稀有金属2017年第5期
稀土基催化材料用于含氯废气催化燃烧的研究进展
王丽 谢鸿凯 戴启广 郭扬龙 郭耘 卢冠忠
华东理工大学化学与分子工程学院工业催化所
摘 要:
针对有毒有害的含氯挥发性有机化合物废气的催化氧化脱除, 从稀土氧化物 (Ce和La) 出发, 以饱和含氯代烃 (二氯甲烷, 二氯乙烷等) 和不饱和氯代烃 (氯苯, 氯乙烯) 为模型反应;详细分析了稀土修饰 (Ce, La) 或以稀土为主的催化剂 (CeO2, La MnO3) 对氯代烃催化燃烧的影响。以催化剂表面的酸性中心和氧化还原性能为主线, 分析了二者在氯代烃催化燃烧反应过程中的作用, 酸中心与催化剂CO选择性、积碳相关;而多氯副产物的生成和分布则受催化剂氧化性能的影响。在此基础上提出氯代烃的催化燃烧催化剂的设计思路, 结合氯代烃分子结构差异, 调控催化剂表面酸性中心和氧化还原性能之间的匹配, 提高催化剂的低温反应活性、兼顾含碳 (氯) 副产物的选择性、避免催化剂积碳、氯中毒的现象, 增加催化剂的稳定性。
关键词:
催化燃烧;氯代烃;酸性;氧化还原;表面性质;
中图分类号: O643.36;X701
作者简介:王丽 (1973-) , 女, 吉林和龙人, 博士, 副教授, 研究方向:催化材料设计与制备;E-mail:wangli@ecust.edu.cn;;卢冠忠, 教授;电话:021-64252827;E-mail:gzhlu@ecust.edu.cn;
收稿日期:2017-04-10
基金:国家重点研发计划项目 (2016YFC0204300);国家自然科学基金项目 (21571061) 资助;
Research Progress in Catalytic Removing Chlorinated Volatile Organic Compounds by Rare Earth Materials
Wang Li Xie Hongkai Dai Qiguang Guo Yanglong Guo Yun Lu Guanzhong
Key Laboratory for Advanced Materials, Research Institute of Industrial Catalysis, East China University of Science and Technology
Abstract:
On the catalytic removal of chlorinated volatile organic compounds, the effects of catalysts from or modified by rare earth oxides on the catalytic combustion of chlorinated volatile organic compounds ( CVOCs) , such as dichloromethane, dichloroethane, vinyl chloride, chorobenzene, were further investigated and discussed in detail. This paper focused on the relationship between the acidity and redox property of the catalyst. The acidity played a key role in CO selectivity and carbon deposition; while redox property dominated in the distribution of chlorinated byproducts. Based on the above dicussion, the way to design new catalysts for CVOCs combustion was suggested by tuning the acidity and redox property. However, the side effects of coke deposition, CO selectivity, the distribution of chlorination of organic compounds needed to be considered when exploring new catalyst with high activity and stability at low temperature.
Keyword:
catalytic combustion; chlorinated volatile organic compounds; acidity; redox; surface property;
Received: 2017-04-10
含氯挥发性有机化合物 (chlorinated volatile organic compounds, CVOCs) 是挥发性有机物 (VOCs) 中的一类。可划分为含氯烃类有机化合物、含氯芳香烃类有机化合物和聚合氯化物三大类化合物。含氯烃类有机化合物被广泛地用作溶剂及化工原料, 而含氯芳香烃类污染物主要来源于氯基氧化剂木纸浆的漂白、含氯化合物的热处理及金属的回收工业[1]。为了消除氯代烃对人体和环境的危害;我国发布了《大气污染物综合排放标准》 (GB19267-1996) , 明确提出了治理排放标准。美国根据《清洁空气法》制定了危险空气污染物国家排放标准 (NESHAP) 。根据CVOCs排放特点, 废气治理处理量高和浓度低的特点、结合治理成本, 催化燃烧法以低温转化成为经济、可行的方法[2]。
