Trans. Nonferrous Met. Soc. China 23(2013) 2977-2983
Synthesis and characterization of fibrous nickel hydroxide obtained from spent nickel catalyst
Pinak PATNAIK1,2, Avijit BISWAL1,2, Bankim Chandra TRIPATHY1,2, Sangitarani PRADHAN1, Barsha DASH1, Ramasamy SAKTHIVEL1, T. SUBBAIAH1,2
1. CSIR- Institute of Minerals and Materials Technology, Bhubaneswar-751 013, India;
2. Academy of Scientific and Innovative Research (AcSIR), Anusandhan Bhavan, Rafi Marg, New Delhi-110001, India
Received 7 January 2013; accepted 31 January 2013
Abstract:
The recovery of nickel from spent nickel catalyst for the preparation of nickel hydroxide was studied. Nickel was extracted from the spent catalyst by acid leaching with 1 mol/L sulfuric acid at 90 °C. Purified nickel solution was used in the preparation of nickel hydroxide. Three different methods, namely urea hydrolysis, conventional, and hydrothermal methods, of precipitation using NaOH were employed to get various nickel hydroxides samples named as Ni(OH)2-U, Ni(OH)2-C, and Ni(OH)2-H, respectively. Hydrothermal treatment induced better crystallinity in the Ni(OH)2 compared with conventional method. Both Ni(OH)2-C and Ni(OH)2-H samples have mixed phases of β-Ni(OH)2 and α*-Ni(OH)2×0.75H2O phases, whereas Ni(OH)2-U has only α*-Ni(OH)2×0.75H2O. TEM image of Ni(OH)2-U sample shows rod-like Ni(OH)2 structures. Among all, Ni(OH)2-U shows the best electrochemical activity.
Key words:
spent nickel catalyst; fibrous nickel hydroxide; recovery;
1 Introduction
Nickel hydroxide is a widely used material having a number of applications starting from rechargeable batteries in cellular phones, powered electric vehicles to being used as a premium product in catalyst formulations. Nickel catalyst is a preferred choice in various industries simply because of its lower costs and better performances in comparison to other materials. The reuse of same catalyst several times unfortunately results in the deactivation process of the catalyst for they require replacement. Over a period of time, tons of spent reforming catalysts get accumulated, leading to pollution to the concerned industry in particular. Therefore, the need is to find some suitable method for utilizing this waste material in the recovery of valuable metals. Recovery of nickel from spent catalyst solves the crisis originating due to rapid depletion of primary metal resources owing to the permanently increasing demand, limited possibilities for locating new ore-bodies and concern about environmental degradation. Recovery of metals from waste/secondary sources not only enhances the resource base but also results in a corresponding decrease in the requirement for mining ores and the supply of energy and also reduces the problem of disposal of linked industrial wastes. Since spent nickel catalysts are very rich in nickel, many attempts have been made to recover nickel from spent catalyst [1,2]. Acid digestion method is commonly practiced for the above purpose. It has been found that sulfuric acid is a better option to extract the nickel [3]. This nickel solution can be used for nickel electro-winning and preparation of other value added materials. Obtaining any value added materials from waste is attractive from the point of environment sustainability and minimization of waste disposal.
Nickel hydroxide finds applications as positive electrode material in nickel-based alkaline rechargeable batteries [4,5]. Ni(OH)2 or precipitate containing this hydroxide as NiCO3Ni(OH)2 is used as starting material to produce NiO [6], which is widely used in ceramic and glass industries [7]. ACHARYA et al [8] studied the effect of different precipitating reagents on physicochemical and electrochemical properties of nickel hydroxide derived from nickel sulfate solution. It has been reported that shape and size of nickel hydroxide particles reflect a drastic change in their electrochemical behavior. The charge–discharge process takes place more easily and more reversibly on the electrodes having nano-sized β-Ni(OH)2 particles compared to those having micro sized spherical β-Ni(OH)2 particles [9]. Therefore, it is necessary to synthesize nano sized nickel hydroxide for better efficiency. In this work, an attempt is made to extract nickel as nickel sulfate solution from its spent catalyst and used as a nickel source for preparation of nickel hydroxide. Different precipitating reagents and conditions are adopted to synthesize fibrous nickel hydroxide. The results from different characterizations of nickel hydroxide samples prepared under different experimental conditions are discussed.
