
J. Cent. South Univ. (2020) 27: 344-355
DOI: https://doi.org/10.1007/s11771-020-4300-7
Fe/N-doped mesoporous carbons derived from soybeans:A highly efficient and low-cost non-precious metal catalyst for ORR
WU Qiu-mei(伍秋美)1, DENG Da-kuan(邓大款)1, HE Yi-lun(何轶伦)1,ZHOU Zhong-cheng(周忠诚)1, SANG Shang-bin(桑商斌)2, ZHOU Zhi-hua(周智华)3
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
3. School of Chemistry and Chemical Engineering, Hunan University of Science and Technology,Xiangtan 411201, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
Oxygen reduction reaction (ORR) plays a crucial role in many energy storage and conversion devices. Currently, the development of inexpensive and high-performance carbon-based non-precious-metal ORR catalysts in alkaline media still gains a wide attention. In this paper, the mesoporous Fe-N/C catalysts were synthesized through SiO2-mediated templating method using biomass soybeans as the nitrogen and carbon sources. The SiO2 templates create a simultaneous optimization of both the surface functionalities and porous structures of Fe-N/C catalysts. Detailed investigations indicate that the Fe-N/C3 catalyst prepared with the mass ratio of SiO2 to soybean being 3:4 exhibits brilliant electrocatalytic performance, excellent long-term stability and methanol tolerance for the ORR, with the onset potential and the half-wave potential of the ORR being about 0.890 V and 0.783 V (vs RHE), respectively. Meanwhile, the desired 4-electron transfer pathway of the ORR on the catalysts can be observed. It is significantly proposed that the high BET specific surface area and the appropriate pore-size, as well as the high pyridinic-N and total nitrogen loadings may play key roles in enhancing the ORR performance for the Fe-N/C3 catalyst. These results suggest a feasible route based on the economical and sustainable soybean biomass to develop inexpensive and highly efficient non-precious metal electrochemical catalysts for the ORR.
Key words:
biomass; oxygen reduction reaction; electro-catalyst; nitrogen-doped carbon;
Cite this article as:
WU Qiu-mei, DENG Da-kuan, HE Yi-lun, ZHOU Zhong-cheng, SANG Shang-bin, ZHOU Zhi-hua. Fe/N-doped mesoporous carbons derived from soybeans: A highly efficient and low-cost non-precious metal catalyst for ORR [J]. Journal of Central South University, 2020, 27(2): 344-355.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4300-71 Introduction
Fuel cells have attracted widespread attention due to their high power generation efficiency, clean fuel (hydrogen, methanol, etc.) and pollution-free emissions. The oxygen reduction reaction (ORR) as the main cathode reaction due to its sluggish kinetics and the main voltage-drop of the cells is one of the crucial factors that determine the overall performance of these energy conversion devices. So far, platinum and its alloys are still the best electrocatalysts used for accelerating the ORR. However, due to their relatively high price, limited reserves and easy to agglomeration resulting in poor stability [1, 2], it is a huge setback for their large-scale commercial applications. Therefore, great efforts have been undertaken to develop cheap, high-activity and durable non-noble metal catalysts.
Among these candidates for non-Pt catalysts, carbon-supported transition metal-nitrogen (M-N/C) materials have been considered as the most promising alternatives to replace the expensive Pt-based catalysts due to the excellent ORR catalytic properties, long-term stability and the application of inexpensive precursors. For M-N/C catalysts, nitrogen sources are usually divided into two categories: one is a ligand containing pyridine or pyrrole, such as polypyrrole [3], 1,10-phenanthroline [4]; the other is a ligand containing no pyrrole or pyridine, such as ethylenediamine, polyaniline, NH3 [5]. The organic compounds used are generally costly and some of them are even toxic. Besides, the carbon sources are mainly derived from expensive and complicated chemicals, such as carbon nanotubes, metal organic frameworks (MOFs) [6], covalent organic frameworks (COMs) [7]. These traditional C/N sources and complex synthesis methods are not conducive to cost-efficient commercialization of fuel cells. Thus, it is of great significance to design an appropriate approach to search for nitrogen- doped carbon catalysts with low cost, environmental friendliness and brilliant catalytic performance.
As reported previously, protein-enriched biomass (like egg white [8], enoki mushroom [9]) can be used to synthesize active and stable electrocatalysts. Soybean is one of the most important crops in the world and obtained abundantly and cheaply. The total protein content in soybean is about 36.5 wt.%, representing 5.9 wt.% nitrogen in the product [10]. The high N-containing content helps to increase the amount of doped nitrogen and the number of active species, while the high fat and carbohydrates make them useful as carbon sources. On the other hand, exposing active sites that participate in the ORR process needs high specific surface area. Continuously multi-stage open-framework structure accelerates the transfer efficiency of reactants and simultaneously increases the utilization of active sites [11, 12]. As one kind of advanced carbon nanomaterials, mesoporous carbons have attracted much attention due to advantages of high porosity, large surface area, excellent electrical conductivity and chemical inertness. Template-based method is one of the most common techniques used for preparing porous carbons. Generally, the particle size and pore-size distribution of the carbon materials can be effectively tuned by simply using templates with various pore sizes [13].
Herein, a series of Fe-N/C catalysts derived from pyrolysis of the hybrids of soybeans, melamine and FeCl3 were efficiently synthesized by adding different quantity of mesoporous silica as templates. The physical properties of the catalysts were carefully examined, and their ORR activities in alkaline media were analyzed in detail. The results show that high percentage of pyridinic-N and the total N content, as well as the high BET specific surface area may be important to enhance the ORR performance. Electrochemical results further suggest that this catalyst may be a good substitute to the Pt-based catalyst.
2 Experiments
2.1 Materials synthesis
The raw soybean purchased from a local market was washed and completely dried at 80 °C in a vacuum drying chamber. The mesoporous silica A200 was supplied by Guangdong Shenna Trading Co. Ltd. Ferric chloride and melamine were analytical reagents and used as received without any further treatment.