氯代烃的催化燃烧反应的过程为:吸附在酸中心上 (L酸、B酸) 的氯代烃通过C-Cl断裂发生脱氯反应, 解离后的氯代烃分子在催化剂表面活性氧物种的进攻下发生氧化反应, 生成含碳产物 (CO, CO2) ;解离的Cl-以HCl或者Cl2 (Deacon反应产物) 形式存在。如何调控催化剂表面酸性中心和氧化还原性能之间的匹配是提供催化剂活性和稳定性的关键, 酸中心与催化剂积碳与CO选择性相关[3];而与多氯副产物的生成和分布则受催化剂氧化性能的影响[4]。氯代烃分子中C-Cl键的解离难易程度与其分子结构相关, 如1, 2-二氯乙烷分子中82 Kcal·mol-1[5], 氯苯96 Kcal·mol-1[6], 一氯甲烷84 Kcal·mol-1[7], 因此催化剂设计时需考虑含氯烃分子结构的影响。
稀土的4f轨道配体有弱的配位效应和弱的路易斯酸性, 对有机污染物的C-H, C-Cl键的活化起着至关重要的作用[8,9,10,11]。稀土中研究广泛的主要以金属镧和铈为主, 以La MO3钙钛矿型复合氧化物形式存在, 具有灵活的可“化学剪裁”和高温稳定性能[12];而Ce优异的氧化还原性在氧化反应起着重要作用[13,14,15,16]。Cl沉积会导致Ce O2催化剂失活[17,18];La MO3的弱氧化还原性能导致多氯副产物的生成[19,20]。因此通过掺杂、调变载体性质提高催化剂的活性、选择性和稳定性。
本文从稀土氧化物 (Ce和La) 出发, 以饱和含氯代烃 (二氯甲烷, 二氯乙烷等) 和不饱和含氯烃 (氯苯, 二氯乙烯) 为模型反应, 考察复合稀土氧化物、稀土氧化物-贵金属、钙钛矿金属氧化物和钙钛矿金属氧化物-金属之间的相互作用对于氯代烃催化燃烧性能的影响。
1 不饱和烯烃的催化燃烧
对于氯代不饱和烃的催化燃烧表明, 催化剂的氧化还原性能比酸性更有利于反应活性的提高;因此在设计和制备催化剂上, 通过对分子筛、La M-n O3、Ce O2载体进行掺杂改性、利用贵金属和载体的相互作用提高催化剂的氧化还原性, 进而提高催化剂的反应性能。
1.1 分子筛基催化剂
分子筛的酸性不仅对C-C键的活化具有促进作用, 而且对不饱和氯代烃的催化燃烧也具有一定的反应活性, 如HZMS分子筛对氯苯T50高于500℃[21];超稳分子筛USY对二氯乙烯T90为310℃[22]。由于在分子筛催化剂表面容易发生积碳现象, 导致催化剂失活;通常会引入具有氧化性能的过渡金属, 如Co, Ce, Cu, Cr等提高催化剂的氧化还原性能, 降低催化剂表面的积碳, 通过二者之间的协同作用提高催化剂的低温反应性能和稳定性。
将Ce O2和超稳分子筛USY混合发现Ce O2-USY (8∶1) 在273℃可将二氯乙烯完全氧化, 由于Ce O2的氧化还原性能与USY酸性之间的协同作用, 使得催化剂具有最佳HCl和CO2选择性。CH3Cl, C2H3Cl, CH3CHO和CH3COOH是二氯乙烯分解的主要副产物, 其中CH3Cl是二氯乙烯在强酸裂解的产物;而C2H3Cl是二氯乙烯在L酸脱氯的产物。CH3CHO和CH3COOH则来源于C2H3Cl进一步氧化。在此基础上进一步引入具有氧化性能的Cu O[23]和Cr2O3[24], 利用它们与Ce O2之间的协同作用提高对氧的活化性能, 以降低反应含氯副产物的生成。与Ce O2-USY相比, Cu O-Ce O2-USY副产物中CH3Cl浓度降低10倍, 且C2H3Cl浓度最大值降低5倍, 且向低温偏移50℃;CH3CHO和CH3COOH产生窗口温度显著变窄, 但最大值增加。由于Cr2O3的强氧化性导致多氯副产物 (CHCl3和C2Cl4) 的浓度远低于Ce O2-USY;但是反应后催化剂存在10%Cr的流失现象, 这是由于部分与Ce O2弱相互作用的Cr2O3与Cl发生反应生成Cr O2Cl2所致。
用稀土元素修饰Co/HMS催化剂, 发现稀土元素修饰后的催化剂依然能够保持HMS的二维六面体介孔结构, 其中Ce的引入有利于Co在HMS上形成Co3O4晶体簇活性中心, 能够提高Co的氧化还原性能;Co和Ce的电子结构有利于氯苯上的氯解离, 形成氯自由基, 加快氯苯氧化[21]。
1.2 La Mn O3钙钛矿催化剂
La Mn O3钙钛矿具有的高温稳定性和良好的氧流动性使得在其催化氧化方面得以广泛应用。La Mn O3在300℃可实现氯苯的完全氧化[25];对氯乙烯转化率90%的温度T90为240℃[26]。非计量比钙钛矿、A位或者B位掺杂均可有效增加催化剂表面氧空穴的数量、提高氧的流动性, 从而提高催化剂的氧化还原性能。对于A位掺杂的钙钛矿而言, La0.8Sr0.2Mn O3表现出最高活性, T90为291℃, 这是由于Sr的引入提高了催化剂的氧化还原性能和表面氧物种的浓度。催化剂在高浓度氯苯气氛下 (1000×10-6) 失活原因是活性位点被吸附的Cl堵塞所致[25]。与之相反, 对于La0.8Sr0.2Mn O3催化剂的氯乙烯的催化燃烧未表现出如对氯苯的催化燃烧的促进作用[26]。B位掺杂的Ni, 氯乙烯的催化燃烧性能提高, T90降低了30℃, 这是由于增加催化剂表面Mn4+的浓度和Mn4+/Mn3+的氧化还原性能所致[27]。对于失活催化剂的分析则表明积碳和有机Cl物种沉积不是导致催化剂失活的主要原因, 而活性位点的Mn4+的显著降低是失活的主因。这是由于在催化剂表面生成难挥发的金属氯化物 (La OCl) 所致[20]。通过微波制备纳米非化学计量钙钛矿催化剂, 由于Mn Ox纳米颗粒高分散于在钙钛矿La Mn O3表面, 不仅有利于三氯乙烯的降解并抑制含氯副产物和CO的生成[28]。