2 Experimental
2.1 Materials
The spent nickel catalyst materials were crushed, ground and screened to provide materials with particle size less than <100 μm. The chemical composition was determined using XRF (X-ray fluorescence) techniques (Model: Philips PW 2440, Magix Pro). The spent catalyst contained 14.6% NiO and 85.4% Al2O3.
2.2 Methods
The spent nickel catalyst was leached and the leach liquor was purified. The purified nickel solution was then used for the synthesis of nickel hydroxide. The detailed procedures are given in the corresponding sections.
2.2.1 Leaching
Spent nickel catalyst was leached with sulfuric acid to generate nickel leaching liquor containing aluminium as an impurity. The optimum dissolution conditions for leaching were 1 mol/L sulfuric acid at temperature of 90 °C for 6-7 h. The leaching experiments were carried out by taking required amount of sulphuric acid along with ground spent catalyst material in 10% pulp density in a water jacketed glass beaker of 1 L capacity fitted to a reflux condenser. The water in the water jacket was circulated through a water bath maintained at 366 K (93°C) so that the temperature inside the beaker was maintained at 90 °C. Nickel was estimated by chemical analysis and the impurities were analyzed by ICP-OES (inductively coupled plasma-optical emission spectrometer, model: Optima 2100 DV, Perkin Elmer).
2.2.2 Nickel analysis
To 5 mL of the nickel ion solution, 25 mL of water was added followed by 3 to 4 drops of freshly prepared murexide indicator. Ammonia solution was added until the pH became ~7, which was indicated by yellow colour of the solution. It was then titrated with 0.1 mol/L EDTA solution until the colour changed from yellow to bluish violet. The nickel concentration is calculated as
1 mL of 0.1 mol/L EDTA = 5.871 mg of Ni2+
2.2.3 Purification
The purification of leach liquor was carried out by precipitating the impurities using NaOH as the precipitating agent. The impurities tend to precipitate at low pH values; hence pH of the solution was varied within the range of 2.0-5.0 by the addition of dilute NaOH to the leach liquor. Purified nickel leaching liquor was obtained by filtration of the insoluble precipitate. The details of the purification process are discussed later.
2.2.4 Precipitation
The purified nickel solution was used for synthesis of three different kinds of nickel hydroxide at pH 10.5. The conventional method of synthesis of nickel hydroxide was carried out by drop wise addition of 1 mol/L NaOH to the purified leaching liquor with stirring simultaneously at room temperature till the pH reached its optimum value of 10.5. The greenish precipitate of nickel hydroxide obtained was aged for 24 h and then filtered. The precipitate was washed with distilled water and dried at 110 °C. The nickel hydroxide obtained by this procedure was denoted as Ni(OH)2-C. Hydrothermal method of synthesis involved treatment of nickel solution at pH 10.5 in a pressurized autoclave reactor maintained at temperature of 180 °C. The experiment was carried out for 8 h, followed by filtration, washing and drying, and the samples obtained were referred as Ni(OH)2-H. The nickel hydroxide prepared by adding sufficient quantity of urea to leaching liquor at 100 °C up on stirring followed by aging, filtration, washing and drying was named Ni(OH)2-U.
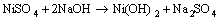 (1)
(1)
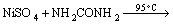
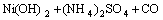 (2)
(2)
2.2.5 Physical characterization
All the three nickel hydroxide samples were characterized for phase identification using a powder X-ray diffractometer (Model: X’pert Pro, Panalytical, Netherlands) equipped with a Cu target (Cu Kα radiation, λ=1.54056 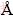 ) operating at 40 kV and 30 mA. Thermo-gravimetric analysis was carried out by Mettler Toledo instrument (TGS/SDTA851e) at a heating rate of 20 °C/min. For functional group identification, Fourier transform infrared (FTIR) spectra were recorded in the wave number range from 400 cm-1 to 4000 cm-1 (spectrum Gx Model,Perkin Elmer). Potassium bromide (KBr) was used as the reference material. PAS detector and glassy carbon as reference were used for photo acoustic FTIR spectrum measurement. General morphology of the samples was investigated with the help of a transmission electron microscope (Technai G2, FEI, The Netherlands) operating at a maximum 200 kV. For this purpose, the sample was dispersed ultrasonically in ethanol and transferred onto a carbon coated copper grid followed by drying under IR lamp in air. Gatan Digital MicrographTM is the application software used to acquire, visualize, analyze and process the digital image data of the samples.
