Trans. Nonferrous Met. Soc. China 25(2015) 1315-1324
Recycling of metals from waste Sn-based alloys by vacuum separation
Bin YANG 1,2,3, Ling-xin KONG 1,2,3, Bao-qiang XU 1,2,3, Da-chun LIU 1,2,3, Yong-nian DAI 1,2,3
1. National Engineering Laboratory for Vacuum Metallurgy, Kunming University of Science and Technology, Kunming 650093, China;
2. Key Laboratory of Vacuum Metallurgy for Nonferrous Metal of Yunnan Province, Kunming University of Science and Technology, Kunming 650093, China;
3. State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Kunming University of Science and Technology, Kunming 650093, China
Received 25 May 2014; accepted 8 December 2014
Abstract: In order to recycle waste Sn-based alloys, the vapor-liquid phase equilibrium composition diagrams of Sn-Pb, Sn-Sb and Sn-Zn binary systems were calculated. The calculated results indicate that Pb, Sb and Zn can be separated from Sn effectively. Based on the above calculation, the industrial experiments of vacuum distillation of Sn-Pb alloy, Sn-Pb-Sb alloy, Sn-Pb-Sb-As alloy, crude Sn and Sn-Zn alloy with different contents were carried out. The experimental results show that Pb (>99% Pb) and Sn (≤0.003% Pb) were obtained simultaneously while Sn-Pb alloy was subjected to vacuum distillation; the crude Sn (>90% Sn, ≤ 2% Pb, ≤6% Sb) and crude Pb (≤2% Sn) were obtained simultaneously while a single vacuum distillation was carried out for Sn-Pb-Sb alloy; the Pb and Bi contents in the Sn ingot (99.99% Sn) achieve the grade A of GB/T 728—2010 standard, more than 50% of As and Sb was removed after vacuum distillation of crude Sn; Zn (<0.002% Sn) and Sn (about 3% Zn) were obtained while vacuum distillation of Sn-Zn alloy was conducted at 1173 K, 20-30 Pa for 8-10 h.
Key words: Sn-based alloys; activity coefficient; vacuum distillation; vapor-liquid phase equilibrium
1 Introduction
Sn is one of the earliest metals that human discovered and used and is widely used in aerospace, military, nuclear industry, electronics information, semiconductors, superconducting alloys, navigation, medicine, food industry and other fields since it has the following characteristics: low melting point, non-toxic, high atmospheric corrosion resistance, good ductility and good weldability. It is an indispensable key material in military industry and modern cutting-edge technology areas [1,2]. The Sn smelting and production level of China are in a leading position in the world. The Sn resources in China are mainly distributed in Yunnan, Guangxi, Hunan, Guangdong and Jiangxi provinces where the recoverable deposits account for 97.3% of the country’s reserves [3]. The Sn production of Yunnan Tin Group Co., Ltd. and Guangxi Tin Group Co., Ltd. accounts for about one-fifth of the total output of the world in 2011. In recent years, a large number of waste Sn resources such as Sn-Pb alloys, multi-Sn alloys, crude Sn and Zn-Sn alloys have been produced from the smelting process of Sn, Pb and Cu, the recycling process of Sn secondary resources and the production and machining process of solders, bearing babbits and Sn alloy coatings due to the development of industry and the decrease of mineral resources [4].
For a long time, many problems such as the waste of Sn, Pb, Sb and Zn resources and other resources, higher energy consumption and environmental pollution have emerged due to lack of efficient processing techniques. In order to further improve the Sn smelting level of China, the developing of more advanced technologies and equipments, therefore, has become imperative for China. A new road to industrialization will thus be realized, an environment-friendly and resource-saving society will be achieved finally. The separation and recovery of waste Sn-based alloys by traditional methods, however, have some problems. Recently, a lot of researches including theoretical basic research and equipment upgrade have been carried out for the recovery of waste Sn-based alloys by vacuum distillation and many achievements have been achieved [5-7]. Vacuum metallurgy has many advantages such as short flow sheet and lower energy consumption. It can eliminate the disadvantages of traditional metallurgical processes. Vacuum distillation has been studied and successfully used in separation and purification of various elements from nonferrous alloys [8-10].
In this work, vacuum distillation experiments for Sn-Pb alloys, multi-Sn alloys, crude Sn and Sn-Zn alloys were carried out based on the theoretical prediction. The corresponding new technology, therefore, was developed and widely used in industries. It provides an important technique for the effective and comprehensive utilization of Sn, Pb, Sb and Zn resources.
2 Calculation and prediction for vacuum distillation of Sn alloys
2.1 Vapor pressure of components of Sn alloys
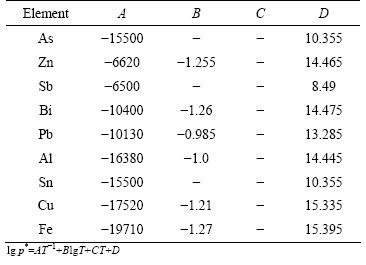
The phase transition will take place when the system pressure is less than the saturated vapor pressure of a substance, and the substance will exist in the form of gas phase. The saturated vapor pressure of the pure metals as a function of temperature was calculated using the equation and related parameters [11] given in Table 1, as shown in Fig. 1. It can be seen from Fig. 1 that the saturated vapor pressure of the components increases with the increase of temperature. The saturated vapor pressure of these components follows the sequence of As > Zn > Sb > Bi > Pb > Al > Sn > Cu > Fe at the same temperature. Therefore, As, Zn, Sb, Bi, Pb and Al will exhibit a higher volatility than Sn, while Cu and Fe exhibit a low volatility.

