
Adjustment on gibbsite and boehmite co-precipitation from supersaturated sodium aluminate solutions
WANG Zhi(王 志)1, YANG Liu(杨 柳)1, 2, ZHANG Juan(张 娟)1, 2,
GUO Zhan-cheng(郭占成)1, ZHANG Yi(张 懿)1
1. National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China;
2. Graduate University of Chinese Academy of Sciences, Beijing 100049, China
Received 6 July 2009; accepted 5 January 2010
Abstract:
Gibbsite is the usual precipitation product from alumina refineries with either Bayer or sintering process. However, the advantage of boehmite precipitation over gibbsite precipitation is the significant energy saving in the subsequent calcination step. The current investigation takes a pragmatic approach to measure precipitation ratios, determine product phase, morphology and particle size distribution, and assess the impacts and adjustment capability of main parameters such as seed, temperature, ethanol medium, and supersaturation on the precipitation kinetics and alumina hydrate type during co-precipitation process. The results clarify that gibbsite and boehmite both can be precipitated from supersaturated sodium aluminate solutions simultaneously, and the competitive formation between Al(OH)3 and γ-AlOOH determines the main precipitate phases from pregnant liquor. Boehmite seeds, high temperature and ethanol addition can promote the boehmite precipitation and improve the mass fraction of boehmite in products. Co-precipitation changes the multimodal distribution of seeds to a normal and well distribution of products, and the particle size is more than several times that of seeds.
Key words:
sodium aluminate solutions; co-precipitation; boehmite; gibbsite;
1 Introduction
According to the thermodynamic data, when temperature is higher than 60 ℃ various aluminum oxide hydrates, including gibbsite, boehmite and diaspore, can be precipitated from supersaturated sodium aluminate solutions. As for the calcinations of the intermediate hydrate to alumina, AlOOH dehydrates to alumina requiring only 72 kJ/mol Al2O3 produced, in contrast to Al(OH)3 which requires more than 170 kJ/mol Al2O3[1]. Gibbsite (Al(OH)3) is the usual precipitation product from alumina refineries with either Bayer or sintering process[2-4]. Thus, 60% energy saving may be achieved by adopting AlOOH calcination instead of Al(OH)3 calcination due to the lower enthalpy and less mass to be calcined. JOANNE et al[2] presented that the calcination of boehmite rather than gibbsite would save approximately 12% of the energy consumed in an Australia alumina refinery.
Many dedications to boehmite precipitation have been carried out in the last decade. MISRA and SIVAKUMAR[5] firstly invented a process for producing pure boehmite by precipitation directly from Bayer liquors at a temperature range of about 115-145 ℃, treating the heated solution with seed material. PANIAS et al[6-9] confirmed that boehmite can be precipitated at 90 ℃ from supersaturated sodium aluminate solution with 120 g/L Na2O, 132 g/L Al2O3 and 300 g/L initial seed concentration. DASH et al[10] found that boehmite precipitation could be possible at normal pressure only when boehmite seed was added to the supersaturated sodium aluminate solution, keeping the alumina-to-caustic ratio at either 1.1 or 1.0 and temperature ≥85 ℃. LI et al[11] investigated on the thermodynamics and experimental parameters of boehmite seeded precipitation, indicating that pure boehmite can be obtained in the temperature range of 90-150 ℃.
In fact, because the starting Bayer liquors were not supersaturated enough and boehmite has higher apparent solubility than gibbsite, pure boehmite direct precipitation has been proven difficult and high recoveries of boehmite are not reached. Slow kinetics means higher retention times and recycling volumes in the precipitation circuit, that is, higher operating costs in this part of the alumina plant. In general, it has to be stated that the boehmite precipitation process is still not comparable to the gibbsite one. Adjusting the content of gibbsite and boehmite in the final product determines the energy-saving capability of this new process[12-13]. Through coupling of seeded-precipitation and carbonization or ethanol medium[14], the new co-precipitation process for producing alumina hydrate containing both gibbsite and boehmite was proposed in this work. The main parameters such as seed ratio, temperature and ethanol solvent, were presented and discussed to improve the fundamental understanding of boehmite and gibbsite co-precipitation.
