文章编号:1004-0609(2010)02-0288-05
MoO3包覆对锂一次电池CuF2正极材料性能的影响
刘修明,王先友,伍 文,王 欣,王国宝,杨顺毅
(湘潭大学 化学学院 环境友好化学与应用教育部重点实验室,湘潭 411105)
摘 要:
通过球磨CuF2和MoO3的混合物,制成CuF2/MoO3复合材料。通过X射线衍射(XRD)、扫描电镜(SEM)和电化学测试等研究CuF2/MoO3复合材料的结构和电化学性能。结果表明:MoO3晶粒均匀地附着在CuF2的表面,形成均匀的导电网,从而使制备的CuF2/MoO3复合材料具有高导电性,可作为锂一次电池正极活性材料。以0.1C倍率放电,截止电压为1.5 V,CuF2/MoO3复合材料放电比容量高达483mA?h/g,放电电位平台为2.5 V,明显高于CuF2正极材料的278 mA?h/g,接近其理论容量528 mA?h/g。
关键词:
锂一次电池;正极材料;CuF2/MoO3复合材料;电化学性能;
中图分类号:TM912 文献标识码:A
Effects of MoO3 encapsulating on performances of CuF2 cathode material for application of lithium primary batteries
LIU Xiu-ming, WANG Xian-you, WU Wen, WANG Xin, WANG Guo-bao, YANG Shun-yi
(Key Laboratory of Environmentally Friendly Chemistry and Applications, Ministry of Education, School of Chemistry, Xiangtan University, Xiangtan 411105, China)
Abstract: The CuF2/MoO3 composite was prepared by milling the mixture of CuF2 and MoO3. The structural and electrochemical properties of the CuF2/MoO3 composite were investigated by X-ray diffraction (XRD), scanning electron microscope (SEM) and electrochemical experiments. The results indicate that the MoO3 grains homogeneously coat on the surface of CuF2 and then form a high conductive matrix, which can be used for cathode material of lithium primary batteries. The specific discharge capacity of CuF2/MoO3 composite is as high as 483 mA?h/g at a rate of 0.1C and the cutoff of 1.5 V, which is apparently higher than that of the lithium primary battery using pure CuF2 (278 mA?h/g) as cathode active material and close to the theoretical specific capacity of CuF2 (528 mA?h/g).
Key words: lithium primary battery; cathode material; CuF2/MoO3 composite; electrochemical performance
锂一次电池具有工作电压和比能量高、工作温度范围宽、自放电率低等优点,在电子工业、医疗器械、远程仪表控制和国防领域有广泛的应用前景。目前的锂一次电池主要有Li-MnO2[1]、Li-I2[2]、Li-Ag2V4O11[3]、Li-SO2[4]、Li-SOCl2[5]、Li-FeS2[6]、Li-(CF)n[7]及Li-CuO[8]等。随着电子技术的发展,对锂一次电池的性能要求越来越高。金属氟化物正极材料是近年来发展起来的新一代有前景的锂电池正极材料,其电极反应不同于传统锂离子嵌入机理,锂离子在这类材料中不但可能发生嵌入反应,还可以和锂发生如式(1)所示的化学转换反应来贮存能量,因此其放电容量远远高于传统锂离子嵌入反应。另外,由于氟的电负性大,金属氟化物正极材料的工作电压远高于其他金属氧化物、金属硫化物等正极材料。近几年,国内外对FeF3/C、BiF3/C、BiOF/C等作为锂二次电池正极材料进行了大量研究,并获得了很好的结果[9?15]。王先友等[16?17]在BiOF和FeF3作为锂离子电池正极材料方面的研究取得了较大 进展。