Fig. 1 Saturated vapor pressure as a function of temperature for some pure metals
2.2 Separation criterion and vapor-liquid phase equilibrium composition diagram for vacuum distillation of Sn-Me binary alloys
For a Sn-Me binary alloy (Me represents Pb, Sb, Zn, As, Cu, Bi, Al and Fe), according to the ideal gas state equation, the relationship between ρ(Sn) and ρ(Me)
can be expressed as:
 (1)
(1)
where ρ(Sn)and ρ(Me) are the vapor densities of Sn and Me in the vapor phase, respectively; wl(Sn) and wl(Me) are the mass fractions of Sn and Me in the liquid phase, respectively. And βSn/Me is the separation coefficient and defined as
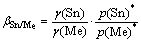 (2)
(2)
where γ(Sn) and γ(Me) are the activity coefficients of Sn and Me, respectively, p(Sn)* and p(Me)* are the saturated vapor pressures of pure Sn and Me, respectively. The separation of binary Sn-based alloys by vacuum distillation can be judged by βSn/Me. If βSn/Me<1, namely, ρ(Sn)/ρ(Sn)<>1(Sn)/w1(Me), Sn will concentrate in the liquid phase, while Me will concentrate in the vapor phase. For the same reason, Sn will concentrate in the gas phase, and Me will concentrate in the liquid phase while βSn/Me>1. The Sn content in the liquid phase equals that in the vapor phase while βSn/Me=1, viz., the separation of Sn and Me cannot happen.
Table 1 Related parameters and calculation equation for saturated vapor pressure [11]
The separation coefficient can only be used as a rough guidance in predicting the possibility of separation of binary alloys. In order to quantitatively predict the separation degree and product composition of vacuum distillation of alloys, the vapor-liquid phase equilibrium composition was introduced. Assuming wg(Sn) and wg(Me) are the mass fractions of Sn and Me in the vapor phase, respectively, then
In the gas phase
wg(Sn)+wg(Me)=1 (3)
In the liquid phase
wl(Sn)+wl(Me)=1 (4)
For gas phase,
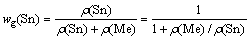 (5)
(5)
Substituting Eqs. (1) and (2) into Eq. (5), wg(Sn) can be represented as