) operating at 40 kV and 30 mA. Thermo-gravimetric analysis was carried out by Mettler Toledo instrument (TGS/SDTA851e) at a heating rate of 20 °C/min. For functional group identification, Fourier transform infrared (FTIR) spectra were recorded in the wave number range from 400 cm-1 to 4000 cm-1 (spectrum Gx Model,Perkin Elmer). Potassium bromide (KBr) was used as the reference material. PAS detector and glassy carbon as reference were used for photo acoustic FTIR spectrum measurement. General morphology of the samples was investigated with the help of a transmission electron microscope (Technai G2, FEI, The Netherlands) operating at a maximum 200 kV. For this purpose, the sample was dispersed ultrasonically in ethanol and transferred onto a carbon coated copper grid followed by drying under IR lamp in air. Gatan Digital MicrographTM is the application software used to acquire, visualize, analyze and process the digital image data of the samples.
2.2.6 Electrochemical characterization
A suitable pellet of Ni(OH)2 was prepared by mixing powdered Ni(OH)2 sample with graphite powder in 2:1 of mass proportion and 2-3 drops of 5% PVA as a binder. The powders were mixed thoroughly by means of mortar and pestle till the mixture became uniform. The powdered mixture was then placed in a stainless steel mesh and pressed using a pelletiser by applying a pressure of 34323.27 kPa for 6 min. The obtained pellet had a diameter of 25 mm. A floating cell arrangement was prepared for evaluating the electrochemical activity of various Ni(OH)2 samples. The charge-discharge profile was recorded by using 6 mol/L KOH as the electrolyte solution at a constant current of 80 mA at room temperature of (27+2) °C. The experimental cell consisted of two nickel strips as counter electrodes and zinc strip as the reference electrode, along with the cathode in the form of pellet that was prepared from the above discussed method of pellet preparation. The pellet was then put into a cell assembly and allowed to equilibrate for 4 h at its open circuit potential before charging for 8 h at a current of 80 mA·h and then discharging at 80 mA·h with a cut-off voltage (COV) of 1.3 V. The studies were carried out using a deep cycle battery tester (BITRODE, USA).
3 Results and discussion
3.1 Leaching
The composition of spent catalyst is 14.6% NiO and 85.4% Al2O3. The leaching liquor generated after leaching of the spent catalyst with 1 mol/L sulphuric acid at 10% pulp density contained 11.8 g/L nickel and 830 mg/L aluminium, indicating a leaching efficiency of 99.25% and 1.84% for nickel and aluminium respectively. Separation of aluminium from the leaching liquor was carried out by precipitating it as sodium aluminate, using NaOH as the precipitating agent. The aluminium content in the leaching liquor was analyzed by the ICP-OES. The results of precipitation of aluminium by NaOH, within the pH range of 2.0-5.0 are shown in Table 1. From Table 1 it is quite evident that at pH=5 all the aluminium was removed from the leaching liquor. It is also observed that with increasing pH, the precipitation of nickel as hydroxides was negligible. The precipitation of nickel started at a pH of 5.5-6 [10]. The composition of the leaching liquor after purification is 11.8 g/L nickel.
3.2 Characterization of nickel hydroxides
3.2.1 XRD studies
Figure 1(a) shows the X-ray diffraction patterns of spent nickel catalyst and residue obtained after acid extraction of nickel. It reveals that spent nickel catalyst has alumina (JCPDS No. 43-1484) as major phase and nickel oxide (JCPDS No. 01-072-1464) as minor phase. But the residue contains alumina (JCPDS No. 43-1484) and very minor quantity of silica phase ((JCPDS No. 86-1630 and 86-1562). The absence of diffraction lines corresponding to nickel oxide phase in the XRD pattern of residue indicates that NiO present in the spent catalyst is leached out during sulfuric acid treatment.