1.3 Ce基金属复合氧化物催化剂
Ce O2具有良好的氧化还原性能, 是氯代烃脱氯的重要活化和氧化中心, 600℃可实现氯苯全转化[29], 但是由于Cl物种在催化剂表面的沉积导致催化剂易失活[30]。通过掺杂Co, Cr, Mn可提高催化剂表面的氧的流动性, 增加催化剂的反应活性和稳定性。Cr Ox与Ce O2的相互作用促进具有强氧化性能的Cr6+物种的增加, 232℃实现二氯乙烯完全氧化;水的引入在高温段有利于Cl物种移除[31]。具有层级结构花状介孔Co3O4-Ce O2比负载型的Co3O4/Ce O2催化剂在消除1, 2, 4-三氯苯反应中具有更低的活化能;这是由于Co3O4和Ce O2之间的协同使得Co-O键松弛, 提高活性氧的流动性所致[32]。在Co3O4中引入Ce, 不仅增加了活性Co2+和Ce3+物种的数量, 而且形成Co2+/Co3+和Ce3+/Ce4+两个氧化循环对;改变了晶格氧的配位环境, 促进了氧空穴的产生[33,34]。Mn和Cu引入Ce O2可形成具有类萤石结构的固溶体Cu-Mn-CeO[35], Mn4+/Mn3+和Cu2+/Cu+两个氧化还原对, 从而进一步提高降解氯苯的性能;同时Cu O和Mn Ox引入可有效抑制有机副产物的形成、提高CO2的选择性, 反应过程如图1所示[36]。
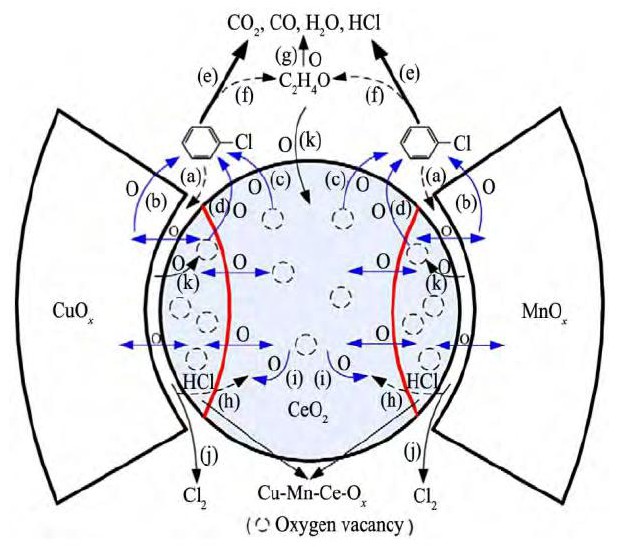
图1 氯苯在Cu-Mn-Ce-O上催化燃烧Fig.1Proposed chlorobenzene combustion route over Cu O-Mn Ox-Ce O2composite catalysts
动力学研究表明表明Cl物种的移除是整个反应的控制步骤[37], 通过改变Cl物种在催化剂表面的吸附、脱附和催化剂的氧化还原性能都可以加速反应性能。Mg引入可增加表面电子密度从而减弱Cl物种吸附强度[38], 利用分子筛的酸性可促进Cl从苯环脱离而且使得苯环更易发生开环氧化反应[4]。在催化剂中引入Ti可抑制Cl与催化剂表面碱性氧空穴和表面羟基交换, 从而使得催化剂表面具有较高比例的活性氧[39];而引入与Ce离子半径、结构相同的Pr, 更容易与Ce形成固溶体, 从而提高催化剂在低温度的还原性和抗氯中毒的性能[40]。
催化剂具有的表面活性氧物种以及快速脱除表面吸附氯的能力也是保持高稳定活性的重要原因[29,41]。对于氯物种的脱除途径一种认为是在活性氧物种的作用下的金属氧化物的氧化循环, 如Mn Cl2和Mn OxCl被快速氧化成Mn Ox, 从而达到高效除氯的作用[42,43];也有认为表面吸附氯的脱除是通过Deacon反应得以实现[30]。对于Sn-Ce-Mn-O体系而言, Sn Ce Ox固溶体更易于与Cl发生反应, 避免了Mn OxCly产生;Sn的引入也促进活性位上的Cl物种通过Deacon反应得以消除, 反应机制如图2所示[44]。
1.4 负载贵金属催化剂
对于负载型贵金属催化剂而言, 贵金属的引入 (Pt, Pd和Ru) 可有效促进催化剂的氧化性能, 一方面有利于降低CO选择性;另一方面降低Deacon反应的温度, 避免HCl在催化剂表面的积累, 提高催化剂的稳定性。贵金属Ru的引入显著促进了催化剂对于氯苯的催化燃烧, Ru/Ce O2催化剂氯苯催化燃烧的T90温度为280℃, 远低于Ce O2, 其T10温度为400℃;且产物中为CO2, HCl和Cl2, 仅有少量的二氯苯的生成, 在Ru/Ce O2表面上氯苯脱除的反应机制如图3所示[45]。

图2 氯苯在Sn修饰Mn-Ce-La催化剂上催化燃烧的机制Fig.2 Proposed reaction mechanism for catalytic combustion of CB over Mn-based and Sn doped Mn-based catalysts
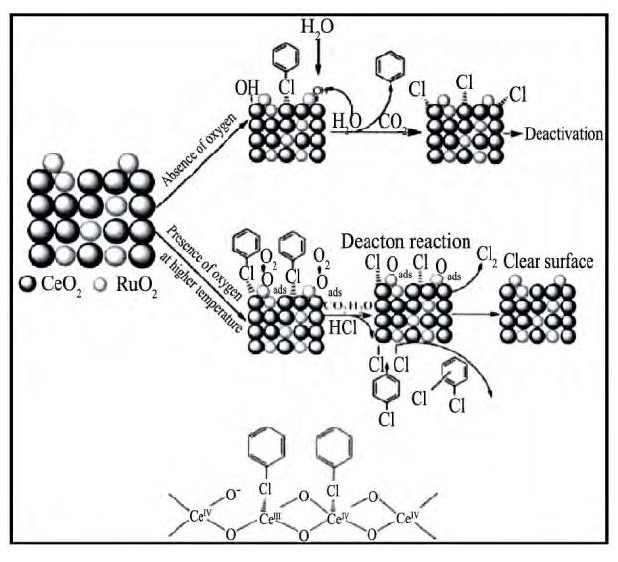
图3 氯苯在Ru/Ce O2催化剂上催化燃烧的机制Fig.3 Proposed reaction mechanism for catalytic combustion of CB over Ru/Ce O2based catalysts
载体的性质对反应活性影响显著。载体化学组成、形貌都会影响贵金属和载体之间的相互作用增强。对于负载型Pd催化剂而言, 钙钛矿载体比氧化铝载体显示出更佳的反应性能。将Pd负载于钙钛矿La BO3 (B=Co, Mn, Fe, Ni) 上, 其对氯苯的燃烧性能影响顺序为:Pd/La Mn O3+δ (243℃) >Pd/La Fe O3 (270℃) >Pd/La Co O3 (360℃) >Pd/La Ni O3 (408℃) , 催化剂在还原过程中生成的Pd0与不同钙钛矿载体之间的重构导致催化剂的活性差异[19]。对于负载型Pt催化剂而言, 复合氧化物 (Al2O3-Ce O2, Al2O3-Ti O2) 比单一金属氧化物 (Al2O3) 更适合做金属整体式催化剂的载体。Pt/Al2O3-Ce O2催化剂在688℃可转化99%的四氯乙烯且HCl的选择性达91%。反应低温区检测到副产物C2HCl3, CHCl3和C2H4[46]。进一步研究表明催化剂的氧化还原能力和活性氧的数量对反应活性的影响远比催化剂的酸性更为重要[47]。