The detailed preparation processes were carried out as follows: the mixture of soybean (30 g), melamine (30 g) and deionized water (800 mL) were ground into homogeneous soymilk by the soymilk maker, and then the mixture was air dried to remove the solvent. On the other hand, ferric chloride (72.4 g) and different amount of SiO2 were dispersed in 150 mL deionized water, respectively. Subsequently, the above prepared two mixtures were mixed and underwent adequately ball-milling at 400 r/min for 12 h, followed by vacuum drying for 12 h. The dried hybrid was heat-treated in tubular furnace under N2 atmosphere at 700 °C for 2 h, and then cooled to room temperature. To remove the excess metal species and silica templates, the produced samples were chemically treated in 1 mol/L HCl and 20 wt.% HF solution stirred for 12 h, respectively, followed by thoroughly washed with de-ionized water. Finally, after being vacuum dried at 80 °C, the catalysts obtained were denoted as Fe-N/C1, Fe-N/C2, Fe-N/C3 and Fe-N/C4,which correspond to the mass ratio of SiO2 to soybean being 1:4, 2:4, 3:4 and 4:4, respectively. In order to evaluate the cost efficiency, the mass yields were calculated. The mass yields after heat and acid treatments are about 50 wt.% and 20 wt.%, respectively. The obtained total mass yields are all higher than 10 wt.%.
2.2 Physical characterizations
X-ray diffraction (XRD) analysis was operated on DX-2700B diffractometer using Cu Ka radiation at 3.6°/min. Transmission electron microscopy (TEM) images were recorded on FEI Tecnai G2 F30 microscope. X-ray photoelectron spectroscopy (XPS) was performed on ThermoFisher-VG Scientific ESCALAB250Xi using a monochromated Al-Ka X-ray source (hυ=1486.6 eV). The specific surface areas were measured by Brunauer–Emmett–Teller (BET) nitrogen adsorption-desorption on Quadrasorb SI-3MP instrument with nitrogen adsorption at 77 K. The particle pore-size distributions were obtained from the nitrogen adsorption isotherms by the density functional theory.
2.3 Electrochemical measurements
The electrochemical properties of the catalysts toward the ORR were characterized by the rotate disk electrode (RDE) and the rotating ring-disk electrode (RRDE) techniques. RDE and RRDE were performed on CHI760E electrochemical workstation (Chenhua Instruments, China) and bipotentiostat (Pine Instrument), respectively. The platinum wire and saturated calomel electrode (SCE) were used as the counter electrode and reference electrode, respectively. The catalyst coated glass carbon electrode (RDE: f 5 mm; RRDE: f 5.7 mm) was applied as the working electrode. The potentials versus the used reference electrode were calibrated with the potentials versus the reversible hydrogen electrode (RHE) in 0.1 mol/L NaOH unless otherwise specification, and the conversion formula should be applied as follows: E(vs.RHE) = E(vs.SCE)+ 0.059pH + 0.241 = E(vs.SCE) + 1.008.
The working electrode was prepared as follows: 5 mg prepared catalysts were dispersed in the solution of 2 mL ethanol and 40 μL Nafion solution (5wt.%) ultrasonically for homogenization for 15 min, then 20 μL dispersion was pipetted onto the glass carbon and then dried at room temperature.
All the linear sweep voltammetry (LSV) and cyclic voltammetry (CV) experiments were conducted in the potential range of 0.208 V to 1.208 V at a scan rate of 10 mV/s in O2- and N2-saturated 0.1 mol/L NaOH solution, respectively. It is known that the electron transfer number (n) involved in the ORR process is an important factor to evaluate the property of the ORR catalysts. In order to detect the electron transfer numbers and the corresponding HO2- yields, the RRDE measurements were also performed with the ring potential biased at 0.20 V (vs SCE). The electron transfer numbers and HO2- yields of the ORR were calculated by the equations n=4-2X(HO2-) and X(HO2-)=(2Iring/N)/(Idisk+Iring/N), respectively, where N is the collection efficiency (0.37), Idisk and Iring are the currents at the disk and ring electrodes, respectively. The stability of the catalyst was evaluated by the chronoamperometric technique at 0.608 V in O2-saturated 0.1 mol/L NaOH aqueous solution for 7200 s. The methanol-tolerance performance of the catalyst was performed as the same as the LSV except for the 1 mol/L methanol in the O2-saturated solution.
3 Results and discussion
3.1 Morphology and structure of Fe-N/C catalysts
Figure 1 presents the XRD patterns of the catalysts prepared with different mass of SiO2 used as template. It shows two carbon peaks with 2θ of 26.6° and 43.9°, which correspond to the crystal planes (002) and (004), respectively. The strong peak at 26.6° implies the existence of graphitic layers with good orderliness [14], whereas the other weak peak at about 43.9° indicates the disordered structure of carbon phase in the catalysts [15]. On the other hand, compared with the PDF (89-8104), the strengths of the characteristic diffraction peak for Fe2O3 are obviously increasing as the SiO2 mass ratio increasing [16, 17].
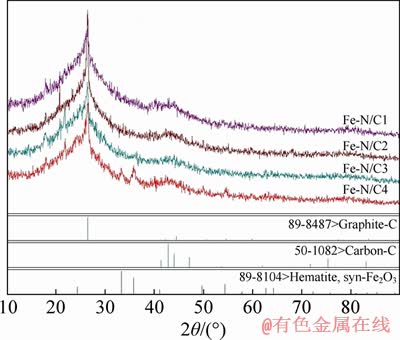
Figure 1 XRD patterns of catalysts prepared with different weight of SiO2
The morphology and microstructure of Fe-N/C catalysts are shown in Figure 2. It can be seen that carbon layers become thin and transparent with increasing amount of the silica added. It might be due to the removed silica particles from the carbon layers during the acid leaching, leaving holes uniformly distributed. It should also be mentioned that no obvious nanoparticles of iron or iron oxide can be observed from the TEM images. These results may imply that most Fe components are reduced by carbon at high temperature and then the soluble Fe species are removed during the acid leaching.