 (6)
(6)
In a similar way,
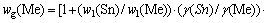
 (7)
(7)
Substituting a series of wl(Me)/wl(Sn), γ(Me), γ(Sn) and p*(Sn), p*(Me) into Eq. (6), the vapor-liquid phase equilibrium composition diagram of wg(Sn)-wl(Sn) can be obtained.
For a Sn-Me binary alloy, in order to obtain the vapor-liquid phase equilibrium composition diagram, the activity coefficients γ(Me) and γ(Sn) are necessary. The needed activity coefficients, however, were not available in the existing monographs.
For a long time, a large number of researches on calculation and prediction of the activity coefficient were conducted, and various prediction models were proposed. Based on free volume theory and lattice theory, a partition function of pure substance and their mixtures was established, and a new thermodynamic model, viz., molecular interaction volume model (MIVM)was established finally by combining with statistical thermo- dynamics and fluid phase equilibrium theory [12-14]. The MIVM has been used to predict the thermodynamic properties of a variety of binary and multi-component alloys, and a good application effect was observed [15-17]. In this work, the activity coefficients of components of Sn-Pb, Sn-Sb and Sn-Zn alloys were calculated by using the MIVM.
Substituting the activity coefficients into Eqs. (5) and (6), the vapor-liquid phase equilibrium diagram of Sn-Pb, Sn-Sb and Sn-Zn binary systems at different temperatures can be predicted, as shown in Figs. 2-4.
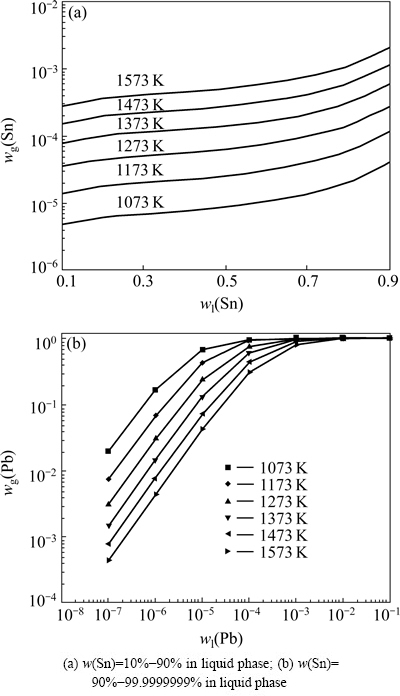
Fig. 2 Vapor-liquid phase equilibrium composition diagram of Sn-Pb alloy
The range of interesting temperature and composition in this work is more wider than those of JIA et al [18] and HAN et al [19]. These diagrams can be used to predict the equilibrium composition of the liquid and vapor phase while the separation of Sn-Pb, Sn-Sb and Sn-Zn alloys is carried out at different temperatures, and to provide the guidance for practice.

Fig. 3 Vapor-liquid phase equilibrium composition diagram of Sn-Sb alloy

Fig. 4 Vapor-liquid phase equilibrium composition diagram of Sn-Zn alloy
As can be seen from Fig. 2 that the ratio of Sn content in the liquid phase to that in the vapor phase is up to 103-107. So, Sn will concentrate in the liquid phase, while Pb volatilizes into the vapor phase, which indicates that Sn-Pb binary alloys can be separated by vacuum distillation. Figure 2(a) shows that Sn content in the vapor phase increases with the increase of temperature while Sn content in the liquid phase is constant. The Sn content in the vapor phase increases gradually with the increase of Sn content in the liquid phase when the vacuum distillation was conducted at 1373 K. Sn content in the vapor phase, for example, will be more than 1% while Sn content in the liquid phase is more than 90%.
Figure 2(b) indicates that deep removal of Pb from Sn will be realized by vacuum distillation, and the standard of Pb-free solder (Pb<0.003%) will also be achieved. Sn content in the vapor phase, however, will be greater than 90% while the distillation temperature is higher than 1373 K. The vacuum distillation, therefore, should be conducted at a relative low temperature to achieve simultaneously the demands that Pb content in the distilland will be less than 0.003% and Sn content in the vapor phase will be as low as possible.
In the same way, as can be seen from Figs. 3 and 4 that the ratio of Sn content in the liquid phase to that in the vapor phase is greater than 103 for both Sn-Sb and Sn-Zn alloys, which indicates that Sn will concentrate in the liquid phase, while Sb(Zn) evaporate into the vapor phase, and metallic Sn and Sb(Zn) will be obtained respectively from liquid and vapor phase after condensation, indicating that Sb(Zn) can be separated effectively from Sn by vacuum distillation. Under a certain temperature and pressure, Sb(Zn) content in Sn can reach a very low level in vacuum distillation.
3 Practice of vacuum distillation of Sn-based alloys
3.1 Vacuum distillation of Sn-Pb alloys
The schematic diagram of the essential components of the vacuum distillation system used is shown in Fig. 5. The experimental procedures and the analysis methods used in the present work are similar to that used by KONG et al [20]. The vacuum distillation experiments were carried out at different soaking time corresponding to the distillation temperature range of 1173-1223 K for two kinds of alloys (Table 2), and the system pressure was controlled in the range of 5-30 Pa. The Pb content in the product Sn was plotted in Fig. 6.
Table 2 Composition of Sn-Pb alloys (mass fraction, %)