mLi++me+MeFn→nLim/nF+Me (1)
CuF2正极材料作为高性能金属氟化物正极材料之一,其工作电压高达3.55 V,质量比能量密度为1 874 W?h/kg,体积比能量密度为7 870 W?h/kg,是有前景的锂一次电池新型正极材料。关于Li-CuF2作为锂一次电池正极材料的研究,在40年前就已开始[18?19]。但早期研究中由于材料含有CuF2?2H2O和CuOHF,其实际可利用比容量非常低,并且和其他金属氟化物材料一样,CuF2有很强的离子键特征和很大的能带间隙,导致其导电性很差,放电平台很低。ELLIOTT等[18]分别采用直接烧结技术、在48%的HF溶液中电沉积、有机溶液涂制和高压压膜等方法制备了CuF2正极材料,但都没有取得实质性进展。最近,AMATUCCI等[20]报道,可将金属氟化物用导电性化合物修饰,制备成如图1所示的混合导电复合物(Mixed conductive matrix, MCM)能显著提高其电化学性能。

图1 新型纳米复合正极材料示意图
Fig.1 Schematic diagram showing nanocomposite as cathode materials
MoO3是由金属原子Mo在中心、O原子在角边构成的MoO6八面体共用O原子形成链连接,每两个相似的链共边连接形成层状的MoO3化学计量结构。由于其结构特殊性,MoO3具有良好的离子和电子导电性,用其作为表面修饰剂对材料进行包覆,可以明显提高材料的导电性[21]。因此,本文作者通过利用MoO3与CuF2球磨,将导电性良好的MoO3包覆在CuF2表面,通过进一步退火处理制备高导电性的MCM材料作为锂一次电池正极材料,并研究其电化学性能。
1 实验
1.1 CuF2/MoO3复合材料的制备
将无水CuF2 (深圳市迈瑞尔化学技术有限公司, AR99%)与MoO3(天津市科密欧化学试剂开发中心, AR99.5%)按质量分数比为85%?15%在ND2?2L行星式球磨机中球磨2 h,再在管式炉中在氩气保护下于200 ℃退火2 h,形成图1所示的混合导电化合物。
1.2 CuF2/MoO3正极材料的结构测试
用日本理学D/Max?3C型X射线衍射仪对正极活性材料进行晶体结构分析,射线源为CuKα,管电流为20 mA,管电压为36 kV,扫描速度为8 (?)/min,扫描范围2θ为10?~80?;用Hitachi X?650扫描电镜测定样品的形貌。
1.3 电池的制备及性能测试
将正极活性物质、乙炔黑和聚偏氟乙烯(PVDF)按质量比77?15?8的比例混合均匀,滴加适量的N-甲基-2-吡咯烷酮(NMP),超声波分散20 min,得糊状物质,将其均匀涂成极片,在70 ℃真空干燥12 h。电解液用1 mol/L LiPF6的EC/DEC(体积比为1?1)的混合液,以金属锂片为负极,Celgard2400为隔膜。将上述材料在充满氩气的手套箱(MIKROUNA 1220/750)中组装成2025型扣式电池。采用深圳新威公司电池充放电测试仪进行恒电流放电测试,放电截止电压1.5 V,使用上海辰华公司的CHI660A电化学工作站进行交流阻抗和循环伏安测试。
2 结果与讨论
2.1 X射线衍射结果分析
图2所示为CuF2和CuF2/MoO3复合材料的XRD谱。从图2中可见,CuF2和CuF2/MoO3结晶度高,其中CuF2晶体主要沿(011)、(110)、(101)晶面方向生长。对照标准卡70?1936可见,样品在晶面(011)、(101)、
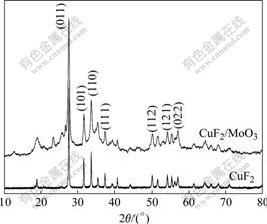
图2 CuF2和CuF2/MoO3复合材料的XRD谱
Fig.2 XRD patterns of CuF2 and CuF2/MoO3 composite
(110)、(111)、(112)、(121)、(022)等处衍射峰与CuF2理论衍射峰所在2θ位置基本吻合,各峰值符合单斜晶系特征。样品CuF2/MoO3的XRD谱中主要衍射峰2θ位置与CuF2基本吻合,表明在球磨过程中,MoO3对CuF2包覆没有改变CuF2的结构,经过球磨制备的样品依然为单斜晶系结构的化合物,晶体主要沿CuF2晶体的(011)、(110)、(101)晶面生长。
此外,图2中CuF2的衍射峰相当尖锐,说明样品结晶性能好,而通过球磨包覆MoO3的样品衍射峰强度略为变小,衍射峰半峰宽度略增大,表明球磨过程中样品的晶粒尺寸变小。