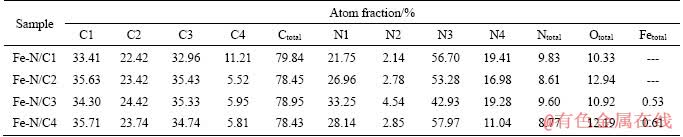
XPS is used to investigate the surface compositions and valence states of the elements on Fe-N/C catalysts and the results are shown in Figure 3. The C 1s spectra can be deconvoluted into four peaks at about 284.7, 285.3, 286.4 and 288.7 eV, which could be assigned to C1(C—C/C=C), C2(C—N), C3(C—O/C=N) and C4(C=O) [16, 18, 19], respectively. The corresponding contents of the carbon species are displayed in Table 1, coupled with the total percentages of C, N, O and Fe on the surface of the catalysts. It can be observed that the total carbon percentages are comparable. For the N 1s spectra, the peaks at about 398.4, 399.2, 400.2 and 401.3 eV are attributed to pyridinic N (N1), pyrrolic N (N2), graphitic N (N3) and oxidized N (N4) [15, 20, 21], respectively. With SiO2 proportion increasing, the atomic ratio of pyridinic-N increases from 21.75 at.% for Fe-N/C1, then decreases to 28.14 at.% for Fe-N/C4, and reaches the maximum value of 33.25 at.% for Fe-N/C3 when the mass ratio of SiO2 to soybean is 3:4. Up to now, the real catalytic active sites of the N species for the ORR are still a controversial issue, though there are tremendous researches on the N-doped materials. However, it is generally recognized that the pyridinic-N species can function as the active centers, implying that high pyridinic-N content exhibits better catalytic performance [22, 23]. In the present study, it can be noticed from Table 1 that the total nitrogen percentage is close to 9 at.%, higher than those on the other nitrogen- doped materials [4, 6]. On the other hand, the total nitrogen percentage of Fe-N/C3 catalyst isn’t the highest (9.60 at.%), a little lower than that of Fe-N/C1 (9.83 at.%), while the pyridinic-N percentage of Fe-N/C3 catalyst is the highest, about 33.25 at.%, apparently higher than those of the other catalysts, which might contribute to its superior ORR activity.
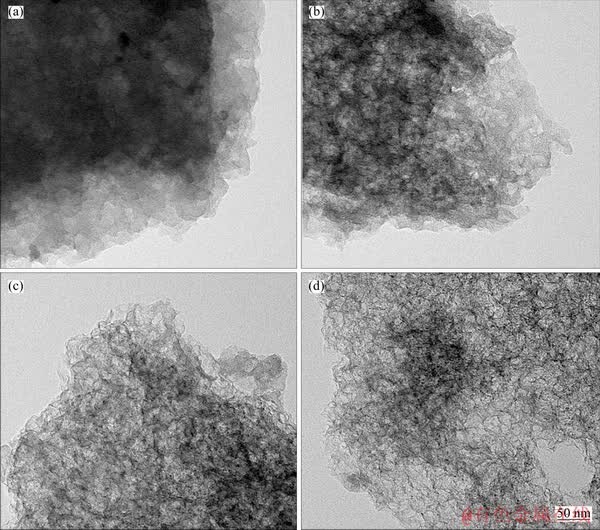
Figure 2 TEM images of Fe-N/C1 (a), Fe-N/C2 (b), Fe-N/C3 (c) and Fe-N/C4 (d) catalysts
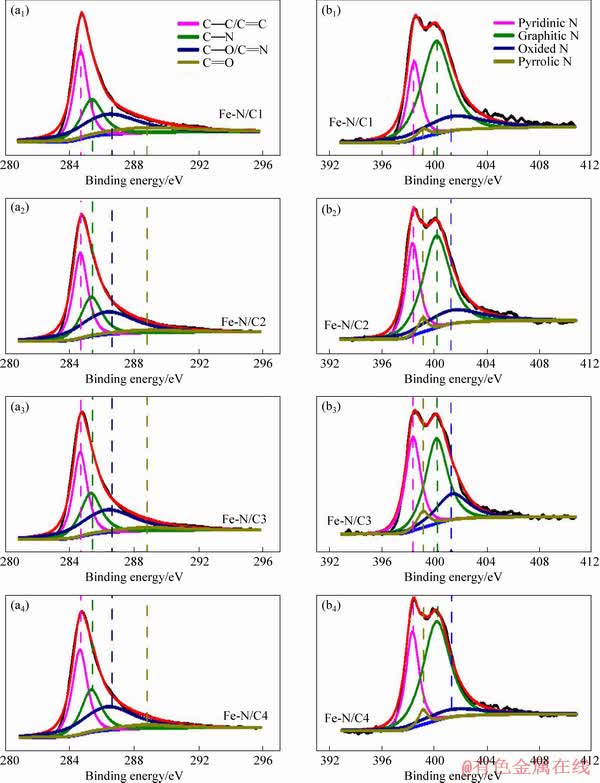
Figure 3 C 1s (a) and N 1s (b) XPS spectra for Fe-N/C catalysts
Table 1 Percentages of C and N species as well as total percentages of C, N, O and Fe on surface of Fe-N/C catalysts
The XPS spectra of Fe 2p for the series of Fe-N/C catalysts are shown in Figure 4. It can be seen that the peak intensity of Fe 2p is not obvious for Fe-N/C1 and Fe-N/C2 catalysts, and then gradually increases for Fe-N/C3 and Fe-N/C4 catalysts with SiO2 mass ratio increasing, which is consistent with the XPS results that the total Fe percentage increases, and the XRD results that the Fe2O3 exists in Fe-N/C3 and Fe-N/C4 catalysts. The fitting Fe 2p peaks located at about 710.9 eV and 724.4 eV can be ascribed to Fe 2p3/2 and Fe 2p1/2, respectively, which can be utilized to qualitatively determine the ionic states of iron. Besides the two peaks, the presence of the satellite peak near 718.1 eV is consistent with the characterization of Fe3+ [24-26]. Moreover, no other obvious peaks are detected for Fe 2p3/2 of Fe2+ at 709.5 eV, suggesting the excellent formation of pure ferric oxides combined with the above XRD patterns [24]. On the other hand, the chemical state of O was also investigated by XPS fitting analysis as shown in Figure 5. The spectra of O 1s correspond with the oxygen species: 531.1 eV (O2-), 532.9 eV (OH-) [17, 27]. Combined with the above Fe 2p spectra, it can be further inferred that the iron species in Fe-N/C exists in the form of Fe2O3.