Fig. 5 Schematic diagram of vacuum furnace
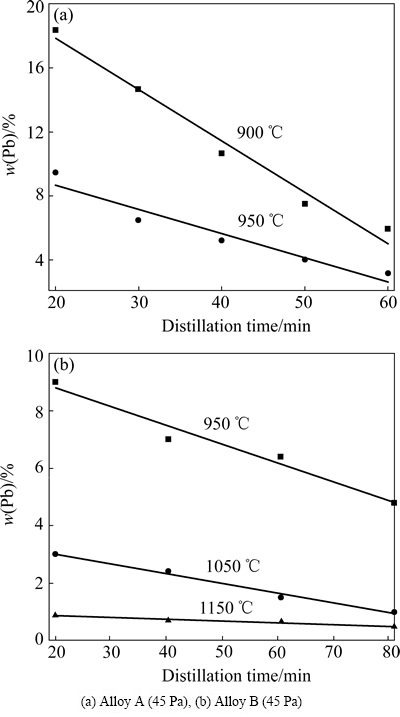
Fig. 6 Pb content in Sn product obtained from vacuum distillation of Sn-Pb alloys with different compositions at different conditions
As can be seen from Fig. 6(a) that Pb content in the vapor phase is larger than 99.5% while Pb content in the liquid phase is reduced from 76% to <4%, viz., wl(Pb)<4×10-2 while a single vacuum distillation is carried out for Alloy A at distillation temperature of 1223 K, vapor pressure of 45 Pa, corresponding to the distillation time of 60 min. However, the predicted results indicate that Pb content in the vapor phase is about 1, viz., wg(Pb)≈1, that is, the vapor phase mainly consists of Pb, the product condensed from volatile, therefore, should have a high purity. The experimental results match the predicted values while a discrepancy was observed because the experiment could not reach the equilibrium state in the limited distillation time. Figure 6(b) shows that Sn content in the vapor phase is 1.14% while Pb content in the liquid phase was reduced to <0.05%, viz. wl(Pb)<5×10-4 after the vacuum distillation is carried out for the Sn-Pb alloy obtained from the first vacuum distillation at the distillation temperature of 1323 K, vapor pressure of 45 Pa, and distillation time of 80 min. However, the predicted results (Fig. 2(b)) indicate that Sn content in the vapor phase is 10%. The experimental result is about one-tenth of the predicted values, which may arise from the accuracy of the experimental technique and conditions and the MIVM.
A newly consecutive vacuum distillation equipment that can dispose of 5 t Sn-Pb alloys one day has been developed, and the industrial experiments on deep removal of Pb from Sn-Pb alloy have been carried out. The raw material compositions are shown in Table 3, and the industrialized production technology indicators are summarized in Tables 4 and 5. The crude Pb (>99% Pb) and crude Sn (<5% Pb) can be obtained while a single vacuum distillation is carried out at 1323 K for Alloy C with low Sn content. The metallic Sn (<0.003% Pb) can be obtained after Alloy D is subjected to three consecutive vacuum distillation in which the crude Pb (>97% Pb) is obtained in the first vacuum distillation, the Sn-Pb alloy (43.32% Sn, 54.7% Pb) and the Sn-Pb alloy (18.7% Sn, 81.2% Pb) are obtained in the second and third vacuum distillation, respectively.
Table 3 Composition of Sn-Pb alloy used in industrial experiments (mass fraction, %)

Table 4 Industrial experiment index for single vacuum distillation of Alloy C
Table 5 Industrial experiment index for single vacuum distillation of Alloy D

Table 6 Comparison between new technology and original vacuum distillation technology for Sn-Pb alloy separation

Combining with the industrial experimental results, a complete set of large consecutive vacuum distillation equipment with handling capacity of 30 t/d in a single unit, comprehensive energy consumption of 280-500 kW·h/t has been developed based on the breakthrough of a series of key and difficult engineering problems such as heating, energy saving, continuous feeding-discharge material, evaporation and condensation. A technology of deep removal of Pb from Sn-Pb alloy, therefore, has been developed. A comparison between the new technology and the original vacuum distillation technology applied in vacuum distillation of Sn-Pb alloys is shown in Table 6. As can be seen form Table 6, the advantage of this new technology lies in the fact that its handing capacity is triple that of the original technology, energy consumption is decreased by more than 20%, and the Pb content in the product Sn is <0.003%. It can dispose of Sn-Pb alloys with any composition.
3.2 Vacuum distillation of Sn-Pb-Sb and Sn-Pb- Sb-As alloys
3.2.1 Vacuum distillation of Sn-Pb-Sb alloys
Two kinds of Sn-Pb-Sb alloys used in this work are listed in Table 7. There is a great difference in the Pb and Sb content while a slight variation in Sn content is observed in these two alloys. The vacuum distillation was carried out for these two alloys at the distillation temperature range of 1373-1523 K and distillation time of 30 min, and the volatilization of Pb and Sb in vacuum distillation was investigated systematically. The removal rate of Sb in the products after vacuum distillation is shown in Fig. 7.
Table 7 Composition of Sn-Pb-Sb alloy (mass fraction, %)