对于Ru/Ce O2催化剂的研究发现, 不同晶面与Ru相互作用强弱有如下关系: (110) > (100) >> (111) , 因此暴露 (110) 和 (100) 晶面更有利于反应的进行。棒状Ce O2-r比八面体Ce O2-O和立方体Ce O2-C更适宜做催化剂载体 (图4) , 这是由于其暴露的 (110) 和 (100) 晶面上Ru更易与Ce形成Ru-O-Ce键, 形成更多的Ru4+物种和氧空穴[48,49]。
引入Ti对Ce O2进行修饰, 可更有效地暴露载体表面的 (110) 和 (100) 晶面, 提高反应性能;催化剂表面Ru O2在低温 (200℃) 通过Deacon反应将生成HCl从催化剂表面脱除, 增加了催化剂的稳定性[50]。

图4 Ru/Ce O2-r与Ru/Ce O2-c和Ru/Ce O2-o的电镜和高倍电镜图Fig.4 TEM and HRTEM images of Ru/Ce O2-r (a, b and c) , Ru/Ce O2-c (d, e and f) and Ru/Ce O2-o (g, h and i)
2 饱和氯代烃的催化燃烧
第一性密度泛函理论计算 (DFT) 二氯乙烷催化氧化反应过程如图5所示:表明二氯乙烷更容易在Ov活性中心上吸附活化, 而不是Ce3+离子上;解离后的二氯乙烷与晶格氧发生部分氧化反应生成乙醛或者CO;进而被表面吸附的氧物种氧化生成CO2, 进一步分析表明Ce O2催化剂失活的原因在于表面消耗的-OH不能及时补充以及HCl快速解离[51]。将Ce0.5Zr0.5O2室温HCl洗涤并高温空气焙烧 (550℃) 预处理以加速氯中毒, 其对二氯乙烷燃烧的性能并未明显降低[52]。研究表明, 催化剂表面氯化后会导致比表面积、Ce O2还原性能的降低, 但同时Cl吸附在Ce4+, Zr4+可生成新的酸性位点, 并且生成了稳定、易还原的Ce OCl物种[53], 从而导致其活性基本保持。与催化剂氯中毒相反, 高温焙烧则导致催化剂活性降低和CO、含氯副产物的生成[54]。
与不饱和烃相比, 酸中心对饱和氯代烃C-Cl键断裂及催化氧化具有更重要的作用。与Ce O2相比, 具有酸性中心H-ZSM5分子筛其氧化二氯乙烷的活性向低温偏移100℃, 但也导致CO选择性高、易发生积碳。利用Ce O2修饰H-ZSM5分子筛, 不仅可以增加催化剂表面B酸量;同时加速催化剂表面氧的流动性, 进而提高CO2选择性, 对于Ce修饰分子筛催化剂其副产物生成机制如图6[55]。

图5 二氯乙烷 (DCE) 反应能垒计算模型Fig.5 Calculated energy profile and structures of key states of C-Cl cleavage of DCE at clean Ce O2 (1 1 1) (1) and reduce Ce O2 (1 1 1) (Ov) (2)
为了避免高温下氯气与含氯卤代烃的进一步反应, 导致更多有毒的多氯副产物生成, 制备了具有三明治结构的Ce O2@H-ZSM-5催化剂, 以控制Ce O2的暴露程度, 减少多氯副产物的生成。结果发现副产物仅为少量的氯乙烯, 低温下仍不可避免发生积碳, 但均为易氧化的白碳 (脂肪烃和环戊二烯基化合物) 。水的存在抑制Deacon反应发生, 从而消除了含氯副产物的生成[55,56]。

图6 Ce O2-H-ZSM-5催化燃烧1, 2-二氯乙烷副产物的形成机制Fig.6Formation pathways of chlorinated by-products during1, 2-dichloroethane oxidation over Ce O2-H-ZSM-5composite catalysts
为了抑制Ce O2基催化剂中含氯副产物的生成、提高催化剂的稳定性能, 通过第二种金属掺杂 (Ti, Fe, V) 提高催化剂的氧化还原性;构造两段式催化剂提高催化剂的稳定性。共同暴露Ce O2和Ti O2催化剂虽然比只暴露Ti O2或者Ce O2的复合氧化物具有较好的活性, 但仍存在CO2选择性低于25%的问题, 并伴有5%左右的含氯副产物 (CxHyClz) [57]。与Ti O2相反, Fe的引入则可有效抑制三氯和四氯副产物的生成, 这是由于Fe有效抑制Cl2的形成所致[58]。具有c-Ce O2和h-Ti O2结构、高度相互分散的Ce O2-Ti O2复合氧化物也显著提高了催化剂的氧化还原性能[59]。
对于VOx/Ce O2催化剂而言, V的引入不仅增加了新的活性中心V5+/V4+, Ce3+-O2--V5+, 二氯乙烷的T90降到230℃;同时改变了氯代烃脱除的反应历程。通过C-H键活化以及VOx物种上的H转移生成反应中间物种CH3CHO是反应的关键步骤, 如图7所示[60]。
构造了两段式Ce/Ti O2-Cu/Ce O2催化剂可进一步降低CO和含氯副产物的选择性, 这种方法实现了C-Cl断裂和CO氧化活性中心的分离;不仅可以避免酸性位的减少, 同时通过Cu O抗Cl性能避免Cl对于Ti O2的毒化作用[61]。