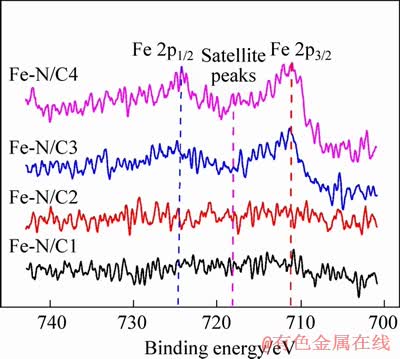
Figure 4 Fe 2p XPS spectra for Fe-N/C catalysts
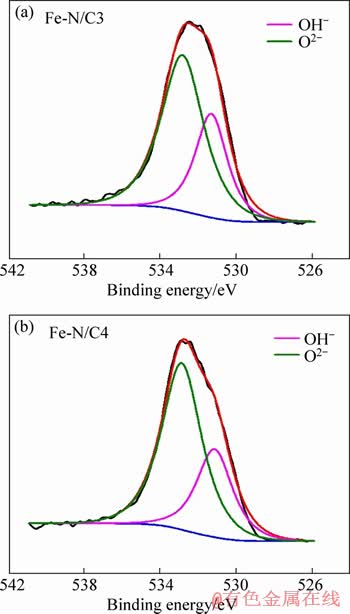
Figure 5 O1s XPS spectra of Fe-N/C3 (a) and Fe-N/C4 catalysts (b)
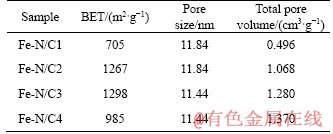
Figure 6 shows the N2 adsorption-desorption isotherms of Fe-N/C catalysts and their corresponding pore size distributions. The BET surface areas, the corresponding pore sizes and the total volumes of the catalysts calculated from Figure 6 are shown in Table 2. As seen from the figure, the significant hysteresis loop occurring at relative pressure on approaching P/P0>0.5 approximately conforms to the type-VI characteristic curve [28], showing the mesoporous structure, which may be attributed to the fact that the silica template was removed during the acid leaching and thus resulted in the hollow mesoporous inside. At the same time, the porous carbons show large uptake at low relative pressure, which is due to the existence of well-developed micro-porosity. It may be due to the escape of the small-molecule gases during the carbonization of the precursors. The pore-size distributions calculated from the adsorption branch on the basis of the DFT model are also shown in the insets. It can be seen that the pore sizes are substantially around 11 nm. The BET surface area of Fe-N/C3 catalyst is the highest, about 1298 m2/g; while the total pore volume for Fe-N/C4 catalyst is the largest, around 1.370 cm3/g.
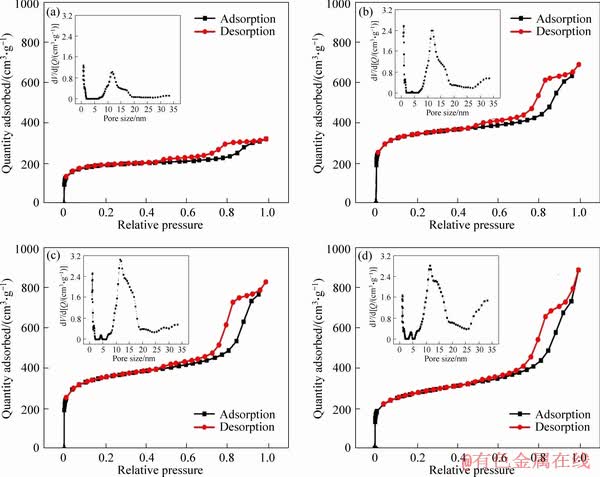
Figure 6 Nitrogen adsorption-desorption isotherms for Fe-N/C1 (a), Fe-N/C2 (b), Fe-N/C3 (c) and Fe-N/C4 (d) (Insets represent the corresponding pore-size distributions curves of catalysts)
Table 2 BET surface areas, corresponding pore sizes and pore volumes of catalysts calculated from Figure 6
3.2 Electrocatalytic activity for ORR
Figure 7(a) presents the CV curves of Fe-N/C catalysts in N2-saturated 0.1 mol/L NaOH solution with the scan rate of 10 mV/s. For all the samples, the CVs show wide reversible electrochemical double-layer curves, and the nearly rectangular shape of the voltammogram indicates that the graphite structure of the catalyst has high electron conductivity and good charge dissemination. It can be seen that the thickness of the electric double layer tends to increase substantially and then decreases as the proportion of the added SiO2 increases, with the maximum value for Fe-N/C3 catalyst, which might be relative with its maximum BET specific surface area.