2 Experimental
The schematic diagram of the experimental apparatus is shown in Ref.[15]. Synthetic sodium aluminate solutions were prepared by dissolving Al(OH)3 into a heated NaOH solution at atmospheric pressure. The liquor was redigested at atmospheric pressure to adjust the molecular ratio to match the initial concentration. The solution was pressure filtered prior to use. 500 mL liquor were pipetted into the crystallizer and equilibrated to the desired temperature for 15 min in constant temperature water bath. Seed or ethanol-water additive was added to the liquor and the reactor was ventilated with the mixture of N2 and CO2 (the volume ratio of 61.5:38.5) after resealing. Ethanol solutions with different concentrations were added into the sodium aluminate solutions with the same volume as mother liquor. Liquid samples were taken from the reactor periodically and were analyzed for the concentrations of alumina (c(Al2O3)), caustic and total caustic using the titration method. At the end of the experiment, the obtained sample was vacuum filtered and the solids were washed and dried at 105 ℃ for 48 h, and then were characterized by X-ray diffractometry (Siemens D5000 X-ray diffractometer), scanning electron microscopy (JEOL JEM 2100F), particle size distribution analysis (Malvern Mastersizer-Laser particle size analyzer) and thermo-gravimetric analysis (ZRY-2P, China). All other chemicals used in this study were of analytical grade.
The co-precipitation ratio of alumina hydrate was presented by η. Seed ratio (RS) was defined as the mass of boehmite added in the solution divided by the mass of alumina contained in the solution. Molecular ratio (RM) was presented by the ratio of alumina to caustic of sodium aluminate solution. w(AlOOH) and w(Al(OH)3) presented the contents of boehmite and gibbsite in the product, respectively, which were determined by thermo-gravimetric analysis according to the loss-of-ignition and X-ray diffractometry. Precipitation selectivity of sodium aluminate solutions meant the ratio of gibbsite or boehmite precipitation ratio to the total precipitation ratio of aluminate solutions. The mass fraction of ethanol in ethanol-water solution, presented by we, changed in the range of 0-1.0, with 0 meaning pure water and 1.0 meaning pure ethanol solvent.
3 Results and discussion
3.1 Adjustment on co-precipitation rate and boehmite ratio in product
3.1.1 Boehmite seed addition
The precipitation kinetics of gibbsite has been already examined and it has been found that this process is controlled by chemical reaction on the surface of the seed particles[16]. Kinetic inhibition has been described as being due to poisoning of the active surface area by hydroxyl ions. To overcome this inhibition, it was proposed that neutralization of caustic would increase the precipitation ratio. This is highly attractive to alumina refinery with carbonization of sintering process, as the neutralized product of sodium carbonate can be recycled to treat bauxite. But, former studies do not draw any sound conclusions as to what is the exact content of boehmite in carbonization products, so several experiments were performed with an aluminate solution that contained 100 g/L Al2O3, RM=1.5, keeping CO2 mixture gas flow rate u(CO2)=200 mL/min.
At the same level of precipitation ratio, products with different boehmite contents were obtained at 85, 90 and 105 ℃, respectively. The results are shown in Fig.1. It is clearly observed that contents of boehmite are lower than 6% in three products, which indicates that the precipitation ratio for gibbsite is much higher than that of boehmite, although they both can be precipitated according to the thermodynamics. Boehmite precipitation leads to a very high apparent solubility. The alumina concentration in a boehmite-seeded solution, left to precipitate for 216 h, is approximately 2.3 times higher than the measured boehmite solubility. This metastability has been noted previously by PANIAS[7]. In terms of industrial carbonization process, gibbsite was also the dominant phase, which supports the above results and the following reaction of precipitation in sodium aluminate solution can be given by Eq.(1):
Al(OH)-4(aq)→γ-AlOOH+β-Al(OH)3+OH-+H2O (1)
In order to control the crystallization process and the content of boehmite in product, we can change the precipitation conditions in an accepted scope.