图7 VOx/Ce O2催化燃烧1, 2-二氯乙烷机制图Fig.7Proposed reaction mechanisms for oxidation of 1, 2-di-chloroethane over pure Ce O2and VOx/Ce O2catalysts
催化剂的酸性中心和氧化中心的匹配与催化剂的组成相关。对于氧化性能和酸性较弱的钙钛矿催化剂而言, 酸性的调节受结构的限制, 因而氧化还原性能的调控对反应的促进作用尤为显著。对于A位掺杂的钙钛矿 (La Mn O3) 而言, 其对还原能力的促进作用显著高于对酸量的促进作用。对于A位掺杂的钙钛矿 (La Mn O3) 而言, A位掺杂对还原能力的促进作用显著高于对酸量的促进作用。高温焙烧 (900℃) La1-xAlxMn O4催化剂其单位活性氧的密度远高于700℃焙烧催化剂, 因此催化剂的结构特性对反应活性的影响更显著[62];负载型钙钛矿催化剂其活性高于钙钛矿催化剂, 活性组分与载体之间的相互作用促进了氧流动性的增加[63]。
与钙钛矿催化剂不同, 贵金属催化剂氧化性能远高于钙钛矿催化剂, 因此增加催化剂表面的酸中心则有利于反应的进一步提高。通过调变载体Al2O3, Al2O3-Ti O2, Al2O3-Ce O2和Ce0.5Zr0.5O2的酸中心, 与具有不同氧化性能的贵金属 (Pt, Pd, Rh, Au) 之间的匹配, 考察二者之间的相互作用对二氯乙烷燃烧性能。贵金属的引入会导致载体的酸中心减少, 降低了对二氯甲烷的吸附活化能力;而贵金属的氧化性会显著降低副产物中CH3Cl, CH2O和CO的浓度[64], Pt显著提高了CO2的选择性。Pt/Al2O3催化剂在420℃可实现二氯甲烷的完全转化, HCl的选择性也高达92%[65];Ce的引入导致Ce-O-Pt固溶体的生成, 进一步提高催化剂的氧化还原性能, 抵消催化剂酸中心减少的副作用[66]。
3 结论与展望
根据氯代烃的催化燃烧反应的特点, 在设计和开发氯代烃燃烧催化剂需要调控催化剂表面酸性中心和氧化还原性能之间的匹配。催化剂表面酸中心的增加, 有利于氯代烃分子的吸附活化, 亦会带来积碳、Cl沉积和CO选择性高的问题;氧化还原性能增强, 有利于氧化反应的发生, 提高CO2选择性并影响到多氯副产物的分布。由于氯代烃分子结构的差异, 导致碳氯键和碳碳键断裂需要的能量不同, 因此还需考虑氯代烃分子结构差异带来的影响。
对于氯代烃的催化氧化脱除;反应活性中心的结构、副产物生成机制的研究目前还未有统一定论;在实际环境下水汽、有机烃 (含氧) 分子、SO2和灰尘等存在对于催化剂结构和活性的影响还需要进一步明确, 因此开展相关方面的研究工作, 对设计和制备高效脱除氯代烃催化剂的研发具有重要的理论意义和实际价值。
参考文献
[1] Finocchio E, Busca G, Notaro M.A review of catalytic processes for the destruction of PCDD and PCDF from waste gases[J].Applied Catalysis B:Environmental, 2006, 62 (1) :12.
[2] Kennes C, Veiga M C.Technologies for the abatement of odours and volatile organic and inorganic compounds[J].Chem.Eng.Trans., 2010, 23:1.
[3] Yokoyama C, Misono M.Catalytic reduction of nitrogen monoxide by propene in the presence of oxygen over cerium ion-exchanged zeolites.I.General characteristics of the reaction and effects of Alkaline earth metal addition[J].Bulletin of the Chemical Society of Japan, 1994, 67 (2) :557.
[4] Sun P F, Wang W L, Dai X X, Weng X L, Wu Z B.Mechanism study on catalytic oxidation of chlorobenzene over MxCe1-xO2/H-ZSM5 catalysts under dry and humid conditions[J].Applied Catalysis B:Environmental, 2016, 198:389.
[5] Huang C C, Lo S L, Tsai S M, Lien H L.Catalytic hydrodechlorination of 1, 2-dichloroethane using copper nanoparticles under reduction conditions of sodium borohydride[J].Journal of Environmental Monitoring, 2011, 13 (9) :2406.