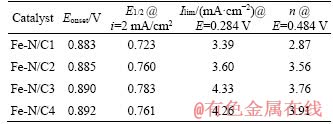
The electrocatalytic activities of Fe-N/C catalysts toward the ORR are compared in Figure 7(b) using the LSV recorded in O2-saturated 0.1 mol/L NaOH solution, and the obtained electrochemical parameters associated with the ORR are all summarized in Table 3. The Fe-N/C3 catalyst displays the higher ORR electrocatalytic activity in terms of the most positive half-wave potential (E1/2) and the comparable onset potential (Eonset) to that of Fe-N/C4, being about 0.783 V and 0.890 V, respectively, all comparable to those on the same type ORR catalysts [4, 6, 7]. This may be mainly attributed to the higher BET specific surface area, the appropriate pore-size, as well as the higher contents for the pyridinic-N and the total nitrogen for Fe-N/C3 catalyst [24, 29-31]. The higher BET specific surface area and the appropriate pore-size would accelerate the transportation of the molecules, and the high specific surface area may increase the exposure probability of the accessible active sites. All these may contribute to the higher activity toward the ORR. As for the higher contents for the pyridinic-N and the total N on the surface of Fe-N/C3, which have been discussed in the former part, would also benefit to enhance the catalytic property toward the ORR for the M-N/C catalysts.
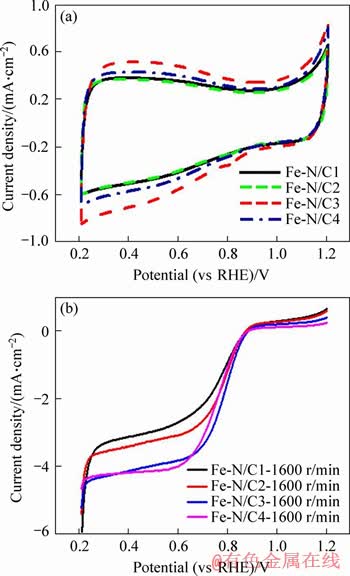
Figure 7 CV curves of Fe-N/C catalysts in N2-saturated 0.1 mol/L NaOH solution (a), LSV curves of Fe-N/C catalysts in O2-saturated 0.1 mol/L NaOH electrolyte with rotation rate of 1600 r/min (b)
Table 3 Electrochemical parameters derived from LSV curves recorded by RDE in O2-saturated 0.1 mol/L NaOH solution
3.3 Reaction mechanism analysis
The LSV curves of Fe-N/C3 catalyst with different rotation rates in 0.1 mol/L O2-saturated NaOH electrolyte are presented in Figure 8(a). It shows that Eonset of the ORR is comparable, and the limited current density (Ilim) increases with the rotation rate increasing. The electron transfer number (n) at different potentials are calculated through the slopes of the curves of j-1 vs. ω-1/2 based on the Koutecky-Levich (K-L) equation, which can be expressed as follows [32]:
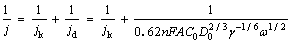
where j is the measured current density; jk and jd are the kinetic and diffusion-limiting current densities, respectively; Co is the concentration of O2 in 0.1 mol/L NaOH solution (1.14×10-6 mol/cm3); Do is the O2 diffusion coefficient in 0.1 mol/L NaOH solution (1.73×10-5 cm2/s); ω is the rotation rate of the disk (ω=2πN, N is the linear rotation speed); n is the overall number of electrons transferred in oxygen reduction; F is the Faraday constant (F=96485 C/mol); γ is dynamic viscosity (0.01 cm2/s) of the electrolyte. The K-L plots of j-1 vs. ω-1/2 for Fe-N/C3 catalyst based on data from 0.284 V to 0.584 V are exhibited in the inset of Figure 8(a). Good linearity and parallelism of the K-L plots imply the first-order ORR kinetics concerning the concentration of dissolved oxygen. Electron transfer numbers calculated from the K-L equation for the series of Fe-N/C catalysts are shown in Figure 8(b). It can be seen that Fe-N/C3 catalyst show the relatively higher number of transferred electrons at various potentials, all comparable to four, which demonstrates a dominant four-electron transfer pathway during the ORR.
To further investigate the electron transfer pathway of the ORR, the RRDE measurements with the rotation speed of 1600 r/min and the scanning rate of 0.010 V/s were also conducted. The electron transfer numbers and the calculated HO2- yields involved in the ORR on Fe-N/C3 catalyst are presented in Figure 8(c). As shown in curves, the electron transfer numbers are mainly higher than 3.8, comparable to the values calculated by the K-L equation. Meanwhile, the relatively low HO2- yields (<8%) can also be observed in the inset. All these results further reflect that oxygen reduction on Fe-N/C3 catalyst goes through the apparent/indirect 4-electron transfer pathway.
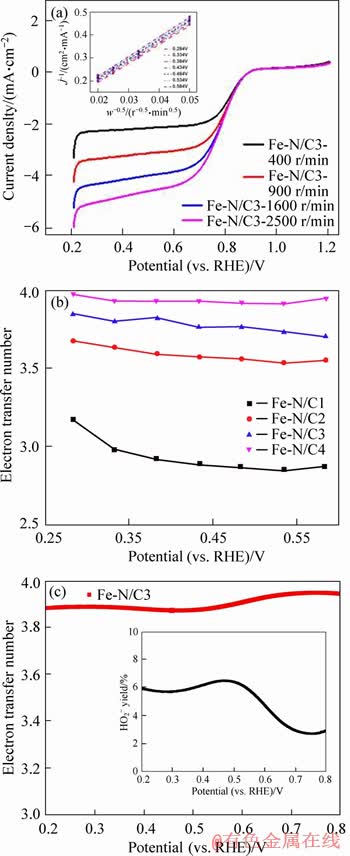
Figure 8 LSV curves of Fe-N/C3 catalyst in O2-saturated 0.1 mol/L NaOH solution at different rotation rates (inset represents the K-L plots of j –1 vs. ω–1/2 at different potentials) (a), electron transfer numbers calculated from K-L equation for Fe-N/C catalysts (b), calculated electron transfer numbers of Fe-N/C3 catalyst recorded by RRDE tests (inset is corresponding HO2- yields)(c)
3.4 Stability and methanol tolerance
Although Fe-N/C3 catalyst shows the excellent activity toward the ORR, the long-term stability and methanol tolerance are also very important to evaluate their performance. The methanol-tolerant for Fe-N/C3 catalyst during the ORR was also investigated in O2-saturated 0.1 mol/L NaOH solution with 1 mol/L methanol. As shown in Figure 9(a), the LSV curve of Fe-N/C3 catalyst doesn’t change significantly when the solution contains 1 mol/L methanol. These results indicate that Fe-N/C3 catalyst is less affected by the cross-effect and has better resistance to methanol oxidation compared to the commercial Pt/C [33].