As can be seen from Fig. 7 that the Sb content in the product, viz., crude Sn was decreased to 0.07% and 0.08% respectively for these two alloys, and the Pb content was lower than 0.01% after vacuum distillation was conducted at 1523 K for 30 min. The removal rate of Sb in Alloy E was equal to that in Alloy F under the same conditions, although the initial Sb content in Alloy E was higher than that of Alloy F. Figure 7 also shows that the removal rate of Sb increases with the increase of distillation temperature. The higher distillation temperature, however, was not suitable because Sn content in the vapor phase continuously increases with the increase of distillation temperature (Fig. 3), that is, it will result in a low recovery rate of Sn and a low purity of the product, viz., Pb-Sb alloy condensed from the volatile.
The crude Sn (>90% Sn, ≤2% Pb and ≤6% Sb) and the crude Pb (≤2% Sn) were obtained simultaneously after a single vacuum distillation was conducted for Sn-Sb-Pb alloys with different contents. The secondary crude Sn (≤0.02% Pb, ≤1% Sb) will be obtained after a consecutive distillation process was carried out for the crude Sn produced from the first distillation, and the Sn-Sb-Pb alloy obtained from the second distillation will be returned to the first distillation. The industrialized experiments were conducted by using the Sn-Pb-Sb alloys (Table 8) as the raw material, and the technical and economic index is summarized in Table 9.

Fig. 7 Sb content in product Sn obtained from vacuum distillation of Sn-Sb-Pb alloys at different temperatures
The results of the industrialized experiments indicate that the crude Sn (98.8% Sn, 0.001% Pb and 0.25% Sb) can be obtained after the secondary distillation was conducted for Sn-Sb-Pb alloy (18.87% Sn, 61.7% Pb and 14.26% Sb). The secondary product, viz., crude Pb (<20% Sb, <2% Sn), will be obtained after disposing of Pb and Sb produced in the first distillation, which can be used to produce Pb anode plate applied in electrolysis. The separation of Pb and Sb from Sn, therefore, will be achieved efficiently.
Table 8 Composition of Sn-Pb-Sb alloy (mass fraction, %)
3.2.2 Vacuum distillation of Sn-Pb-Sb-As alloys
More and more arsenic-bearing Sn-Pb-Sb alloys with As content of 0.1%-2% are produced in the metallurgical process. A condenser used for the condensation of As was added in the transformation process of the internal structure of the equipment based on the knowledge of great differences in condensation temperature of As, Pb and Sb and the results of the industrialized vacuum distillation of Sn-Pb-Sb alloys. The fractional condensation of As from Pb and Sb, therefore, was achieved finally.
In order to demonstrate the feasibility of separation of As from Sn, Pb and Sb, an industrialized experiment was carried out on the Sn-Pb-Sb-As alloys with different As content (Table 10), and the results were summarized in Table 11. The crude Sn (≤0.02% Pb, ≤1% Sb and ≤ 0.07% As), Pb-Sb-Sn alloy (~50% Pb, ~20% Sb and ~1.3% As) and simple substance As (≥95% As) were obtained simultaneously while the first vacuum distillation was carried out at 1573-1673 K and vapor pressure of less than 10 Pa. The crude Sn (90% Sn), Pb-Sn alloy ( ≤1% Sn) and a bit of simple substance As were obtained simultaneously after the secondary vacuum distillation was carried out at 1573-1673 K and vapor pressure of about 1 Pa for Pb-Sb-Sn alloy obtained in the first vacuum distillation. The crude Sn with high Sb content and about 90% Sn was returned to the first vacuum distillation. The processing capacity of the equipment is about 7-8 t/d, the energy consumption is 350-500 kW·h/t. The efficient separation of Pb, Sb and As from Sn along with a high metal recovery rate of 99% was achieved. As was recycled in the form of simple substance, the environment pollution caused by As was thus relieved greatly.
The equipment capacity is 18-20 t/a,and the comprehensive energy consumption remains in a range of 400-600 kW·h/t on the basis of different Sb contents.
3.3 Vacuum distillation of crude Sn
The crude Sn produced from Sn smelting was used to carry out the industrial trials in the consecutive vacuum distillation equipment which is designed to deal with Sn-Pb-Sb-As alloys. The experimental conditions are as follows: distillation temperature of 1623 K; residual pressure of less than 10 Pa; feeding materials of 18 t/a. Table 12 shows the composition of raw material. Tables 13 and 14 show the experimental data and economic and technical index, respectively.
Table 9 Technical and economic index for industrialized vacuum distillation of Sn-Pb-Sb alloy