[6] Zell T, Feierabend M, Halfter B, Radius U.Stoichiometric and catalytic C-Cl activation of Aryl Chlorides using an NHC-stabilized nickel (0) complex[J].Journal of Organometallic Chemistry, 2011, 696:1380.
[7] Urbano F J, Marinas J M.Hydrogenolysis of organohalogen compounds over palladium supported catalysts[J].Journal of Molecular Catalysis A:Chemical, 2001, 173 (1) :329.
[8] Lin S, Yang L Y, Yang X, Zhou R X.Redox behavior of active Pd Oxspecies on (Ce, Zr) xO2-Al2O3mixed oxides and its influence on the three-way catalytic performance[J].Chemical Engineering Journal, 2014, 247:42.
[9] Cen W L, Liu Y, Wu Z B, Liu J, Wang H Q, Weng X L.Cl species transformation on Ce O2 (111) surface and its effects on CVOCs catalytic abatement:a firstprinciples investigation[J].Journal of Physical Chemistry C, 2014, 118 (13) :6758.
[10] Dinh M T N, Giraudon J M, Vandenbroucke A M, Morentb R, Geyterb N D, Lamonier J F.Post plasmacatalysis for total oxidation of trichloroethylene over CeMn based oxides synthesized by a modified“redox-precipitation route”[J].Applied Catalysis B:Environmental, 2015, 172-173:65.
[11] Fan Y, Lu X B, NI Y W, Zhang H J, Zhao L, Chen J P, Sun C L.Destruction of polychlorinated aromatic compounds by spinel-type complex oxides[J].Environment Science Technology, 2010, 44 (8) :3079.
[12] Taherian S, Entezari M H, Ghows N.Sono-catalytic degradation and fast mineralization of p-chlorophenol:La0.7Sr0.3Mn O3as a nano-magnetic green catalyst[J].Ultrasonics Sonochemistry, 2013, 20:1419.
[13] He F, Chen Y, Zhao P, Liu S T.Effect of calcination temperature on the structure and performance of Ce OxMn Ox/Ti O2nanoparticles for the catalytic comnbustion of chlorobenzene[J].J.Nanopart.Res., 2016, 18 (5) :19.
[14] Cao S, Wang H Q, Yu F X, Shi M P, Chen S, Weng X L, Liu Y, Wu Z B.Catalyst performance and mechanism of catalytic combustion of dichloromethane (CH2Cl2) over Ce doped Ti O2[J].Journal of Colloid and Interface Science, 2016, 463:233.
[15] He C, Men G S, Yu Y K, Pan H.Chlorobenzene destruction over mesostrucured Cu O and Mn OxCo-modified Ce O2catalyst:activity and activation route[J].Water Air Soil Pollut., 2015, 226 (3) :57.
[16] He C, Xu B T, Shi J W, Qiao N L, Hao Z P, Zhao J L.Catalytic destruction of chlorobenzene over mesoporous ACe Ox (A=Co, Cu, Fe, Mn, or Zr) composites perpared by inorganic metal precursor spontaneous percipitation[J].Fuel Processing Technology, 2015, 130:179.
[17] Vivier L, Duprez D.Ceria-based solid catalysts for organic chemistry[J].Chemsuschem.2010, 3 (6) :654.
[18] Mullins D R.The surface chemistry of cerium oxide[J].Surface Science Report, 2015, 70 (1) :42.
[19] Giraudon J M, Elhachimi A, Leclercq G.Catalytic oxidation of chlorobenzene over Pd/perovskites[J].Applied Catalysis B:Environmental, 2008, 84 (1) :251.
[20] Zhang C H, Wang C, Hua W C, Guo Y L, Lu G Z, Gil S, Giroir-Fendler A.Relationship between catalytic deactivation and physicochemical properties of La Mn O3perovskite catalyst during catalytic oxidation of vinyl chloride[J].Applied Catalysis B:Environmental, 2016, 186:173.
[21] Zhao W, Cheng J, Wang L N, Chu J L, Wang J C.Catalytic combustion of chlorobenzene on the Ln modified Co/HMS[J].Applied Catalysis B:Environmental, 2012, 127:246.
[22] Huang Q Q, Xue X M, Zhou R X.Decomposition of1, 2-dichloroethane over Ce O2modified USY zeolite catalysts:effect of acidity and redox property on the catalytic behavior[J].Journal of Hazardous Materials, 2010, 183 (1-3) :694.
[23] Huang Q Q, Xue X M, Zhou R X.Catalytic behavior and durability of Ce O2or/and Cu O modified USY zeolite catalysts for decomposition of chlorinated volatile organic compounds[J].Journal of Molecular Catalysis A:Chemical, 2011, 344 (1) :74.
[24] Huang Q Q, Meng Z H, Zhou R X.The effect of synergy between Cr2O3-Ce O2and USY zeolite on the catalytic performance durability of chromium and cerium modified USY catalysts for decomposition of chlorinated volatile organic compound[J].Applied Catalysis B:Environmental, 2012, 115-116:179.
[25] Lu Y J, Dai Q G, Wang X Y.Catalytic combustion of chlorobenzene on modified La Mn O3catalyst[J].Catalysis Communications, 2014, 54:114.
[26] Zhang C H, Wang C, Hua W C, Guo Y L, Guo Y, Lu G Z.The effect of A-site substitution by Sr, Mg and Ce on the catalytic performance of La Mn O3catalysts for the oxidation of vinyl chloride emission[J].Applied Catalysis B:Environmental, 2013, 134:310.