The I-t curve in O2-saturated 0.1 mol/L NaOH solution for Fe-N/C3 catalyst, with the constant potential and disk rotation speed being 0.608 V and 900 r/min, respectively, is shown in Figure 9(b). The relative current density for Fe-N/C3 catalyst toward the ORR suffers a severe degradation within the first 100 s, and then the falling speed is slower, which still retains 84.39% of the initial current density after 7200 s.
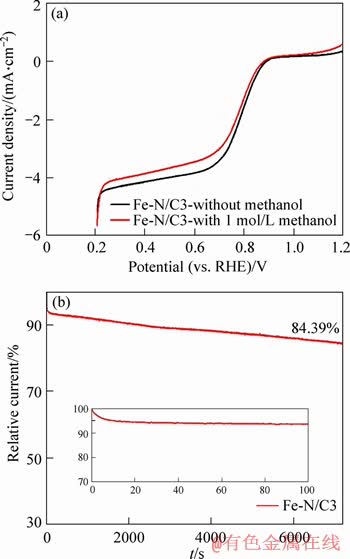
Figure 9 LSV curves of Fe-N/C3 catalyst in O2-saturated 0.1 mol/L NaOH solution with or without 1 mol/L methanol (a), I-t curve of Fe-N/C3 catalyst at 0.608 V in O2 saturated 0.1 mol/L NaOH solution with rotation speed of 900 r/min (inset shows local magnification of curve)(b)
4 Conclusions
In summary, we developed a facile and cost-effective procedure on the basis of templating method to synthesize an iron-modified mesoporous nitrogen-doped carbons (Fe-N/C) using the cheap and environmentally friendly soybean-biomass as the raw materials. Among these prepared catalysts, Fe-N/C3 sample with the mass ratio of SiO2 to soybean being 3:4 exhibits the relatively favorable ORR catalytic activity. The enhancement in the electrocatalytic activity might be closely related to the higher BET specific surface area and hierarchical mesoporous structure owing to the removal of SiO2 templates. Especially, the higher contents of pyridinic-N and total nitrogen on the surface of Fe-N/C3 catalyst may play another crucial role in functioning as the active sites for the ORR. It is quite impressive that Fe-N/C3 catalyst could considerably facilitate the kinetics of the ORR and the O2 reduction on it goes through the four-electron pathway in alkaline media. Hence, this study demonstrates a feasible strategy to design highly efficient and durable N-doped non-precious catalysts for the large-scale commercial application with the combination of hard-templating synthesis and the precursor pyrolysis.
References
[1] KAKATIN, MAITI J, LEE S H, JEE S H, VISWANATHAN B, YOON Y S. Anode catalysts for direct methanol fuel cells in acidic media: Do we have any alternative for Pt or Pt-Ru? [J]. Chemical Reviews, 2014, 114(24): 12397-12429. DOI: org/10.1021/cr400389f.
[2] XU You, ZHANG Bin. Recent advances in porous Pt-based nanostructures: synthesis and electrochemical applications [J]. Chemical Society Reviews, 2014, 43(8): 2439-2450. DOI: 10.1039/C3CS60351B.
[3] BASHYAM R, ZELENAY P. A class of non-precious metal composite catalysts for fuel cells [C]// Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group. 2011: 247-250.
[4] LEFEVRE M, PROIETTI E, JAOUEN F, DODELET J P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells [J]. Science, 2009, 324(5923): 71-74. DOI: 10.1126/science.1170051.
[5] TAN Hai-bo, LI Yun-qi, JIANG Xiang-fen, TANG Jing, WANG Zhong-li, QIAN Hua-yu, MEI Peng, MALGRAS V, BANDO Y, YAMAUCHI Y. Perfectly ordered mesoporous iron-nitrogen doped carbon as highly efficient catalyst for oxygen reduction reaction in both alkaline and acidic electrolytes [J]. Nano Energy, 2017, 36: 286-294. DOI: 10.1016/j.nanoen.2017.04.014.
[6] ZHANG Lin-jie, SU Zi-xue, JIANG Fei-long, YANG Ling-ling, QIAN Jin-jie, ZHOU You-fu, LI Wen-mu, HONG Mao-chun. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions [J]. Nanoscale, 2014, 6(12): 6590-6602. DOI: 10.1039/ c4nr00348a.
[7] BISWAL B P, CHANDRA S, KANDAMBETH S, LUKOSE B, HEINE T, BANERJEE R. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks [J]. Journal of the American Chemical Society, 2013, 135(14): 5328-5331. DOI: 10.1021/ja4017842.
[8] WANG Ke-liang, WANG Hui, JI Shan, FENG Han-qing, LINKOV V, WANG Rong-fang. Biomass-derived activated carbon as high-performance non-precious electrocatalyst for oxygen reduction [J]. RSC Advances, 2013, 3(30): 12039-12042. DOI: 10.1039/c3ra41978a.