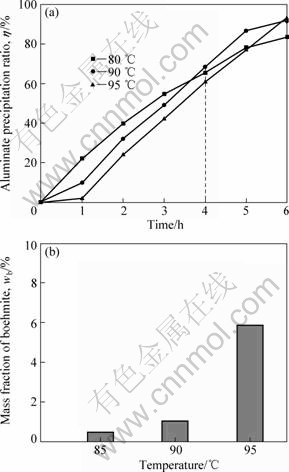
Fig.1 Carbonization precipitation ratio of sodium aluminate solution (a) and boehmite content in product (b) under normal carbonization condition
The supersaturated alkaline solutions of aluminium are known for their long-term stability[17]. Since the interfacial tension between Al(OH)3 and sodium aluminate solution is as high as 1.25 N/m, seed is usually added to initiate the precipitation and allow the particle size of the product to be controlled. Under this condition, the electrical interactions between the surface of the hydrate particles and the aluminate ions in the solution are attenuated. In order to enhance the rate of boehmite precipitation, boehmite seed was also added in these solutions at a ratio of seed Al2O3 content to the solution Al2O3 content from 0.1?1 to 0.8?1. The experimental conditions were as follows: t=90 ℃, c(Al2O3)=104 g/L, RM=1.48, CO2 mixture gas flow rate u(CO2)=200 mL/min. The comparison of effects of the different boehmite seed ratios is shown in Fig.2.
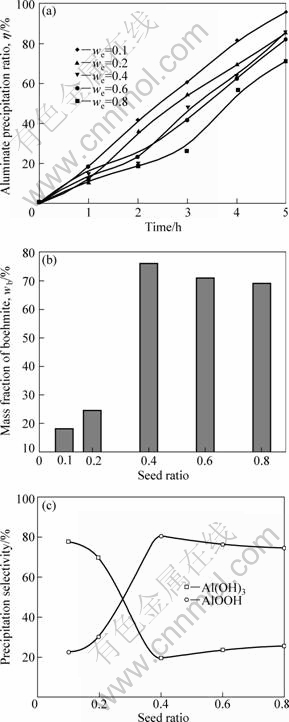
Fig.2 Effect of seed ratio on aluminate precipitation ratio (a), boehmite fraction in product (b) and precipitation selectivity of sodium aluminate solution (c)
Despite the fact that those experiments lasted for 5 h, about ten times shorter than Bayer process, the precipitation ratio of alumina hydrate, between 70% and 90%, was as high as carbonization process, as shown in Fig.2(a). The kinetic inhibition responsible for the low efficiency of seeded precipitation of boehmite disappeared in this new co-precipitation process. But, alumina hydrate co-precipitation ratio was restrained as the seed ratio increases, different from normal Bayer seeded precipitation. Fig.2(b) revealed that a mixture of boehmite and gibbsite was precipitated from solutions, having the composition of the sintering process liquors; and boehmite contents first increased sharply and then decreased a little with the seed ratio increasing. The product was predominantly gibbsite with a few amount of boehmite when seed ratio was below 0.4, however, the product was predominantly boehmite with a few amount of gibbsite when seed ratio was above 0.4. When RS changed from 0.1-0.4,boehmite contents increased rapidly and got the maximal value of 76% when RS=0.4. Boehmite contents decreased to 70% when RS increased to 0.8. Combining the data in Fig.2(a) and Fig.2(b), the precipitation selectivity of boehmite and gibbsite can be obtained, as shown in Fig.2(c). The general trend was that boehmite content increased with increasing seed ratio, while the gibbsite content decreased. These results indicate an “inhibitory effect” of boehmite seed on gibbsite nucleation under co-precipitation conditions, especially when the seed ratio exceeds 0.4.
3.1.2 Precipitation temperature control
Temperature has strong impacts on reaction rate, nucleation and growth of crystallization, aluminate ions transport and seed/solution interfacial tension, so precipitation ratio can be changed through regulating temperature. The experimental conditions was RS=0.4, c(Al2O3)=105 g/L, RM=1.53, CO2 mixture gas flow rate u(CO2)=150 mL/min. The results are shown in Fig.3.