[27] Zhang C H, Wang C, Zhan W C, Guo Y L, Guo Y, Lu G Z.Catalytic oxidation of vinyl chloride emission over La Mn O3and La B0.2Mn0.8O3 (B=Co, Ni, Fe) catalysts[J].Applied Catalysis B:Environmental, 2013, 129:509.
[28] Maghsoodi S, Towfighi J, Khodadadi A, Mortazavi Y.The effects of excess manganese in nano-size lanthanum manganite perovskite on enhancement of trichloroethylene oxidation activity[J].Chemical Engineering Journal, 2013, 215-216:827.
[29] Wang X Y, Kang Q, Li D.Catalytic combustion of chlorobenzene over Mn Ox-Ce O2mixed oxide catalysts[J].Applied Catalysis B:Environmental, 2009, 86 (3) :166.
[30] Dai Y, Wang X Y, Dai Q G.Catalytic combustion of chlorobenzene over Mn-Ce-La-O mixed oxide catalysts[J].Journal of Hazaordous Material.2011, 188 (1-3) :132.
[31] Yang P, Yang S S, Zhi Z Y, Meng Z H, Zhou R X.Deep oxidation of chlorinated VOCs over Ce O2-based transition metal mixed oxide catalysts[J].Applied Catalysis B:Environmental, 2015, 162:227.
[32] Lin S J, Su G J, Zheng M H, Ji D K, Jia M K, Liu Y X.Synthesis of flower-like Co3O4-Ce O2composite oxide and its application to catalytic degradation of 1, 2, 4-trichlorobenzene[J].Applied Catalysis B:Environmental, 2012, 123-124:440.
[33] Wang C, Zhang C H, Hua W C, Guo Y L, Lu G.Z, Gil S, Giroir-Fendler A.Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts[J].Chemical Engineering Journal, 2017, 315:392.
[34] Konsolakis M, Sgourakis M, Carabineiro S A C.Surface and redox properties of cobalt-ceria binary oxides:on the effect of Co content and pretreatment conditions[J].Applied Surface Science, 2015, 341:48.
[35] He C, Yu Y K, Shi J W, Shun Q, Chen J S, Liu H X.Mesostructued Cu-Mn-Ce-O composites with homogeneous bulk composition for chloroenzene removal:catalytic performance and microactivation course[J].Materials Chemistry and Physics, 2015, 157:87.
[36] He C, Yu Y K, Shi J W, Shun Q, Chen J S, Niao N L.Catalytic behavior and synergistic effect of nanostructured mesoporous Cu O-Mn Ox-Ce O2catalysts for chlorobenzene destruction[J].Applied Surface Science, 2014, 297:59.
[37] Dai Y, Wang X Y, Dai Q G, Li D.Effect of Ce and La on the structure and activity of Mn Oxcatalyst in catalytic combustion chlorobenzene[J].Applied Catalysis B:Environmental, 2012, 111-112:141.
[38] Wu M, Wang X Y, Dai Q G, Li D.Catalytic combustion of chlorobenzene over Mn-Ce/Al2O3catalyst promo-ted by Mg[J].Catalysis Communications, 2010, 11 (12) :1022.
[39] Deng W, Dai Q G, Lao Y J, Shi B B, Wang X Y.Low temperature catalytic combustion of 1, 2-dichlorobenzene over Ce O2-Ti O2mixed oxide catalysts[J].Applied Catalysis B:Environmental, 2016, 181:848.
[40] Rivas B de, Guillén-Hurtado N, López-Fonseca R, Coloma-Pascual F, García-García A, Gutiérrez-Ortiz J I, Bueno-López A.Activity, selectivity and stability of praseodymium-doped Ce O2for chlorinated VOCs catalytic combustion[J].Applied Catalysis B:Environmental, 2012, 121-122:162.
[41] Wu M, Wang X Y, Dai Q G, Gu Y X, Li D.Low temperature catalytic combustion of chlorobenzene over Mn-Ce-O/γ-Al2O3mixed oxides catalyst[J].Catalysis Today, 2010, 158:336.
[42] Li H F, Lu G Z, Dai Q G, Wang Y Y, Guo Y, Guo Y L.Efficient low-temperature catalytic combustion of trichloroethylene over flower-like mesoporous Mn-doped Ce O2microspheres[J].Applied Catalysis B:Environmental, 2011, 102 (3-4) :475.
[43] Wang X Y, Ran L, Dai Y, Lu Y.J, Dai Q G.Removal of Cl adsorbed on Mn-Ce-La solid solution catalysts during CVOC combustion[J].Journal of Colloid and Interface Science.2014, 426 (27) :324.
[44] Mao D, He F, Zhao P, Liu S T.Enhancement of resistance to chlorine poisoning of Sn-modified Mn Ce La catalysts for chlorobenzene oxidation at low temperature[J].The Royal Society of Chemistry, 2015, 5 (13) :10040.
[45] Huang H, Dai Q G, Wang X Y.Morphology effect of Ru/Ce O2catalysts for the catalytic combustion of chlorobenzene[J].Applied Catalysis B:Environmental, 2014, 158-159:96.
[46] Pitkaho S, Matejova L, Ojala S, Gaalova J, Keiski R L.Oxidation of perchloroethylene Activity and selectivity of Pt, Pd, Rh, and V2O5catalysts supported on Al2O3, Al2O3-Ti O2and Al2O3-Ce O2[J].Applied Catalysis B:Environmental, 2012, 113-114:150.
[47] Pitkaho S, Matejova L, Ojala S, Gaalova J, Keiski R L.Oxidation of perchloroethylene activity and selectivity of Pt, Pd, Rh, and V2O5catalysts supported on Al2O3, Al2O3-Ti O2and Al2O3-Ce O2part 2[J].Applied Catalysis B:Environmental, 2012, 126:215.