Table 1 Effect of pH on precipitation of aluminium from leaching liquor
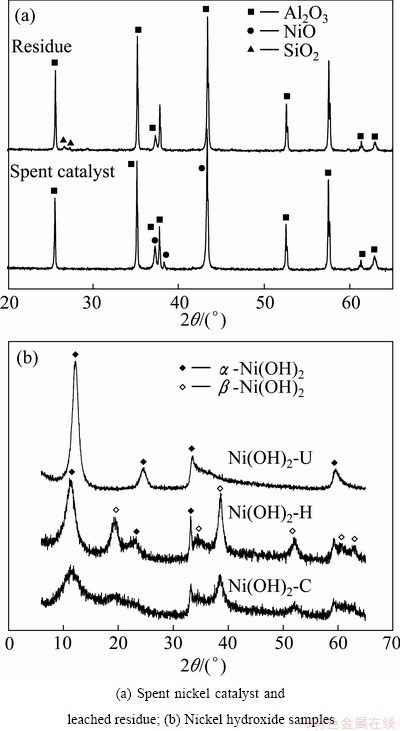
Fig. 1 XRD patterns of samples
Figure 1(b) depicts the X-ray powder diffraction patterns of Ni(OH)2-C, Ni(OH)2-H and Ni(OH)2-U. The diffraction patterns of Ni(OH)2-C and Ni(OH)2-H samples look similar with an increase in intensity of diffraction lines in the later case. This indicates the better crystallinity of the Ni(OH)2-H sample compared to the Ni(OH)2-C sample. However, the diffraction pattern of Ni(OH)2-U sample is found to be different from those of the above two samples. X-ray diffraction patterns of all three samples are matched with standard powder diffraction data of α- and β-Ni(OH)2 (JCPDS No. 38-0715 and 14-0117). It is observed that both Ni(OH)2-C and Ni(OH)2-H samples have a mixed phases of β-Ni(OH)2 and α*-Ni(OH)2×0.75H2O, whereas Ni(OH)2-U has only α*-Ni(OH)2×0.75H2O. The XRD pattern of Ni(OH)2-U is very much similar to that of α-nickel hydroxide reported in Ref. [11].
3.2.2 TGA studies
Thermo-gravimetric analytical (TGA) data of Ni(OH)2-C, Ni(OH)2-H and Ni(OH)2-U are shown in Fig. 2. It indicates that both Ni(OH)2-C and Ni(OH)2-H undergo a similar process of mass loss. The first stage takes place within the temperature range from room temperature to 300 °C, indicating slow dehydration of bound water molecule. The second stage occurs between 300 to 420 °C and the third stage is between 740 and 970 °C corresponding to slow dehydroxylation of Ni(OH)2. However, the dehydroxylation step in the case of Ni(OH)2-U is quite fast taking place at 300-330 °C.
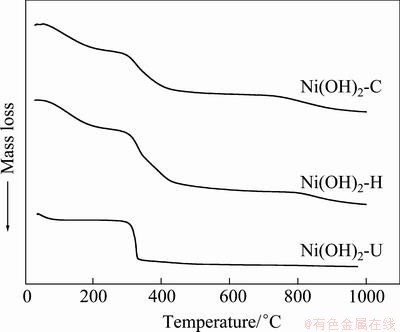
Fig. 2 Thermo-gravimetric curves of different nickel hydroxide samples
3.2.3 FTIR studies
FTIR spectra of all the three samples are shown in Fig. 3. It shows stretching vibration band for hydroxyl group of water molecules at 3400 cm-1 and bending vibration band at 1640 cm-1. The bands observed at 3600 and 780 cm-1 are corresponding to respective stretching and bending vibration of hydroxyl group present in the Ni(OH)2 [12,13]. The other bands observed between 1000 and 1500 cm-1 correspond to anionic species like carbonates intercalated to nickel hydroxide or adsorbed on its surface [8]. The strong band observed at 2280 cm-1 for Ni(OH)2-U sample indicates the adsorption of CO2 on nickel hydroxide surface. The CO2 may come from decomposition of urea during hydrolysis. Photo-acoustic FTIR spectra of all the three samples have been recorded to see the resolved peaks and they are shown in Fig. 3(b). It shows similar band position as has been noticed in normal FTIR spectra.