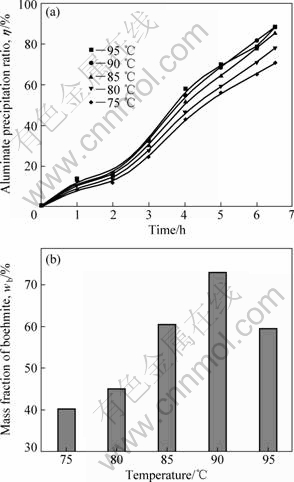
Fig.3 Effect of temperature on precipitation ratio (a) and boehmite content (b)
As shown in Fig.3(a), the co-precipitation ratio increased significantly with an increase in temperature, especially in the range of 75-85 ℃, also quite different from normal Bayer seeded precipitation. Although high temperature results in the decline of supersaturation and is harmful to gibbsite precipitation, low initial supersaturation enables the boehmite precipitation. In addition, the magnitude of the increase in the precipitation rates in boehmite and gibbsite is different. The contents of boehmite shown in Fig.3(b) firstly increased and then had a slight decline with temperature rising. Precipitation at lower temperature produced mainly gibbsite; however, the product contained significant amounts of boehmite above 80 ℃. Low temperature and high initial supersaturation promoted gibbsite nucleation, thus the gibbsite content was more than 50% when temperature was lower than 80 ℃. The maximal boehmite content of 74% was got at 90 ℃, and the increased precipitation ratio can be attributed to boehmite formation, which accounted for more than 60% of the sample at 85 ℃. The kinetic analysis reveals that the activation energy of boehmite precipitation is higher than that of gibbsite[10]. Higher temperature is of advantage to the reaction with higher activation energy. As a result, the boehmite could take the advantage of high temperature and the contents of boehmite increased with temperature rising. However, the fluctuation of boehmite content with temperature needs to be further investigated in our next study.
3.1.3 Ethanol medium for caustic extraction function
Alumina hydrate was prepared from supersaturated sodium aluminate solutions with ethanol addition instead of CO2 charge at RS=0.4. The effects of mass fraction of ethanol in the ethanol-water solution on the precipitation ratio and phase compositions of alumina hydrate were studied. Ethanol could increase significantly the precipitation ratio of sodium aluminate solutions, and the precipitation ratio scaled up with both ethanol mass fraction and precipitation time. Three distinct stages can be observed in Fig.4(a). Firstly, a very fast initial precipitation stage has duration of about 2 h. Secondly, in a transition stage, the precipitation rate continuously decreased until an apparent equilibrium stage was achieved. Finally, the precipitation reaction evolved with very low rate and reached an equilibrium stage, but this equilibrium stage was not a real equilibrium stage as the alumina concentration in the solution was far away from its real equilibrium value[6]. Fig.4(b) indicated that the product was the mixture of boehmite and gibbsite, and the mass fraction of AlOOH in the product increased significantly with the increase of mass fraction of ethanol because the precipitation of gibbsite was inhibited. When we=1.0, the mass fraction of AlOOH in the product reached the peak of 90%.
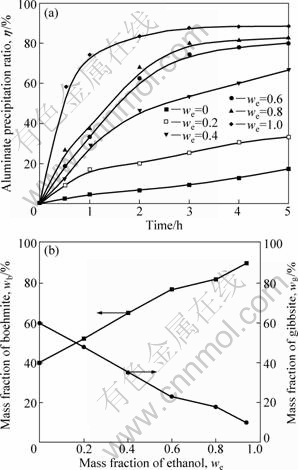
Fig.4 Effect of mass fraction of ethanol (we) on precipitation ratio (a) and composition of products (b)
Boehmite was the main phase in the product, indicating that gibbsite nucleation had been partially suppressed. Just as Eq.(1) shows, precipitation occurs with an increment of OH- concentration; therefore, the seed surface is covered with hydroxyl groups and it repels with negatively charged aluminate ions, inhibiting the precipitation from the aluminate liquors. Due to the extraction of caustic by ethanol, an increase in the concentration of the medium causes a substantial reduction on the specific surface charge of the boehmite particles, then, the inhibition is eliminated.
3.2 Adjustment on alumina hydrate size distribution and morphology
The particle size distributions (PSD) of seed and product co-precipitated at 90 ℃, RS=0.4 are presented in Fig.5. It is indicated that the mean particle size of seed is 10 μm and has a multimodal distribution, while the products of precipitation for 5 h have normal particle size distribution, and the average particle size of product is approximately three times that of the seed. The PSD comparison between seed and product at mass fraction of ethanol of 0.6 is shown in Fig.6. Co-precipitation of gibbsite and boehmite significantly changes the PSD of the seed to a normal distribution of product. The results indicate that fine seed particles decrease through growth and agglomeration with the help of aluminate ions, and there is a large increase in the percentage of particles of larger than 10 μm in size.