[48] Dai Q G, Bai S X, Wang X Y, Lu G Z.Catalytic combustion of chlorobenzene over Ru-doped ceria catalysts:mechanism[J].Applied Catalysis B:Environmental, 2013, 129:580.
[49] Dai Q G, Huang H, Zhu Y, Deng W., Bai S X, Wang X Y, Lu G Z.Catalysis oxidation of 1, 2-dichloroethane and ethyl acetate over ceria nanocrystals with welldefined crystal planes[J].Applied Catalysis B:Environmental, 2012, 117-118:360.
[50] Dai Q G, Bai S X, Wang J W, Li M, Wang X Y, Lu G Z.The effect of Ti O2doping on catalytic performances of Ru/Ce O2catalysts during catalytic combustion of chlorobenzene[J].Applied Catalysis B:Environmental, 2013, 142-143:222.
[51] Dai Q G, Yin L L, Bai S X, Wang W, Wang X Y, Gong X Q, Lu G Z.Catalytic total oxidation of 1, 2-dichloroethane over VOx/Ce O2catalysts[J].Applied Catalysis B:Environmental, 2016, 182:598.
[52] Rivas B de, López-Fonseca R, Gutiérrez-Ortiz M A, Gutiérrez-Ortiz J I.Impact of induced chlorine-poisoning on the catalytic behaviour of Ce0.5Zr0.5O2and Ce0.15Zr0.85O2in the gas-phase oxidation of chlorinated VOCs[J].Applied Catalysis B:Environmental, 2011, 104:373.
[53] Farra R, Girgsdies F, Frandsen W, Hashagen M, Schl9gl R, Teschner D.Synthesis and catalytic performance of Ce OCl in deacon reaction[J].Catalysis Leters, 2013, 143 (10) :1012.
[54] Rivas B de, López-Fonseca R, Sampedro C, GutiérrezOrtiz J I.Catalytic behaviour of thermally aged Ce/Zr mixed oxides for the purification of chlorinated VOC-containing gas streams[J].Applied Catalysis B:Environmental, 2009, 90 (3-4) :545.
[55] Dai Q G, Wang W, Wang X Y, Lu G Z.Sandwichstructured Ce O2@ZSM-5 hybrid composites for catalytic oxidation of 1, 2-dichloroethane:an integrated solution to coking and chlorine poisoning deactivation[J].Applied Catalysis B:Environmental, 2017, 203:31.
[56] De Rivas B, Sampedro C, Ramos-Fernández E V, López-Fonseca, R, Gascon J, Makkee M, Gutiérrez-Ortiz J I.Influence of the synthesis route on the catalytic oxidation of 1, 2-dichloroethane over Ce O2/H-ZSM5 catalysts[J].Applied Catalysis A:General, 2013, 456:96.
[57] Cao S, Wang H Q, Shi M P, Chen S, Wu Z B.Impacts of structure of Ce O2/Ti O2mixed oxides catalysts ontheir performances for catalytic combustion of dichloromethane[J].Catalysis Letter, 2016, 146:1591.
[58] Wang W, Zhu Q, Dai Q G, Wang X Y.Fe doped Ce O2, nanosheets for catalytic oxidation of 1, 2-dichloroethane:effect of preparation method[J].Chemical Engineering Journal, 2017, 307:1037.
[59] Shi Z N, Yang P, Tao F, Zhou R X.New insight into the structure of Ce O2-Ti O2, mixed oxides and their excellent catalytic performances for 1, 2-dichloroethane oxidation[J].Chemical Engineering Journal, 2016, 295:99.
[60] Dai Q G, Bai S X, Li H, Liu W, Wang X Y, Lu G Z.Catalytic total oxidation of 1, 2-dichloroethane over highly dispersed vanadia supported on Ce O2nanobelts[J].Applied Catalysis B:Environmental, 2015, 168-169:141.
[61] Cao S, Wang H Q, Yu F X, Shi M, Chen S, Weng X L, Liu Y, Wu Z B.A two-stage Ce/Ti O2-Cu/Ce O2catalyst with separated catalytic functions for deep catalytic combustion of CH2Cl2[J].Chemical Engineering Journal, 2016, 290:147.
[62] Chen S X, Wang Y, Jia A P, Liu H H, Luo M F, Lu J Q.Enhanced activity for catalytic oxidation of 1, 2-dichloroethane over Al-substituted La Mn O3perovskite catalysts[J].Applied Surface Science, 2014, 307:178.
[63] Zhang C H, Wang C, Gil S, Boreave A, Retailleau L, Guo Y L, Valverde J L, Giroir-Fendler A.Catalytic oxidation of 1, 2-dichloropropane over supported La Mn Ox oxides catalysts[J].Applied Catalysis B:Environmental, 2017, 201:552.
[64] MatějováL, Topka P, Kalu6a L, Pitkaho S, Ojala S, GaálováJ, Keiski R L.Total oxidation of dichloromethane and ethanol over ceria-zirconia mixed oxide supported platinum and gold catalysts[J].Applied Catalysis B:Environmental 2013, 142-143:54.
[65] Pitkaho S, NevanperT, Matejova L, Ojala S, Keiski R L.Oxidation of dichloromethane over Pt, Pd, Rh, and V2O5, catalysts supported on Al2O3, Al2O3-Ti O2, and Al2O3-Ce O2[J].Applied Catalysis B:Environmental, 2013, 138-139 (14) :33.
[66] Chen Q Y, Li N, Luo M F, Lu J Q.Catalytic oxidation of dichloromethane over Pt/Ce O2-Al2O3catalysts[J].Applied Catalysis B:Environmental, 2012, 127:159.









