
J. Cent. South Univ. (2020) 27: 1197-1210
DOI: https://doi.org/10.1007/s11771-020-4360-8

Performance of a high-rate anammox reactor under high hydraulic loadings: Physicochemical properties,microbial structure and process kinetics
SONG Yu-xia(宋雨夏)1, 2, 3, M. Ali2, 3, FENG Fan(冯帆)2, 3, CHAI Xi-lin(柴喜林)3,
WANG Shuo(王硕)4, WANG Yun-yan(王云燕)2, 3, TANG Chong-jian(唐崇俭)2, 3
1. College of Environmental Science and Engineering, Central South University of Forestry and Technology, Changsha 410004, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
3. National Engineering Research Centre for Control and Treatment of Heavy Metal Pollution,Changsha 410083, China;
4. Jiangsu Key Laboratory of Anaerobic Biotechnology, Jiangnan University, Wuxi 214122, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract:
In this study, a lab-scale upflow anaerobic sludge blanket (UASB) reactor was applied to studying the high-rate nitrogen removal of granule-based anammox process. The nitrogen removal rate (NRR) finally improved to 15.77 kg/m3/d by shortening hydraulic retention time (HRT) to 1.06 h. Well-shaped red anammox granules were extensively enriched inside the reactor. The results of nitrogen removal kinetics indicated that the present bioreactor has great nitrogen removal potential, because the maximum rate of substrate utilization (Umax) predicted by Stover-Kincannon model is suggested as 55.68 kg/(m3·d). Analysis of the microbial community showed that the anammox genus Candidatus Kuenenia dominated the bacterial communities. The relative abundance of Candidatus Kuenenia rose from 12.29% to 36.95% after progressively shorter HRT and higher influent substrate concentrations, illustrating the stability of nitrogen removal performance and biomass enrichment offered by the UASB in carrying out high-rate anammox process.
Key words:
anammox; UASB reactor; kinetics; granular sludge; microbial structure;
Cite this article as:
SONG Yu-xia, M. Ali, FENG Fan, CHAI Xi-lin, WANG Shuo, WANG Yun-yan, TANG Chong-jian. Performance of a high-rate anammox reactor under high hydraulic loadings: Physicochemical properties, microbial structure and process kinetics [J]. Journal of Central South University, 2020, 27(4): 1197-1210.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-020-4360-81 Introduction
Ammonium-rich wastewater derives from a variety of sources such as fermentation, pharmacy, food production, and agricultural activities [1-3]. Discharge of nitrogen containing wastewater into water bodies causes eutrophication and other serious environmental problems. Anammox (anaerobic ammonium oxidation) is an innovative microbial process where ammonium can be directly converted into dinitrogen gas by using nitrite as an electron acceptor under anoxic conditions [4]. Since its first discovery in early 1990s, anammox has been widely considered as the most economical and sustainable process for nitrogen removal from high strength nitrogenous wastewater [5]. As reported, the nitrogen removal rate (NRR) of conventional nitrogen removal biotechnologies was usually less than 0.5 kg/(m3·d). But for anammox process, the nitrogen removal rate (NRR) higher than 5 kg/(m3·d) can be easily obtained [1, 6]. Especially, the highest NRRs in laboratory- and full-scale reactors were reported to be 77 kg/(m3·d) and 9.5 kg/(m3·d), respectively [7]. Thus, anammox process has been considered as an alternative to conventional nitrogen removal processes because of the significant reduction of operational cost and space requirement. Because anammox can be applied in combination with short-cut nitrification, the whole process can achieve an efficient autotrophic nitrogen removal system [8].
However, the dominant drawbacks of anammox process is the slow growth rate (reported doubling time was 2.1-14 d) and low cellular yield of the autotrophic anammox bacteria [9]. Reports also mentioned environmental conditions could affect anammox bacteria easily, such as low temperature, extreme pH, high salinity, and the presence of organic matters and other inhibitors [10, 11], among which nitrite was frequently reported to inhibit anammox performance [12]. Thus, the process stability is crucial for anammox reactor especially under high-rate conditions. Because the overloading of nitrite/ammonia can easily cause substrate inhibition.