[9] GUO Chao-zhong, LIAO Wen-li, LI Zhong-bin, SUN Ling-tao, CHEN Chang-guo. Easy conversion of protein-rich enoki mushroom biomass to a nitrogen-doped carbon nanomaterial as a promising metal-free catalyst for oxygen reduction reaction [J]. Nanoscale, 2015, 7(38): 15990-15998. DOI: 10.1039/c5nr03828f.
[10] LIU Ying-ying, RUAN Jian-ming, SANG Shang-bin, ZHOU Zhong-cheng, WU Qiu-mei. Iron and nitrogen co-doped carbon derived from soybeans as efficient electro-catalysts for the oxygen reduction reaction [J]. Electrochimica Acta, 2016, 215:388-397.DOI:10.1016/j.electacta.2016.08.090.
[11] LIANG Ji, ZHENG Yao, CHEN Jun, LIU Jian, HULICOVA- JURCAKOVA D, JARONIEC M, QIAO Shi-zhang. Facile oxygen reduction on a three-dimensionally ordered macroporous graphitic C3N4/carbon composite electrocatalyst [J]. Angewandte Chemie: International Edition, 2012, 51(16): 3892-3896. DOI: 10.1002/anie. 201107981.
[12] SUN Zhen-kun, LIU Yong, LI Bin, WEI Jing, WANG Ming-hong, YUE Qin, DENG Yong-hui, KALIAGUINE S, ZHAO Dong-yuan. General synthesis of discrete mesoporous carbon microspheres through a confined self-assembly process in inverse opals [J]. ACS Nano, 2013, 7(10): 8706-8714. DOI: 10.1021/nn402994m.
[13] LEE J, KIM J, HYEON T. Recent progress in the synthesis of porous carbon materials [J]. Advanced Materials, 2006, 18(16): 2073-2094. DOI:10.1002/adma.200501576.
[14] WANG Zhong-li, ZHANG Xin-bo, LIU Xiao-juan, LV Min-feng, YANG Kui-yue, MENG Jian. Co-gelation synthesis of porous graphitic carbons with high surface area and their applications [J]. Carbon, 2011, 49(1): 161-169. DOI: 10.1016/j.carbon.2010.08.056.
[15] PENG Hong-liang, HOU San-ying, DANG Dai, ZHANG Bing-qing, LIU Fang-fang, ZHENG Rui-ping, LUO Fan, SONG Hui-yu, HUANG Pei-yan, LIAO Shi-jun. Ultra-high- performance doped carbon catalyst derived from o-phenylenediamine and the probable roles of Fe and melamine [J]. Applied Catalysis B: Environmental, 2014, 158-159: 60-69. DOI: 10.1016/j.apcatb.2014.03.033.
[16] MA Zhao-ling, HUANG Xiao-bing, DOU Shuo, WU Jiang-hong, WANG Shuang-yin. One-Pot synthesis of Fe2O3 nanoparticles on nitrogen-doped graphene as advanced supercapacitor electrode materials [J]. Journal of Physical Chemistry C, 2014, 118(31): 17231-17239. DOI: 10.1021/ jp502226j.
[17] SUN Meng, DONG You-zhen, ZHANG Gong, QU Jiu-hui, LI Jing-hong. Alpha-Fe2O3 spherical nanocrystals supported on CNTs as efficient non-noble electrocatalysts for the oxygen reduction reaction [J]. Journal of Materials Chemistry A, 2014, 2(33): 13635-13640. DOI: 10.1039/ c4ta02172j.
[18] HAN Zhuo, TANG Zhi-hong, LI Peng, YANG Guang-zhi, ZHENG Qing-bin, YANG Jun-he. Ammonia solution strengthened three-dimensional macro-porous graphene aerogel [J]. Nanoscale, 2013, 5(12): 5462-5467. DOI: 10.1039/c3nr00971h.
[19] LIN Zi-yin, SONG Min-kyu, DING Yong, LIU Yan, LIU Mei-lin, WONG Ching-ping. Facile preparation of nitrogen-doped graphene as a metal-free catalyst for oxygen reduction reaction [J]. Physical Chemistry Chemical Physics, 2012, 14(10): 3381-3387.DOI:10.1039/c2cp00032f.
[20] LI Xiao-lin, WANG Hai-liang, ROBINSON J T, SANCHEZH, DIANKOV G, DAI Hong-jie. Simultaneous nitrogen doping and reduction of graphene oxide [J]. Journal of the American Chemical Society, 2009, 131(43): 15939-15944. DOI:10.1021/ja907098f.
[21] CHOUSHURY D, DAS B, SARMA D D, RAO C N R. XPS evidence for molecular charge-transfer doping of graphene [J]. Chemical Physics Letters, 2010, 497(1): 66-69. DOI: 10.1016/j.cplett.2010.07.089.
[22] GUO Dong-hui, SHIBUYA R, AKIBA C, SAJI S, KONDO T, NAKAMURA J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts [J]. Science, 2016, 351(6271): 361-365. DOI: 10.1126/science.aad0832.
[23] YU Ding-shan, ZHANG Qiang, DAI Li-ming. Highly efficient metal-free growth of nitrogen-doped single-walled carbon nanotubes on plasma-etched substrates for oxygen reduction [J]. Journal of the American Chemical Society, 2010, 132(43): 15127-15129. DOI:10.1021/ja105617z.
[24] YAMASHITA T, HAYES P. Analysis of XPS spectra of Fe2+, and Fe3+, ions in oxide materials [J]. Applied Surface Science, 2008, 254(8): 2441-2449. DOI: 10.1016/j.apsusc.2007.09. 063.
[25] MILLS P, SULLIVAN J L. A study of the core level electrons in iron and its three oxides by means of X-ray photoelectron spectroscopy [J]. Journal of Physics D: Applied Physics, 2000, 16(5): 723. DOI: 10.1088/0022- 3727/16/5/005.