The experimental results show that the contents of Pb and Bi in the product (99.99% Sn) can meet grade A of GB/T 728—2010 standard and more than 50% of As and Sb can be removed. A novel process of Sn refining, therefore, was proposed based on the vacuum distillation technology and the Sn refining pyrometallurgy. The pressure of numerous material recycling in Sn refining process was significantly released because the deep removal of As, Sb and Pb from crude Sn was achieved. And this new process is likely to reduce three quarters of Al-As slag quantity compared with the current refining process.
Table 10 Composition of Sn-Pb-Sb-As alloys

Table 11 Technical and economic index of industrial vacuum distillation of Sn-Pb-Sb-As alloy
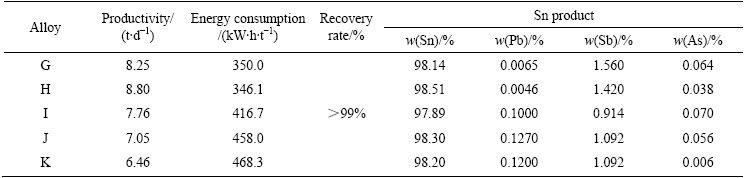
Table 12 Composition of crude Sn (mass fraction, %)

Table 13 Metal balance of industrial vacuum distillation experiment of crude Sn

Table 14 Industrial vacuum distillation experimental index of crude Sn

3.4 Vacuum distillation of Sn-Zn alloys
With the development of solder industry, numerous waste Sn-Zn alloys are accumulated because Pb-Sn solders are gradually replaced by Sn-Zn solders. Therefore, it is imperative for us to reproduce Sn and Zn from these secondary resources. Based on the separation theory of Sn-Zn alloys, Zn can be concentrated and purified in the gas phase by vacuum distillation, while Sn in the liquid phase. A complete set of vacuum distillation equipments were developed to support the specific treatment of the raw materials provided by a company of China. The chemical composition of the material was listed in Table 15. Table 16 shows the results of a typical industrial experiment. As can be seen from Table 16, Sn content in the vapor phase and Zn content in the liquid phase are less than 0.002% and 3%, respectively, while feeding material is 1.2 t, residual pressure is 20-30 Pa, distillation temperature is 1173 K and soaking time is 8-10 h. The technical indexes of industry trials for Sn-Zn alloy (80% Zn) are shown in Table 17. It indicates that the purity of Zn and Sn is larger than 99% and 98%, respectively, the processing capacity in a single-furnace is 1.5 t and the energy consumption is less than 1600 kW·h/t.
Table 15 Composition of Sn-Zn alloy (mass fraction, %)

Table 16 Vacuum distillation experiment data of Sn-Zn alloys
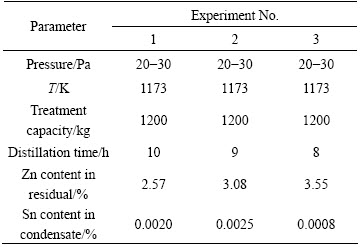
Table 17 Industrial experiment index for vacuum distillation of Sn-Zn alloys