In addition, the performance of anammox process is mainly influenced by the reactor configuration and sludge properties [13]. Several types of reactor such as upflow biofilter (UBF) [14], sequencing batch reactor (SBR) [15, 16], gas-lift reactor and membrane bioreactor (MBR) [7] have been used to carry out anammox process. But the nitrogen removal performance was relatively low because the anammox sludge inside the reactor was always flocculent and thus was difficult to settle down. With the efficient biomass retention and rapid formation of bio-granules, upflow anaerobic sludge blanket (UASB) reactor has been considered as a good choice to develop high-rate bioreactors [17-20]. The granular biomass instead of flocculent sludge possesses better settling or solid-liquid separation property, compacter aggregate structure, higher biomass concentration, greater tolerance to shock loadings and better resistance to toxicants in wastewaters [21], which is especially important for the extremely slow-growing anammox bacteria. High hydraulic condition is reported to be a crucial reason for sludge granulation in bioreactors, because of the higher shear force could enhance the mass transfer which caused a higher bacterial growth rate and resulted in accelerated nucleation (the first step for granulation)[20,22]. Although some researchers have paid attention to the performance of anammox UASB reactors, the related information including microbial structure and process bio-kinetics are still limited in granule-based anammox UASB reactors under high hydraulic loading condition.
In the present study, a lab-scale UASB reactor with long-term operation under increasing hydraulic loading was developed to enrich the anammox granules. Through a continuous hydraulic retention time (HRT) shortening, the reactor performance, physicochemical and microbial community characteristics of the anammox granules were investigated accordingly. In order to better explain the high-rate performance and also provide better guidance to the reactor operation, the substrate removal kinetics based on the long-term operational performance was also implemented.
2 Materials and methods
2.1 Seed sludge and influent
2.1.1 Seed sludge
The seed anammox sludge was taken from lab-scale reactor that have been stable operated over one year at a nitrogen loading rate (NLR) of 3-4.5 kg/(m3·d), which started up by anaerobic granular sludge. The suspended solids(SS), volatile suspended solids (VSS) of the seed sludge were 27.9 and 21.3 g/L, respectively, which corresponded to VSS/SS of 0.73. Due to the good settling property of the anammox granules in UASB reactor, the sludge retention time (SRT) of the reactor was calculated to be higher than 40 d.
2.1.2 Synthetic wastewater
The anammox reactor was constantly fed with synthetic wastewater based on tap water and the compositions of synthetic wastewater were shown as below (g/L): (NH4)2SO4 (nitrogen source), 0.47-1.53; NaNO2 (nitrogen source), 0.59-1.92; KHCO3 (carbon/alkalinity source); 1.25, MgSO4·7H2O, 0.30; NaHPO4, 0.01; CaCl2·2H2O, 0.0056. The composition of trace elements I and II were the same as described by YU et al [6]. The substrate was fed through a peristaltic pump at a flow rate of 5.04-22.68 L/d corresponding to the HRT of 4.76-1.06 h. The influent pH was maintained at 6.9-7.2 using 1:1 HCl.
2.2 Anammox reactor
A plexiglas UASB reactor with an internal diameter of 5 cm corresponding a working volume of 1.0 L was used in this study. The reactor was covered with black cloth to avoid the possible oxygen production caused by light. The synthetic wastewater was pumped through the bottom of the reactor by peristaltic pump. To create desired anaerobic conditions for anammox bacteria, the influent was purged with 95% Ar-5% CO2. During the operation period, the sludge granules settled well at the bottom of column and the gas escaped from the top through three-phase separator.
