [26]. Similarly, the presence of N 2p and Zn 3d electrons in the upper valence band provides p–d repulsion for the valence band maximum, which results in a narrowing of the band gap for the solid solutions of GaN and ZnO [26]. According to the previous study, the CB and VB edge potentials of ZnGaNO are at -0.9 and 1.94 eV, respectively [26]. In our case, the optical band gap for ZnGaNO was determined to be 2.77 eV, which is narrower than the 2.84 eV reported in Ref. [26]. Thus, the VB position rise of ZnGaNO induces the 0.07 eV decrease in band gap, and allows us to adjust the VB edge potential of 1.94 eV reported in Ref. [26] to 1.87 eV. Since the CB edge potential of CNIC (-1.17 eV) is more negative than that of ZnGaNO (-0.9 eV), the photo-induced electrons on CNIC particle surfaces transfer more easily to ZnGaNO via the well developed interface. Similarly, the photo- induced holes on the ZnGaNO surface move to CNIC due to the large difference in VB edge potentials. The scheme for electron–hole separation and transport at the visible–light-driven ZnGaNO–CNIC composite photocatalyst interface is shown in Fig. 7. This reduces the probability of electron–hole recombination and leads to a larger amount of electrons on the ZnGaNO surface and holes on the CNIC surface, respectively, which promotes the photocatalytic degradation of MO.

Fig. 7 Schematic illustration for electron-hole separation and transport at visible light-driven CNIC–ZnGaNO composite photocatalyst interface
Photoluminescence (PL) analysis is an effective method to study the migration and recombination efficiency of photogenerated carriers in semiconductors, due to PL emission probably resulting from the band edge recombination mechanism [16, 20]. To further confirm the above-proposed mechanism, the PL spectra of the ZnGaNO–CNIC composite photocatalysts at an excitation wavelength of 330 nm are investigated and shown in Fig. 8. For pure CNIC, the emission band is centered at about 460 nm, which is close to the band gap energy of 2.7 eV for carbon nitride intercalation compound, which is indicative of the band edge recombination of electron–hole pairs [7]. The positions of the ZnGaNO–CNIC emission peaks are similar to pure CNIC. Obviously, compared with pure CNIC, the emission intensity of the ZnGaNO–CNIC composite samples is much lower, which indicates that the composite sample has a lower recombination rate of electrons and holes under visible-light irradiation. The process can be assumed that the electrons were excited from the valence band to the conduction band of CNIC and then transferred to ZnGaNO, preventing a direct recombination of electrons and holes, thus effectively increasing the separation of photogenerated electron– hole pairs, which conforms to the discussion about the separation of charge carriers and the results of photocatalytic degradation experiments.
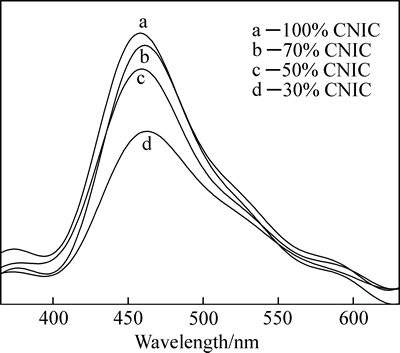
Fig. 8 Photoluminescence spectra of CNIC–ZnGaNO composite photocatalysts
4 Conclusions
1) A series of ZnGaNO–CNIC composite photocatalysts have been fabricated by introducing carbon nitride intercalation compound via a mixing and heating method.
2) Compared with single-phase CNIC or ZnGaNO, the ZnGaNO–CNIC composite photocatalysts exhibited a significantly enhanced photocatalytic performance in degrading MO, and the highest efficiency was observed with 50% ZnGaNO–CNIC composite.
3) Our results clearly indicates that the visible- light-driven ZnGaNO–CNIC composite catalyst has a good photo-oxidation performance, which resulted from the suitably matching conduction and valance band levels and thus improved the separation efficiency of photogenerated electron–hole pairs.
References
[1] Lianos P. Production of electricity and hydrogen by photocatalytic degradation of organic wastes in a photoelectrochemical cell. The concept of the photofuelcell: A review of a re-emerging research field [J]. J Hazardous Mater, 2011, 185(2, 3): 575–590.
[2] Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting [J]. Chem Soc Rev, 2009, 38(1): 253–278.
[3] PELAEZ M, NOLAN N T, PILLAI S C, SEERY M K, FALARAS P, KONTOS A G, DUNLOP P S M, HAMILTON J W J, BYRNE J A, O’SHEA K, ENTEZARI M H, DIONYSIOU D D. A review on the visible light active titanium dioxide photocatalysts for environmental applications [J]. Appl Catal B: Environ, 2012, 125: 331–349.
[4] OSTERLOH F E. Inorganic materials as catalysts for photochemical splitting of water [J]. Chem Mater, 2008, 20(1): 35–54.