[26] MOU Fang-zhi, GUAN Jian-guo, XIAO Zhi-dong, SUN Zhi-gang, SHI Wei-dong, FAN Xi-an. Solvent-mediated synthesis of magnetic Fe2O3 chestnut-like amorphous-core/ γ-phase-shell hierarchical nanostructures with strong As(V) removal capability [J]. Journal of Materials Chemistry, 2011, 21(14): 5414-5421.DOI:10.1039/c0jm03726e.
[27] KAWABE T, SHIMOMURA S, KARASUDA T, TABATAK, SUZUKI E, YAMAGUCHI Y. Photoemission study of dissociatively adsorbed methane on a pre-oxidized SnO2, thin film [J]. Surface Science, 2000, 448(2): 101-107. DOI: 10.1016/S0039-6028(99)00997-8.
[28] KRUK M, JARONIEC M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials [J]. Chemistry of Materials, 2001, 13(10): 3169-3183. DOI: 10.1021/cm0101069.
[29] CHMIOLA J, YUSHIN G, GOGOTSI Y, PORTET C, SIMON P, TABERNA P L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer [J]. Science, 2006, 313(5794): 1760-1763. DOI: 10.1126/science. 1132195.
[30] PAN Fu-ping, CAO Zhong-yue, ZHAO Qiu-ping, LIANG Hong-yu, ZHANG Jun-yan. Nitrogen-doped porous carbon nanosheets made from biomass as highly active electrocatalyst for oxygen reduction reaction [J]. Journal of Power Sources, 2014, 272: 8-15. DOI: 10.1016/j.jpowsour. 2014.07.180.
[31] GOU Chao-zhong, LIAO Wen-li, CHEN Chang-guo. Design of a non-precious metal electrocatalyst for alkaline electrolyte oxygen reduction by using soybean biomass as the nitrogen source of electrocatalytically active center structures [J]. Journal of Power Sources, 2014, 269(4): 841-847. DOI:10.1016/j.jpowsour.2014.07.024.
[32] TONG Feng, LIAO Wen-li, LI Zhong-bin, SUN Ling-tao, SHI Dong-ping, GUO Chao-zhong, HUANG Yu, WANG Yi, CHENG Jing, LI Yan-rong, DIAO Qi-zhi. Heavily graphitic-nitrogen self-doped high-porosity carbon for the electrocatalysis of oxygen reduction reaction [J]. Nanoscale Research Letters, 2017, 12(1): 595. DOI: 10.1186/s11671- 017-2364-6.
[33] PAN Si-yu, CAI Zhuang, DUAN Ya-qiang, YANG Liu, TANG Bo, JING Bao-jian, DAI Ying, XU Xin, ZOU Jin-long. Tungsten diselenide/porous carbon with sufficient active edge-sites as a co-catalyst/Pt-support favoring excellent tolerance to methanol-crossover for oxygen reduction reaction in acidic medium [J]. Applied Catalysis B: Environmental, 2017, 219: 18-29. DOI: 10.1016/j.apcatb. 2017.07.011.
(Edited by YANG Hua)
中文导读
基于大豆的Fe-N掺杂的介孔碳:一种高效低成本的非贵金属氧还原反应电催化剂
摘要:氧还原反应(ORR)在许多储能和能量转换装置中起着至关重要的作用。目前,在碱性介质中开发廉价、高性能的碳基非贵金属ORR催化剂仍受到广泛关注。本文以生物质大豆为氮源和碳源,SiO2为模板剂合成了介孔Fe-N/C催化剂。模板剂SiO2可同时对Fe-N/C催化剂的表面功能和多孔结构进行优化。研究表明,SiO2与大豆质量比为3:4时制备的Fe-N/C3催化剂具有优异的电催化性能、长期稳定性和甲醇耐受性,其ORR的起始电位和半波电位相对于可逆氢电极(RHE)分别约为0.890 V和0.783 V。同时,可以观察到ORR在催化剂表面是四-电子转移路径。结果表明,高BET比表面积和合适的孔径以及较高的吡啶氮和总氮含量对提高Fe-N/C3催化剂的ORR性能具有重要作用。这些结果表明,开发基于经济和可持续的生物质材料大豆的廉价且高效的非贵金属ORR电化学催化剂具有较大的可行性。
关键词:生物质;氧还原反应;电催化剂;氮掺杂碳
Foundation item: Project(21406273) supported by the National Natural Science Foundation of China
Received date: 2019-04-10; Accepted date: 2019-10-22
Corresponding author: WU Qiu-mei, PhD, Associate Professor, Tel: +86-731-88830908; E-mail: wuqiumei20073@126.com; ORCID: 0000- 0002-0941-9655
Abstract: Oxygen reduction reaction (ORR) plays a crucial role in many energy storage and conversion devices. Currently, the development of inexpensive and high-performance carbon-based non-precious-metal ORR catalysts in alkaline media still gains a wide attention. In this paper, the mesoporous Fe-N/C catalysts were synthesized through SiO2-mediated templating method using biomass soybeans as the nitrogen and carbon sources. The SiO2 templates create a simultaneous optimization of both the surface functionalities and porous structures of Fe-N/C catalysts. Detailed investigations indicate that the Fe-N/C3 catalyst prepared with the mass ratio of SiO2 to soybean being 3:4 exhibits brilliant electrocatalytic performance, excellent long-term stability and methanol tolerance for the ORR, with the onset potential and the half-wave potential of the ORR being about 0.890 V and 0.783 V (vs RHE), respectively. Meanwhile, the desired 4-electron transfer pathway of the ORR on the catalysts can be observed. It is significantly proposed that the high BET specific surface area and the appropriate pore-size, as well as the high pyridinic-N and total nitrogen loadings may play key roles in enhancing the ORR performance for the Fe-N/C3 catalyst. These results suggest a feasible route based on the economical and sustainable soybean biomass to develop inexpensive and highly efficient non-precious metal electrochemical catalysts for the ORR.