2.2 扫描电镜(SEM)分析
图3所示为CuF2和CuF2/MoO3复合材料的SEM像。从图3(a)中可以看出,CuF2正极材料的颗粒大小均匀,平均粒径约为0.5 mm,每个粒子表面光滑,而且有团聚现象,大量一次粒子聚集成二次粒子。而被MoO3包覆着的CuF2/MoO3样品相对于CuF2,其颗粒表面明显有更小的微粒附着,由于球磨后微小粒子的尺寸效应,微粒之间的团聚明显增加,形成了类似于图1所示的混合导电化合物(MCM)。
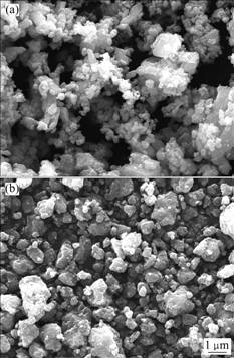
图3 CuF2 (a)和CuF2/MoO3(b)复合材料的SEM像
Fig.3 SEM images of CuF2 (a) and CuF2/MOO3 composite (b)
2.3 电化学性能分析
2.3.1 循环伏安测试
图4所示为CuF2和CuF2/MoO3复合材料在扫描速率为0.1 mV/s时的循环伏安曲线。从图4可以看出,还原峰对应于嵌锂过程,氧化峰对应于脱锂过程。从图4中还可观察到很明显的还原峰,对应于Cu2+/Cu的还原。放电(嵌锂)过程形成了纳米晶的Cu粉和LiF[20],由于纳米晶的Cu粉具有高的导电性,使得反应能不断进行,反应方程式如下:
2Li++2e+CuF2→Cu+2LiF (2)
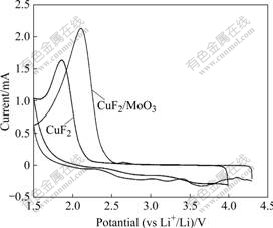
图4 CuF2和CuF2/MoO3的循环伏安曲线
Fig.4 CV curves of CuF2 and CuF2/MoO3
由图4可以发现,CuF2/MoO3复合材料还原峰明显比CuF2的高,还原峰覆盖的面积也明显大得多。虽然MoO3不参与电池反应,对电池的容量没有贡献,但从图4可见,MoO3包覆能明显增强材料的导电性,从而提高材料的电化学性能,显著增大材料的放电比容量。
2.3.2 放电性能测试
为了研究MoO3包覆对Li-CuF2电池放电性能的影响,分别用CuF2/MoO3和CuF2作正极活性物质制备2025型扣式锂一次电池,以理论容量的0.1C倍率,截止电压为1.5 V进行放电测试,结果如图5所示。
从图5可以看出,CuF2/MoO3作正极活性物质的锂一次电池性能明显优于未包覆的电池,Li-CuF2的放电比容量为278 mA?h/g,而Li-CuF2/MoO3的放电容量高达483 mA?h/g,接近理论比容量528 mA?h/g。另外,包覆后的电池放电平台为2.5 V,而未包覆电池放电平台只有2.1 V左右,这说明包覆MoO3明显改善了CuF2的导电性,从而提高了其放电性能。从图5还可以看出,在放电过程中,放电曲线平稳,只出现一个放电平台,这个平台为Cu2+与Cu的相互转化,与式(2)一致。
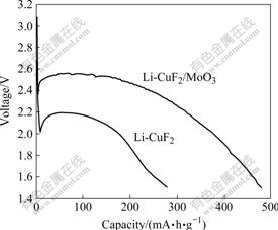
图5 Li-CuF2和Li-CuF2/MoO3的放电曲线
Fig.5 Discharge curves of Li-CuF2 and Li-CuF2/MoO3
2.3.3 交流阻抗测试
图6所示为CuF2与包覆MoO3的CuF2材料制成 的2025型扣式电池的交流阻抗谱。交流信号幅度为5 mV,频率范围为0.01~100 kHz。由图6可以看出,曲线均由高频区的半圆弧及低频区与实轴几乎呈45?的直线组成,其中高频区的半圆代表电荷转移反应电阻,低频区(0.01~10 Hz)直线部分描述的是锂离子在固相活性物质中扩散Warburg阻抗。由图6可知,包覆MoO3材料的电荷转移电阻明显小于未包覆材料的电荷转移电阻。从图6中的插图可见,包覆MoO3的Li-CuF2/MoO3电池的欧姆电阻明显比Li-CuF2电池小。这说明MoO3的包覆不仅改善了电池的导电性,而且能降低电池的电化学反应阻抗,从而改善其电化学性能。
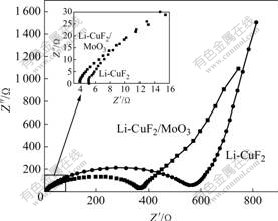
图6 Li-CuF2和Li-CuF2/MoO3的交流阻抗谱
Fig.6 AC impedance spectra of Li-CuF2 and Li-CuF2/MoO3
3 结论
1) 通过掺入15%的MoO3球磨,使其均匀包覆在CuF2表面,形成了高导电性混合导电化合物,并用作锂一次电池新型正极材料。
2) MoO3的包覆不影响材料的结构,包覆后的高导电性复合材料仍具有典型CuF2的单斜晶系结构,其电化学活性明显增加。
3) MoO3的包覆能有效地改善CuF2正极材料的电化学性能,降低欧姆电阻,CuF2/MoO3正极材料的电荷转移阻抗也明显比CuF2低,因而具有更高的放电比容量,放电比容量高达483 mA?h/g,远高于未包覆的CuF2和其它锂一次电池正极材料。因此,该材料是一种有前景的锂一次电池新型正极材料。