4 Conclusions
1) The vapor-liquid phase equilibrium composition of Sn-Pb, Sn-Sb and Sn-Zn alloys were calculated. It can be used to predict the distribution of components of alloys between vapor and liquid phase during vacuum distillation.
2) The experimental results show that the Pb content in Sn was reduced to 0.05% while vacuum distillation was conducted for Sn-Pb alloy (4% Pb) at distillation temperature of 1323 K, chamber pressure of 45 Pa, corresponding to the soaking time of 80 min.
3) The crude Sn (>90% Sn, ≤2% Pb, ≤6% Sb) and crude Pb (≤2% Sn) were obtained simultaneously while a single vacuum distillation was carried out for Sn-Pb-Sb alloy; the Pb and Bi contents in the Sn ingot (99.99% Sn) achieve the grade A of GB/T 728—2010 standard, more than 50% of As and Sb were removed after vacuum distillation of crude Sn; Zn (<0.002% Sn) and Sn (about 3% Zn) were obtained while vacuum distillation was conducted at 1173 K, system pressure of 20-30 Pa for 8-10 h.
4) The experimental results match with the predicted values while a discrepancy was observed because the experiment could not reach the equilibrium state in the limited distillation time.
References
[1] LIU Meng-fei. The “Sn” dream of China [J]. China Metal Bulletin, 2011, 32: 16-18. (in Chinese)
[2] National Development and Reform Commission, Ministry of Land and Resources, Commerce Department, State Environmental Protection Administration, State Administration for Industry and Commerce, State Quality Inspection Administration, General Administration of China Customs. The advice on strengthening management of W, Sn and Sb industry [J]. Advanced Materials Industry, 2005, 8: 30-32. (in Chinese)
[3] ZHANG Mei. Global Sn resource and development situation [J]. China Metal Bulletin, 2011, 32: 19-21. (in Chinese)
[4] HUANG Wei-sen. Sn [M]. Beijing: Metallurgical Industry Press, 2000. (in Chinese)
[5] KONG Ling-xin, YANG Bin, XU Bao-qiang, LI Yi-fu. Application of molecular interaction volume model for phase equilibrium of Sn-based binary system in vacuum distillation [J]. Metallurgical and Materials Transactions A, 2014, 45A: 4405-4410.
[6] YANG Bu-zheng, ZHAO Xiang-sheng, DAI Yong-nian, YANG Bin, MA Wen-hui, HAN Long. Separation of zinc and enrichment of tin from waste Zn-Sn alloys by vacuum distillation [J]. Journal of Kunming University of Science and Technology, 2006, 31 (3): 15-18. (in Chinese)
[7] KONG Ling-xin, YANG Bin, XU Bao-qiang, LI Yi-fu, LIU Da-chun, DAI Yong-nian. Application of MIVM for Pb-Sn-Sb ternary system in vacuum distillation [J]. Vacuum, 2014, 101: 324-327.
[8] GOPALA A, KIPPHARDT H, MATSCHAT R, PANNE U. Process methodology for the small scale production of m6N5 purity zinc using a resistance heated vacuum distillation system [J]. Materials Chemistry and Physics, 2010, 122: 151-155.
[9] YANG Hong-wei, XU Bao-qiang, YANG Bin, MA Wen-hui, TAO Dong-ping. Calculation of phase equilibrium in vacuum distillation by molecular interaction volume model [J]. Fluid Phase Equilibria, 2011, 314: 78–81.
[10] ALI S T, PRASAD D S, MUNIRATHNAM N R, PRAKASH T L. Purification of tellurium by single-run multiple vacuum distillation technique [J]. Separation and Purification Technology, 2005, 43: 263-267.
[11] DAI Yong-nian, YANG Bin. Vacuum metallurgy of nonferrous materials [M]. Beijing: Metallurgical Industry Press, 2000. (in Chinese)