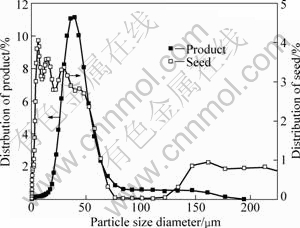
Fig.5 Size distribution of seed and obtained product under 90 ℃ and RS=0.4
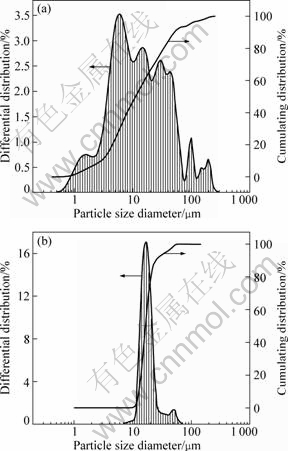
Fig.6 Size distributions of seed (a) and product (b) at mass fraction of ethanol we=0.6
The SEM images of crystals from co-precipitation are shown in Fig.7. It is indicated that the products have uniform morphology and particle sizes of seeds are about 10 μm (Fig.7(a)). The product of pure carbonization without seeds consists of large aggregates (Fig.7(b)), and the hexagonal and blocky shape suggests that the crystals could be gibbsite. The main products of precipitation are boehmite of quadrangle crystals when RS=0.4 (Fig.7(c)), with smaller aggregates of diamond-shaped particles. Precipitation occurs through the addition of growth units onto the surface or through the re-arrangement of a cluster near the surface. Boehmite precipitation occurs with the addition of boehmite growth unit onto a boehmite seed surface. This indicates that adding boehmite seeds can induce the precipitation of boehmite while inhibit the formation of gibbsite.
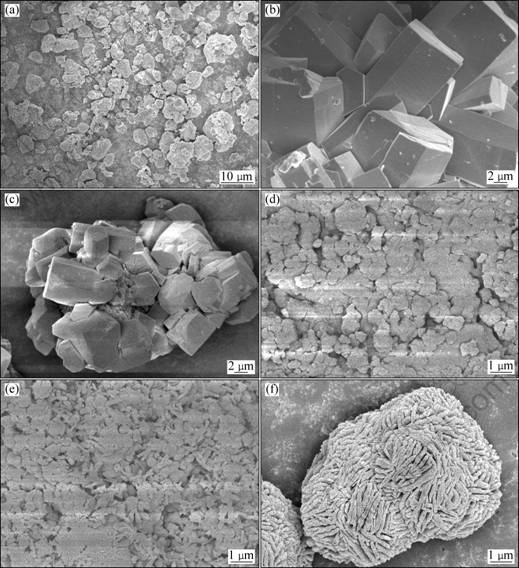
Fig.7 SEM images of seed (a) and products at different seed ratio (b-c) and mass fraction of ethanol (d-f): (a) Seed; (b) RS=0 and t=90 ℃; (c) RS=0.4; (d) we=0; (e) we=0.2; (f) we=0.6
Significant morphological changes occurred in the products with changes in ethanol content as shown in (Fig.7(d-f)). The product with ethanol additive is plate-like particles with uniform morphology (Fig.7(d)) compared with no additive. Increasing the ethanol mass fraction increased the amount of boehmite present in the product. Fig.7(f) revealed that, when the ethanol content is larger than 0.6, the product consists of large hollow agglomerated particles having a different crystal structure to gibbsite. The effect of the ethanol appears to be through the promotion of boehmite precipitation rather than through the disruption of the gibbsitic clusters formation.
4 Conclusions
1) New process of substituting gibbsite with boehmite as an intermediate product needs to be developed to eliminate the distance surpassed by Bayer process. The kinetic inhibition responsible for the low efficiency of the normal boehmite seeded precipitation process has disappeared in this new co-precipitation process, different from the present one only in its precipitation circuit.
2) Gibbsite is the dominant phase in carbonization products, and the content of boehmite is lower than 6%. In co-precipitation, boehmite seed ratio and temperature have a strong impact on the boehmite and gibbsite co-precipitation from sodium aluminate solution. With the seed ratio increasing, the ratio of alumina hydrate precipitation from sodium aluminate solution decreases but the absolute content of boehmite in products increases while that of gibbsite decreases. When caustic is extracted by ethanol, the mass fraction of boehmite in products is increased significantly with the increase of mass fraction of ethanol.