[1] JOHNSON C S. Development and utility of manganese oxides as cathodes in lithium batteries[J]. Journal of Power Sources, 2007, 165(2): 559?565.
[2] OWENS B B, SKARTAD P M. Ambient temperature solid state batteries[J]. Solid State Ionics, 1992, 53/56(1): 665?672.
[3] BENINATI S, FANTUZZI M, MASTRAGOSTINO M, SOAVI F. MW-assisted synthesis of SVO for ICD primary batteries[J]. Journal of Power Sources, 2006, 157(1): 483?487.
[4] ABRAHAM K M. Chemical and electrochemical processes in some lithium?liquid cathode batteries[J]. Journal of Power Sources, 1991, 34(2): 81?101.
[5] SPOTNITZ R M, YEDUVAKA G S, NAGASUBRAMANIAN G, JUNGST R. Modeling self-discharge of Li/SOCl2 batteries[J]. Journal of Power Sources, 2006, 163(1): 578?583.
[6] MONTORO L A, ROSOLEN J M. Gelatin/DMSO: A new approach to enhancing the performance of a pyrite electrode in a lithium battery[J]. Solid State Ionics, 2003, 159: 233?240.
[7] NAGASUBRAMANIAN G, SANCHEZ B. A new chemical approach to improving discharge capacity of Li/(CFx)n cells[J]. Journal of Power Sources, 2007, 165(2): 630?634.
[8] 邢雪坤, 肖 明, 李 川, 吴昊清. 锂?氧化铜电池及其反应机理[J]. 化学学报, 1984, 42(3): 220?226.
XING Xue-kun, XIAO Ming, LI Chuan, WU Hao-qing. Reaction mechanisms in Li/CuO and Li/Cu2O cells[J]. Acta Chimica Sinica, 1984, 42(3): 220?226.
[9] PLITZ I, BADWAY F, AL-SHARAB J, DUPASQUIER A, COSANDEY AMATUCCI G G. Structure and electrochemistry of carbon-metal fluoride nanocomposites fabricated by solid-state redox conversion reaction[J]. Journal of the Electrochemical Society, 2005, 152(2): A307?A315.
[10] BERVAS M, BADWAY F, KLEIN L C, AMATUCCI G G. Bismuth fluoride nanocomposite as a positive electrode material for rechargeable lithium batteries[J]. Electrochemical and Solid State Letters, 2005, 8(4): A179?A183.
[11] BERVAS M, KLEIN L C, AMATUCCI G G. Reversible conversion reactions with lithium in bismuth oxyfluoride nanocomposites[J]. Journal of the Electrochemical Society, 2006, 153(1): A159?A170.