[12] TAO Dong-ping, YANG Bin, LI Duen-fang. Prediction of the thermodynamic properties of quinary liquid alloys by modified coordination equation [J]. Fluid Phase Equilibria, 2002, 193: 167-177.
[13] TAO Dong-ping. A thermodynamic model for solid solutions and its application to the C-Fe-Co, C-Fe-Ni and Mn-Cr-Pt solid dilutions [J]. Materials Science and Engineering A, 2004, 366(2): 239-247.
[14] TAO Dong-ping. A comparison of the molecular interaction volume model with the sub-regular solution model in multicomponent liquid alloys [J]. Metallurgical and Materials Transaction A, 2004, 35A: 419-424.
[15] YANG Hong-wei, YANG Bin, XU Bao-qiang, LIU Da-chun, TAO Dong-ping. Application of molecular interaction volume model in vacuum distillation of Pb-based alloys [J]. Vacuum, 2012, 86(9): 1296-1299.
[16] YANG Hong-wei, XU Bao-qiang, YANG Bin, MA Wen-hui, TAO Dong-ping. Calculation of phase equilibrium in vacuum distillation by molecular interaction volume model [J]. Fluid Phase Equilibria, 2012, 314: 78-81.
[17] JIA Guo-bin. Research on separation and purification of certain Pb based alloys by vacuum distillation [D]. Kunming: Kunming University of Science and Technology, 2010. (in Chinese)
[18] JIA Guo-bin, YANG Bin, LIU Da-chun. A study on vacuum distillation of waste Pb and Sn alloy [J]. Resource Recycling, 2008(1): 30-32. (in Chinese)
[19] HAN Long, YANG Bin, YANG Bu-zheng. Industrial experiment on recovering metals from waste Zn-Sn alloy by vacuum distillation [J]. China Nonferrous Metallurgy, 2007(2): 55-57. (in Chinese)
[20] KONG Ling-xin, YANG Bin, LI Yi-fu, XU Bao-qiang, LIU Da-chun, JIA Guo-bin. Application of MIVM for Pb-Sn system in vacuum distillation [J]. Metallurgical and Materials Transactions B, 2012, 43B: 1649-1656.
废弃锡合金真空分离回收金属
杨 斌1,2,3, 孔令鑫1,2,3, 徐宝强1,2,3, 刘大春1,2,3, 戴永年1,2,3
1. 昆明理工大学 真空冶金国家工程实验室,昆明 650093;
2. 昆明理工大学 云南省有色金属真空冶金重点实验室,昆明 650093;
3. 昆明理工大学 复杂有色金属资源清洁利用国家重点实验室,昆明 650093
摘 要:
以废弃锡合金的回收利用为目标,计算绘制了Sn-Pb、Sn-Sb及Sn-Zn二元合金的气-液相平衡成分图。结果表明:Pb、Sb及Zn能够有效地与Sn分离。以此为指导,对不同成分的Sn-Pb合金、Sn-Pb-Sb合金、Sn-Pb-Sb-As合金、粗Pb合金以及Sn-Zn合金开展真空蒸馏工业化实验研究。实验结果表明:Sn-Pb合金在1323 K条件下经真空蒸馏可获得含Pb>99%的粗Pb和含Pb≤0.003%的Sn;Sn-Pb-Sb合金经一次真空蒸馏,可得到含Sn量>90%、含Pb量≤2%、含Sb量≤6%的粗Sn和含Sn≤2%的粗Pb;粗Sn经过真空蒸馏后,产品中Pb和Bi含量达到Sn锭GB/T 728—2010中Sn99.99A级标准,超过50%的As和Sb得到脱除;Sn-Pb合金在1173 K。体系压力20-30 Pa条件下真空蒸馏8-10 h,得到的产品Zn中含Sn<0.002%,Sn中含Zn约3%。
关键词:
(Edited by Yun-bin HE)
Foundation item: Project (2014HA003) supported by the Cultivating Plan Program for the Technological Leading Talents of Yunnan Province, China; Project (51474116) supported by the National Natural Science Foundation of China; Project (IRT1250) supported by the Program for Innovative Research Team in University of Ministry of Education of China; Project (20140355) supported by the Analytical Test Fund of Kunming University of Science and Technology, China; Project supported by the First-class Doctoral Dissertation Breeding Foundation of Kunming University of Science and Technology, China
Corresponding author: Bin YANG; Tel: +86-871-65163583; E-mail: kgyb2005@126.com
DOI: 10.1016/S1003-6326(15)63730-X
摘 要:以废弃锡合金的回收利用为目标,计算绘制了Sn-Pb、Sn-Sb及Sn-Zn二元合金的气-液相平衡成分图。结果表明:Pb、Sb及Zn能够有效地与Sn分离。以此为指导,对不同成分的Sn-Pb合金、Sn-Pb-Sb合金、Sn-Pb-Sb-As合金、粗Pb合金以及Sn-Zn合金开展真空蒸馏工业化实验研究。实验结果表明:Sn-Pb合金在1323 K条件下经真空蒸馏可获得含Pb>99%的粗Pb和含Pb≤0.003%的Sn;Sn-Pb-Sb合金经一次真空蒸馏,可得到含Sn量>90%、含Pb量≤2%、含Sb量≤6%的粗Sn和含Sn≤2%的粗Pb;粗Sn经过真空蒸馏后,产品中Pb和Bi含量达到Sn锭GB/T 728—2010中Sn99.99A级标准,超过50%的As和Sb得到脱除;Sn-Pb合金在1173 K。体系压力20-30 Pa条件下真空蒸馏8-10 h,得到的产品Zn中含Sn<0.002%,Sn中含Zn约3%。