3) Experimental results confirm that boehmite can be precipitated at temperatures lower than 100 ℃ and under atmosphere conditions in the presence of boehmite seed particles or ethanol medium. Co-precipitation changes the multimodal distribution of seeds to a normal and well distribution of products, and the particle size is more than several times that of seeds.
References
[1] FILIPPOU D, PASPALIARIS I. From Bayer process liquors to boehmite and then to alumina: An alternative route for alumina production [J]. Light Metals, 1993: 119-123.
[2] JOANNE L, CHRIS V, MELISSA L, GRETA B. Boehmite and gibbsite precipitation [J]. Light Metals, 2005: 203-207.
[3] MARIA L, HELEN S, PERSIO S. Characterization of the aluminum hydroxide microcrystals formed in some alcohol-water solutions [J]. Materials Chemistry and Physics, 2002, 76: 243-249.
[4] TSUCHIDA T. Hydrothermal synthesis of submicrometer crystals of boehmite [J]. Eur Ceram Soc, 2000, 20(11): 1759-1762.
[5] MISRA C, SIVAKUMAR T J. Boehmite production by precipitation from sodium aluminate solution at elevated temperatures. US 4595581[P]. 1986.
[6] PANIAS D, ASIMIDIS P, PASPALIARIS I. Solubility of boehmite in concentrated sodium hydroxide solutions: Model development and assessment [J]. Hydrometallurgy, 2001, 59: 15-29.
[7] PANIAS D. Role of boehmite/solution interface in boehmite precipitation from supersaturated sodium aluminate solutions [J]. Hydrometallurgy, 2004, 74: 203-212.
[8] PANIAS D, KRESTOUL A. Effect of synthesis parameters on precipitation of nanocrystalline boehmite from aluminate solutions [J]. Powder Technology, 2007, 175(33): 163-173.
[9] SKOUFADIS C, PANIAS D, PASPALIARIS I. Kinetics of boehmite precipitation from supersaturated sodium aluminate solutions[J]. Hydrometallurgy, 2003, 68: 57-68.
[10] DASH B, TRIPATHY B C, BHATTACHARYA I N, DAS S C, MISHRA C R, PANI B S. Effect of temperature and alumina/caustic ratio on precipitation of boehmite in synthetic sodium aluminate liquor [J]. Hydrometallurgy, 2007, 88: 121-126.
[11] LI Xiao-bin, LIU Xiang-ming, GOU Zhong-ru, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng, DING An-ping, LI Ming, LIU Ye-xiang. Thermodynamics of carbonization of aluminate solution [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 1005-1010. (in Chinese)
[12] WANG Z, BI S, YANG Y, YUAN Z. Evolution of particle size and strength of hydrargillite from carbonization in seeded sodium aluminate liquors [J]. Journal of Crystal Growth, 2005, 274(1/2): 218-225.
[13] DEMOPOULOS G P. Aqueous precipitation and crystallization for the production of particulate solids with desired properties [J]. Hydrometallurgy, 2009, 96: 199-214.
[14] WANG Z. GUO Z. Method for boehmite precipitation from supersaturated sodium aluminates solutions by carbonation decomposition. CN 10065124[P]. 2007.
[15] WANG Z, BI S, YANG Y, YUAN Z. The Application of Al(OH)3 seed in carbonization of sodium aluminate liquors [J]. Light Metals, 2005: 223-227.
[16] HUIXIN L, JONAS A M, JOHN T, ANDREA R G. The crystallization mechanism of Al(OH)3 from sodium aluminate solutions[J]. Journal of Crystal Growth, 2005, 279: 508-520.
[17] MOOLENAAR R J, EVANS J C. The structure of the aluminate ion in solutions at high PH [J]. Phys Chem, 1970,74: 3629-3636.
Foundation item: Projects(50704030) supported by the National Natural Science Foundation of China; Project(KGCX2-YW-321-2) supported by the Knowledge Innovation Program of the Chinese Academy of Sciences
Corresponding author: WANG Zhi; Tel/Fax: +86-10-62558489; E-mail: zwang@home.ipe.ac.cn
DOI: 10.1016/S1003-6326(09)60172-2