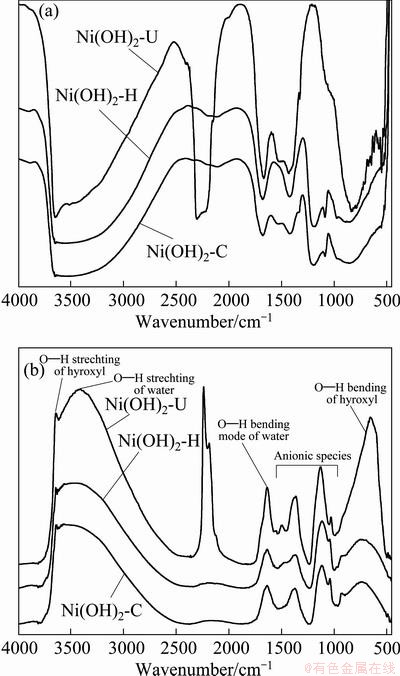
Fig. 3 FTIR spectra of different nickel hydroxide samples (a) and photo-acoustic FTIR spectra of different nickel hydroxide samples (b)
3.2.4 TEM studies
TEM pictures of Ni(OH)2-C, Ni(OH)2-H and Ni(OH)2-U are shown in Figs. 4, 5 and 6 respectively. Ni(OH)2-C sample show fibrous structures, whereas rod like features are seen in Ni(OH)2-H sample. This indicates that hydrothermal treatment brings a change in the morphology of sample. Ni(OH)2-U shows fully grown rod-shaped fibrous structures. Similar features have been reported for Ni(OH)2 obtained using sonochemical method [10]. Selected area electron diffraction (SAED) patterns of nickel hydroxides are also shown in these figures. The d-values obtained from SAED patterns are well match with those from XRD pattern of nickel hydroxide.
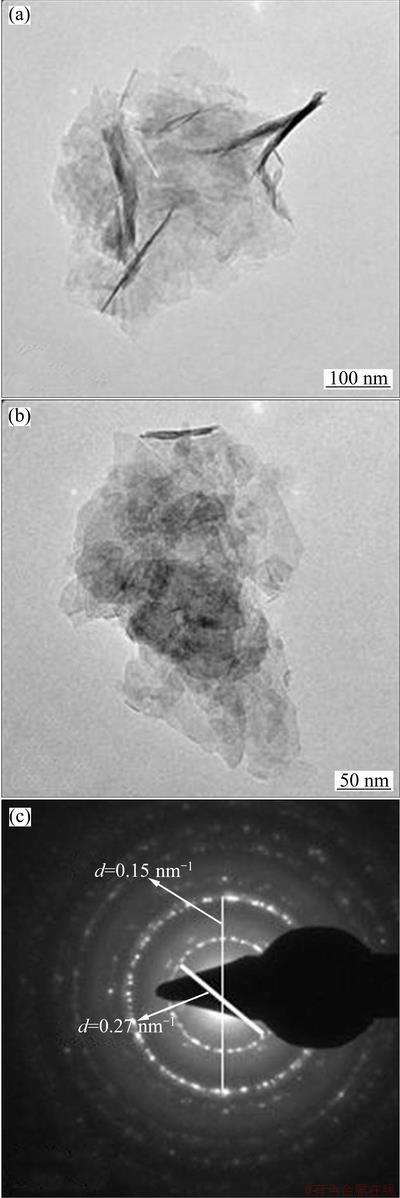
Fig. 4 TEM images and SAED pattern of Ni(OH)2-C
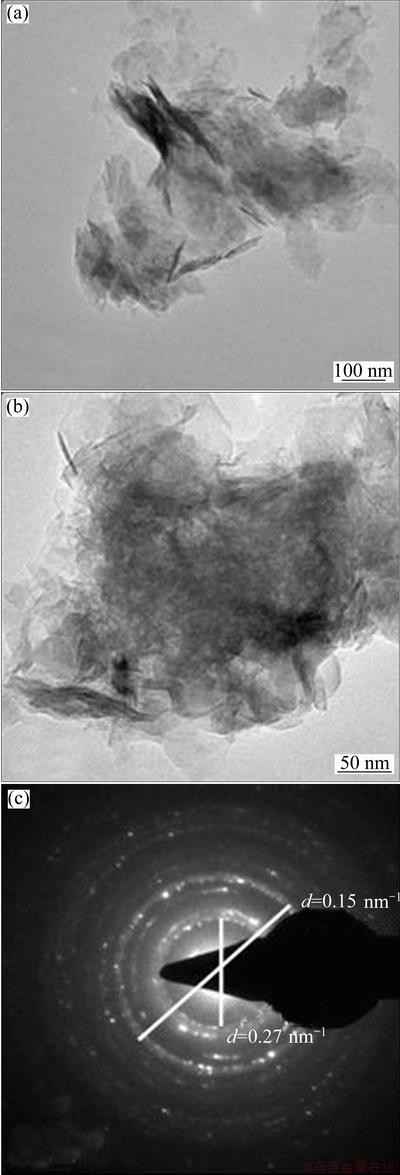
Fig. 5 TEM images and SAED pattern of Ni(OH)2-H
3.3 Electrochemical activity
Nickel hydroxide was used as an active material in all nickel-based secondary batteries. The cell reaction involves the oxidation of Ni(OH)2 to NiO·OH during charging and the reduction of oxidized product back to Ni(OH)2 during discharging. The charging–discharging reactions are given below:
NiO·OH+H2O+e-  Ni(OH)2+OH- (3)
Ni(OH)2+OH- (3)
The active component of the battery is NiO·OH. The reaction mechanism involves diffusion of hydrogen ions through the solid-state lattices of Ni(OH)2 and NiO·OH to give a continuous change in the composition of active material. The discharge curves and discharge