[5] LIN Xin-ping, XING Jing-cheng, WANG Wen-deng, SHAN Zhi-chao, XU Fang-fang, HUANG Fu-qiang. Photocatalytic activities of heterojunction semiconductors Bi2O3/BaTiO3: A strategy for the design of efficient combined photocatalysts [J]. J Phys Chem C, 2007, 111(49): 18288–18293.
[6] WANG Xin-chen, MAEDA K, THOMAS A, TAKANABE K, XIN Gang, CARLSSON J M, DOMEN K, ANTONIETTI M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light [J]. Nat Mater, 2009, 8(1): 76–80.
[7] WU Zhao-chun, GAO Hong-lin, YAN Shi-cheng, ZOU Zhi-gang. Synthesis of carbon black/carbon nitride intercalation compound composite for efficient hydrogen production [J]. Dalton Trans, 2014, 43(31): 12013–12017.
[8] LIU Gang, NIU Ping, SUN Cheng-hua, SMITH S C, CHEN Zhi-gang, LU Gao-qing, CHENG Hui-ming. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4 [J]. J Am Chem Soc, 2010, 132(33): 11642–11648.
[9] YAN Shi-cheng, LI Zhao-sheng, ZOU Zhi-gang. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation [J]. Langmuir, 2010, 26(6): 3894–3901.
[10] ZHANG Yuan-jian, MORI T, YE Jin-hua, ANTONIETTI M. Phosphorus-doped carbon nitride solid: Enhanced electrical conductivity and photocurrent generation [J]. J Am Chem Soc, 2010, 132(18): 6294–6295.
[11] YUE Bing, LI Qiu-ye, IWAI H, KAKO T, YE Jin-hua. Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light [J]. Sci Technol Adv Mater, 2011, 12(3): 034401.
[12] GAO Hong-lin, YAN Shi-cheng, WANG Jia-jia, HUANG Yu-an, WANG Peng, LI Zhao-sheng, ZOU Zhi-gang. Towards efficient solar hydrogen production by intercalated carbon nitride photocatalyst [J]. Phys Chem Chem Phys, 2013, 15(41): 18077– 18084.
[13] YAN Hong-jian, YANG Hao-xin. TiO2–g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation [J]. J Alloys Compd, 2011, 509(4): L26–L29.
[14] Zhang Zhen-yi, Huang Jin-dou, Zhang Ming-yi, Yuan Qing, Dong Bin. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity [J]. Appl Catal B: Environ, 2015, 163: 298–305.
[15] YAN Shi-cheng, LV Shu-bai, LI Zhao-sheng, ZOU Zhi-gang. Organic–inorganic composite photocatalyst of g-C3N4 and TaON with improved visible light photocatalytic activities [J]. Dalton Trans, 2010, 39(6): 1488–1491.
[16] Ge Lei, Han Chang-cun, Liu Jing. Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange [J]. Appl Catal B: Environ, 2011, 108–109: 100–107.
[17] Kang H W, Lim S N, Song Dong-su, Park S B. Organic-inorganic composite of g-C3N4–SrTiO3:Rh photocatalyst for improved H2 evolution under visible light irradiation [J]. Inter J Hydro Energy, 2012, 37(16): 11602–11610.
[18] Fu Jie, Tian Yan-long, Chang Bin-bin, Xia Feng-na, Dong Xiao-ping. BiOBr–carbon nitride heterojunctions: Synthesis, enhanced activity and photocatalytic mechanism [J]. J Mater Chem, 2012, 22(39): 21159–21166.
[19] XU Hui, YAN Jia, XU Yuan-guo, SONG Yan-hua, LI Hua-ming, XIA Jie-xiang, HUANG Chuan-jing, WAN Hui-lin. Novel visible-light-driven AgX/graphite-like C3N4 (X=Br, I) hybrid materials with synergistic photocatalytic activity [J]. Appl Catal B: Environ, 2013, 129: 182–193.
[20] Xiang Qian-juan, Yu Jia-guo, Jaroniec M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 [J]. J Phys Chem C, 2011, 115(15): 7355–7363.
[21] Ge Lei, Han Chang-cun. Synthesis of MWNTs/g-C3N4 composite photocatalysts with efficient visible light photocatalytic hydrogen evolution [J]. Appl Catal B: Environ, 2012, 117–118: 268–274.
[22] YANG Ming, JIN Xiao-qi. Towards improved visible light-induced photocatalytic performance by Cr-doped SrTiO3–carbon nitride intercalation compound (CNIC) composite [J] Journal of Central South University, 2016, 23(2): 310–316.
[23] Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y. Visible-llight photocatalysis in nitrogen-doped titanium oxides [J]. Science, 2001, 293(5528): 269–271.
[24] Maeda K, Teramura K, Lu D L, Takata T, Saito N, Inoue Y, Domen K. Photocatalyst releasing hydrogen from water [J]. Nature, 2006, 440(7082): 295–296.