[12] MAKIMURA Y, ROUGIER A, TARASCON J M. Pulsed laser deposited iron fluoride thin films for lithium-ion batteries[J]. Applied Surface Science, 2006, 252(13): 4587?4592.
[13] LI Hong, BALAYA P, MAIER J. Li-storage via heterogeneous reaction in selected binary metal fluorides and oxides[J]. Journal of the Electrochemical Society, 2004, 151(11): A1878?A1885.
[14] FU Zhen-wen, LI Chi-lin, LIU Wen-yuan, MA Jun, WANG Ying, QIN Qi-zong. Electrochemical reaction of lithium with cobalt fluoride thin film electrode[J]. Journal of the Electrochemical Society, 2005, 152(2): E50?E55.
[15] ZHOU Yong-ning, LIU Wen-yuan, XUE Ming-zhe, YU Le, WU Chang-liang, WU Xiao-jing, FU Zhen-wen. LiF/Co nanocomposite as a new Li storage material[J]. Electrochemical and Solid-state Letters, 2006, 9(3): A147?A150.
[16] 王 欣, 王先友, 曹俊琪, 伍 文, 李 娜, 魏建良, 杨顺毅. 锂二次电池新型正极材料BiO0.1F2.8的制备及性能研究[J]. 功能材料, 2009, 5(40): 774?778.
WANG Xin, WANG Xian-you, CAO Jun-qi, WU Wen, LI Na, WEI Jian-liang, YANG Shun-yi. The synthesis and electrochemical performance of BiO0.1F2.8 as a new positive electrode for rechargeable lithium battery[J]. Journal of Functional Materials, 2009, 5(40): 774?778.
[17] 伍 文, 王先友, 王 欣, 王国宝, 杨顺毅, 魏建良, 李 娜. 锂二次电池新型正极材料FeF3(H2O)0.33的制备及电化学性能研究[J]. 功能材料, 2008, 11(39): 1824?1827.
WU Wen, WANG Xian-you, WANG Xin, WANG Guo-bao, YANG Shun-yi, WEI Jian-liang, LI Na. Studies on preparation and electrochemical performances of FeF3(H2O)0.33 cathode material for the application of lithium rechargeable battery[J]. Journal of Functional Materials, 2008, 11(39): 1824?1827.
[18] ELLIOTT W E, HUFF J R, SIMMONS G L, MCDONALD G D, JAMROZY J L, TOWLE W L. A program to develop a high-energy-density primary battery with a minimum of 200 watt hours per pound of total battery weight[R]. Washington, D.C: National Aeronautics and Space Administration, 1965.
[19] ABENS S G, MAHY T X, MERZ W C. Development of high-energy-density primary batteries[R]. Washington D.C: National Aeronautics and Space Administration, 1965.
[20] AMATUCCI G G, PEREIRA N. Fluoride based electrode materials for advanced energy storage devices[J]. Journal of Fluorine Chemistry, 2007, 128(4): 243?262.
[21] BADWAY F, MANSOUR A N, PEREIRA N, AL-SHARAB J F, CONSANDEY F, PLITZ I, AMATUCCI G G. Structure and electrochemistry of copper fluoride nanocomposites utilizing mixed conducting matrices[J]. Chemistry of Materials, 2007, 19(17): 4129?4141.
基金项目:国家自然科学基金资助项目(20871101,20673092)
收稿日期:2009-02-03;修订日期:2009-09-28
通信作者:王先友,教授,博士;电话:0731-58293043;E-mail: wxianyou@yahoo.com
摘 要:通过球磨CuF2和MoO3的混合物,制成CuF2/MoO3复合材料。通过X射线衍射(XRD)、扫描电镜(SEM)和电化学测试等研究CuF2/MoO3复合材料的结构和电化学性能。结果表明:MoO3晶粒均匀地附着在CuF2的表面,形成均匀的导电网,从而使制备的CuF2/MoO3复合材料具有高导电性,可作为锂一次电池正极活性材料。以0.1C倍率放电,截止电压为1.5 V,CuF2/MoO3复合材料放电比容量高达483mA?h/g,放电电位平台为2.5 V,明显高于CuF2正极材料的278 mA?h/g,接近其理论容量528 mA?h/g。