potentials (initial discharge potentials) of the three samples in the first cycle are shown in Fig. 7. The discharge potentials of the samples are found to be 140, 120 and 100 mA·h/g for Ni(OH)2-U, Ni(OH)2-H, Ni(OH)2-C, respectively. The charge-discharge profiles shown in Fig. 7(b) for the above samples are also recorded four cycles from which it is inferred that all the samples almost retain their capacity relative constant (except Ni(OH)2-C) even at the completion of the fourth cycle. Among all the samples the Ni(OH)2-U shows better electrochemical activity than others. The higher activity of this sample is due to the formation of α-Ni(OH)2 phase, which has larger interlayer separation around 8 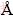 along c-axis due to the intercalation of significant quantity of water molecules and anions compared with β-nickel hydroxide. Mobility of such water molecules and hydroxides ions can easily be facilitated within the largely separated layers of α-nickel hydroxide, making it more electrochemically active [14,15]. Urea can be considered a better precipitating reagent for synthesis of nickel hydroxide with respect to its electrolytic characteristics.
along c-axis due to the intercalation of significant quantity of water molecules and anions compared with β-nickel hydroxide. Mobility of such water molecules and hydroxides ions can easily be facilitated within the largely separated layers of α-nickel hydroxide, making it more electrochemically active [14,15]. Urea can be considered a better precipitating reagent for synthesis of nickel hydroxide with respect to its electrolytic characteristics.