[25] Hara M, Takata T, Kondo J N, Domen K. Photocatalytic reduction of water by TaON under visible light irradiation [J]. Catal Today, 2004, 90(3, 4): 313–317.
[26] Yan Shi-cheng, Wang Zhi-qiang, Li Zhao-sheng, Zou Zhi-gang. Two-step reactive template route to a mesoporous ZnGaNO solid solution for improved photocatalytic performance [J]. J Mater Chem, 2011, 21(15): 5682–5686.
[27] YAN Shi-cheng, LI Zhao-sheng, YU Tao, ZOU Zhi-gang. Photodegradation performance of g-C3N4 fabricated by directly heating melamine [J]. Langmuir, 2009, 25(17): 10397–10401.
[28] DRESSELHAUS M S, DRESSELHAUS G. Intercalation compounds of graphite [J]. Adv Phys, 2002, 51(1): 1–186.
[29] Chen Hai-yan, Wen Wen, Wang Qi, Hanson J C, Muckerman J T, Fujita E, Frenkel A I, Rodriguez J A. Preparation of (Ga1-xZnx)(N1-xOx) photocatalysts from the reaction of NH3 with Ga2O3/ZnO and ZnGa2O4: In situ time-resolved XRD and XAFS studies [J]. J Phys Chem C, 2009, 113(9): 3650–3659.
[30] Hu C C, Teng H. Gallium oxynitride photocatalysts synthesized from Ga(OH)3 for water splitting under visible light irradiation [J]. J Phys Chem C, 2010, 114(47): 20100–20106.
[31] LIN-VIEN D, COLTHUP N B, FATELLEY W G, GRASSELLI J G. The handbook of infrared and Raman characteristic frequencies of organic molecules [M]. San Diego, CA: Academic Press Inc, CA, 1991.
[32] Hyo B J, Carsten R, Wilson H. MOCVD of BN and GaN thin films on silicon: New attempt of GaN growth with BN buffer layer [J]. J Cryst Growth, 1998, 189: 439–444.
[33] Ai Yu-jie, Xue Cheng-shan, Sun Chuan-wei, Sun Li-li, Zhuang Hui-zhao, Wang Fu-xue, Li Hong, Chen Jin-hua. Synthesis of GaN nanowires through Ga2O3 films’ reaction with ammonia [J]. Mater Lett, 2007, 61(13): 2833–2836.
[34] Yang Ming, Huang Qiao, Jin Xiao-qi. ZnGaNO solid solution–C3N4 composite for improved visible light photocatalytic performance [J]. Mater Sci and Eng B, 2012, 177(8): 600– 605.
[35] Yoon S H, Lee J H. Oxidation mechanism of As(III) in the UV/TiO2 system: Evidence for a direct hole oxidation mechanism [J]. Environ Sci Technol, 2005, 39(24): 9695–9701.
[36] LIU Guang-ming, LI Xiang-zhong, ZHAO Jin-cai, HORIKOSHI S, HIDAKA H. Photooxidation mechanism of dye alizarin red in TiO2 dispersions under visible illumination: an experimental and theoretical examination [J]. J Mol Catal A: Chem, 2000, 153(1, 2): 221–229.
(Edited by YANG Hua)
Cite this article as:
YANG Ming, WAN Li-juan, JIN Xiao-qi. Synthesis of ZnGaNO solid solution–carbon nitride intercalation compound composite for improved visible light photocatalytic activity [J]. Journal of Central South University, 2017, 24(2): 276-283.
DOI:https://dx.doi.org/10.1007/s11171-017-3428-1Foundation item: Project(51208102) supported by the National Natural Science Foundation of China
Received date: 2015-09-21; Accepted date: 2016-05-11
Corresponding author: YANG Ming, Associate Professor, PhD; Tel: +86-25-83794100; Fax: +86-25-83795406; E-mail: bartty_ym@sina.com
Abstract: Visible-light-driven ZnGaNO solid solution–carbon nitride intercalation compound (CNIC) composite photocatalyst was synthesized via a mixing and heating method. The composite photocatalyst was characterized by X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM), Fourier transform infrared (FT-IR) spectroscopy, UV-vis diffuse reflection spectroscopy, X-ray photoelectron spectroscopy (XPS), photoluminescence (PL) spectroscopy and BET surface area measurements. The activity of ZnGaNO–CNIC composite photocatalyst for photodegradation of methyl orange (MO) is higher than that of either single-phase CNIC or ZnGaNO solid solution. The as-prepared composite photocatalysts exhibit an improved photocatalytic activity due to enhancement for the separation and transport of photo-generated electron–hole pairs.
- Synthesis of ZnGaNO solid solution–carbon nitride intercalation compound composite for improved visible light photocatalytic activity