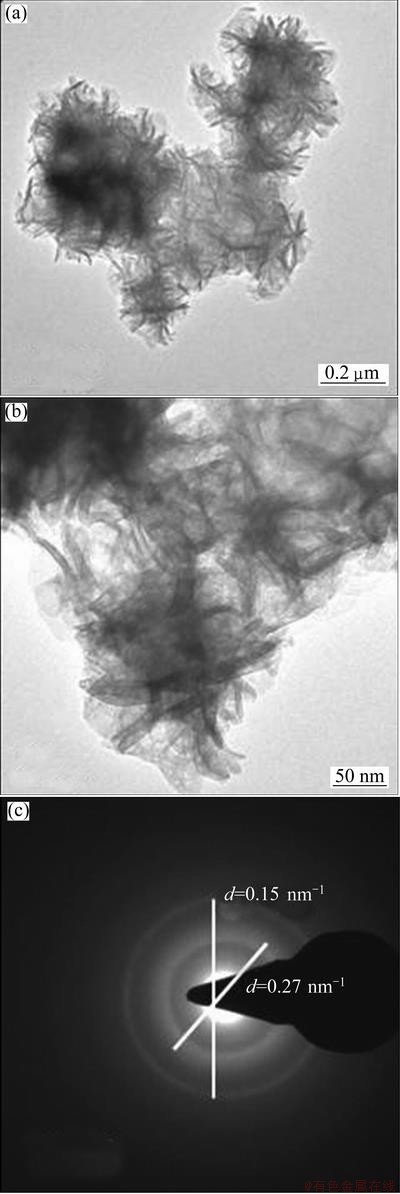
Fig. 6 TEM images and SAED pattern of Ni(OH)2-U
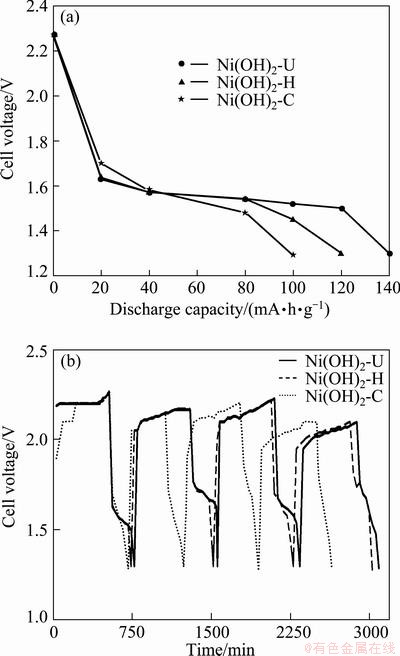
Fig. 7 Plots for cell voltage vs discharge capacities in the first cycle for different samples (a) and charge-discharge behavior of different samples within 4 cycles (b)
4 Conclusions
The nickel spent catalyst has hazardous effects and poses serious threat to the ecosystem. The real challenge in front of us is to develop a cost-effective method of production of nickel hydroxide that will not only solve issues related to the environment, but help meeting the huge supply demands of the material without the depletion of primary resources. The method described here is simple, safe, economically viable and suitable for industrial applications. This work demonstrates the extraction of nickel from spent nickel catalyst and utilization of extracted nickel for preparation of nickel hydroxide. Nickel hydroxide obtained from urea hydrolysis method is unique and different from that by other two methods (hydrothermal and conventional) in terms of morphology, single phase nature and electrochemical activity. Urea hydrolysis method yields selective α-nickel hydroxide owing to good discharge capacity whereas other two methods produce both α- and β- nickel hydroxide.
Acknowledgements
The authors would like to thank the Director, CSIR-IMMT for his encouragement to publish the work.
References
[1] SAHU K K, AGARWAL A, PANDEY B D. Nickel recovery from spent nickel catalyst [J]. Waste Management and Research, 2005, 23: 148-154
[2] OSSMAN M E, SHETA W, ELTAWEEL Y. Linear genetic programming for prediction of nickel recovery from spent nickel catalyst [J]. American Journal of Engineering and Applied Sciences, 2010, 3(2): 482-488.
[3] LEE J Y, RAO S V, KUMAR B N, KANG D J, REDDY B R. Nickel recovery from spent Raney nickel catalyst through dilute sulfuric acid leaching and soda ash precipitation [J]. Journal of Hazardous Materials, 2010, 176: 1122-1125.
[4] FALK S U, SALKIND A J. Alkaline storage batteries [M]. New York: Wiley, 1969.
[5] BREEN J M, WHITE R E, BOCKRIS J O, CONWAY B E. Modern aspect of electrochemistry [M]. New York: Plenum, 1990: 29.
[6] XIANG L, DENG X Y, JIN Y. Experimental study on synthesis of NiO nano-particles [J]. Scripta Materialia, 2002, 47: 219-224.
[7] GUALTIERI A F, MAZZUCATO E, VENTURELLI P, VIANI A, ZANNINI P, PETRAS L. X-ray powder diffraction quantitative analysis performed in situ at high temperature: Application to the determination of NiO in ceramic pigments [J]. Journal of Applied Crystallography, 1999, 32: 808-813.
[8] ACHARYA R, SUBBAIAH T, ANAND S, DAS R P. Effect of precipitating agents on the physicochemical and electrolytic characteristics of nickel hydroxide [J]. Materials Letters, 2003, 57: 3089-3095.
[9] GUAN Xiao-yan, DENG Jian-cheng. Preparation and electrochemical performance of nano-scale nickel hydroxide with different shapes [J]. Materials Letters, 2007, 61: 621-625.
[10] RAMESH T N, KAMATH P V. Synthesis of nickel hydroxide: Effect of precipitation conditions on phase selectivity and structural disorder [J]. J Power Sources, 2006, 156: 655-661.
[11] JEEVANANDAM P, KOLTYPIN Y U, GEDANKEN A. Synthesis of nanosized α-nickel hydroxide by a sonochemical method [J]. Nano Letters, 2001, 1: 263-266.
[12] KAMATH P V, SUBBANA G N. Electroless nickel hydroxide: Synthesis and characterisation [J]. J Appl Electrochem, 1992, 22: 478-482.
[13] PORTEMER F, DELAHAYE-VIDAL A, FIGLARZ M. Characterization of active material deposited at the nickel hydroxide electrode by electrochemical impregnation [J]. J Electrochem Soc, 1992, 139: 671-678.
[14] AKINC M, JONGEN N, LEMAITRE J, HOFMANN H. Synthesis of nickel powders by urea decomposition [J]. J Eur Ceram Soc, 1998, 18: 1559-1564.
[15] DELAHAYE-VIDAL A, FIGLARZ M. Textural and structural studies on nickel hydroxide electrodes. II. Turbostatic nickel (II) hydroxide submitted to electrochemical redox cycling [J]. J Appl Electrochem, 1987, 17: 589-599.
废镍催化剂回收制备纤维状氢氧化镍
Pinak PATNAIK1,2, Avijit BISWAL1,2, Bankim Chandra TRIPATHY1,2, Sangitarani PRADHAN1, Barsha DASH1, Ramasamy SAKTHIVEL1, T. SUBBAIAH1,2
1. CSIR- Institute of Minerals and Materials Technology, Bhubaneswar-751 013, India;
2. Academy of Scientific and Innovative Research (AcSIR), Anusandhan Bhavan, Rafi Marg, New Delhi-110001, India
摘 要:研究从废旧镍催化剂中回收镍以制备氢氧化镍。采用酸浸法,在90 °C下用1 mol/L硫酸浸没废镍催化剂,从中提取镍。向净化后的含镍溶液中加入NaOH,分别采用3种不同的方法,即尿素水解、传统方法和水热方法制得3种不同的氢氧化镍,分别命名为Ni(OH)2-U, Ni(OH)2-C和Ni(OH)2-H。与传统方法相比,采用水热方法制得的氢氧化镍具有更好的结晶度。Ni(OH)2-C和Ni(OH)2-H都含有b-Ni(OH)2与a*-Ni(OH)2×0.75H2O的混合相,而Ni(OH)2-U只含有a*-Ni(OH)2×0.75H2O相。TEM观察显示Ni(OH)2-U样品具有棒状结构。在这3种样品中,Ni(OH)2-U表现出最好的电化学活性。
关键词:废镍催化剂;纤维状氢氧化镍;回收
(Edited by Hua YANG)
Corresponding author: Pinak PATNAIK; E-mail: pinakpatnaik@gmail.com
DOI: 10.1016/S1003-6326(13)62823-X
Abstract: The recovery of nickel from spent nickel catalyst for the preparation of nickel hydroxide was studied. Nickel was extracted from the spent catalyst by acid leaching with 1 mol/L sulfuric acid at 90 °C. Purified nickel solution was used in the preparation of nickel hydroxide. Three different methods, namely urea hydrolysis, conventional, and hydrothermal methods, of precipitation using NaOH were employed to get various nickel hydroxides samples named as Ni(OH)2-U, Ni(OH)2-C, and Ni(OH)2-H, respectively. Hydrothermal treatment induced better crystallinity in the Ni(OH)2 compared with conventional method. Both Ni(OH)2-C and Ni(OH)2-H samples have mixed phases of β-Ni(OH)2 and α*-Ni(OH)2×0.75H2O phases, whereas Ni(OH)2-U has only α*-Ni(OH)2×0.75H2O. TEM image of Ni(OH)2-U sample shows rod-like Ni(OH)2 structures. Among all, Ni(OH)2-U shows the best electrochemical activity.