2.3 Operational strategies
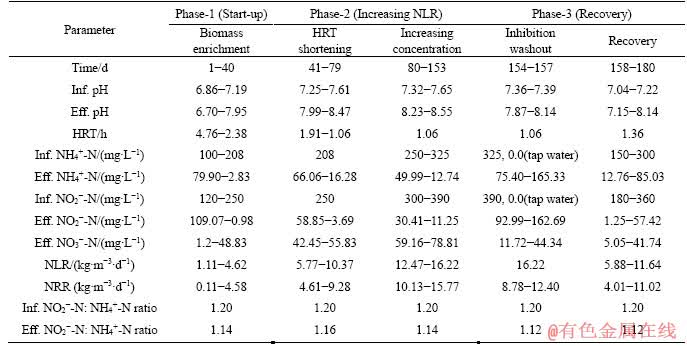
The whole operating period (180 d) was consisted of three phases based on the pattern of reactor performance and operational conditions (Table 1). The reactor was operated at the temperature of (30±1) °C by using a thermostatic water bath. Initially the UASB was commenced by inoculating anammox sludge and introducing ammonium and nitrite into the reactor. In initial 40 d, the nitrogen loading rate (NLR) was raised by adjusting the substrates concentrations (100- 180 mg/L NH4+-N, 120-208 mg/L NO2--N) and the HRT (4.76-2.38 h). Thereafter (41-153 d), the NLR of the UASB reactor was increased in two consecutive steps. Firstly, the NLR was increased by shortening the HRT from 2.38 to 1.06 h by setting NH4+-N and NO2--N at 208 and 250 mg/L, respectively. The subsequent elevation of NLR was conducted through increasing the NH4+-N and NO2--N concentration up to 325 and 390 mg/L while the HRT was maintained at 1.06 h. After inhibition, the reactor performance was recovered by the simultaneous prolongation of HRT and reduction of substrates concentrations. The pH was adjusted at 7.04-7.65 in the influent wastewater during the recovery phase.
2.4 Nitrogen removal kinetics
Many substrate removal models have been reported to evaluate the nitrogen removal kinetics of anammox bioreactors, such as anammox upflow filter [14], anammox EGSB [23] and anammox non-woven membrane reactor [24]. Three types of substrate removal models, i.e., Grau second-order model, Stover-Kincannon (S-K) model and Monod model were chosen to predict the process kinetics of high-rate anammox UASB at present work since they can appropriate descript the performance of anammox bioreactor [14, 23, 24]. The detailed descriptions and equivalence formulas of the three models are presented in Table 2.
Table 1 Performance of UASB at different operating HRTs during whole phase
2.5 Analytical methods
The influent and effluent samples were collected and analyzed daily. The changes in the concentration of ammonium, nitrite and nitrate were measured through the spectrophotometric method of salicylate-hypochlorous acid, N-(1-Naphthyl) ethylenediamine dihydrochloride and ultraviolet spectrophotometry with UV-spectrophotometer (UV-4100, Hitachi, Japan) [25], respectively. The pH values were determined using a pH meter (PHS-3E, INESA Scientific Instrument Co., Ltd., China) [26]. The temperature in the reactor was measured by a mercurial thermometer. Suspended sludge (SS), volatile suspended sludge (VSS) were determined by gravimetric analysis. SS was measured by drying the filtrated residue overnight (24 h) at 105 °C and VSS was detected by burning the dried residue in an oven at 550 °C for 1 h. The sludge settleability and sludge volume index (SVI) were measured according to SONG et al [27]. Settled granular sizes were measured using standard scale. The high-throughput sequencing of microbial community composition in the UASB was analyzed by an Illumina MiSeq platform of Novogene (Beijing, China) according to previous reports [27].
3 Results and discussion
3.1 Reactor performance
In this study, the UASB was operated for 180 d with an aim to evaluate the reactor performance at increasing NLR conditions. The reactor performance was divided into three phases based on the data obtained from the reactor operation which has been described below accordingly.
3.1.1 Start-up and stabilization of performance (Phase-1: 1-40 days)
Through inoculating the anammox sludge (0.6 L) and keeping the influent wastewater of 100 mg/L NH4+-N and 120 mg/L NO2--N with an HRT of 4.76 h, the UASB reactor was progressive started up. The reactor demonstrated anammox reaction from the beginning of reactor start-up with initial NLR of 1.11 kg/m3/d. Other operational parameters such as temperature and influent pH were keeping at constant level as mentioned in materials and methods, the NLR was elevated either by adjusting the substrate concentrations or shortening the HRT. It’s noteworthy to mention that the anammox sludge was floated frequently in the initial one week of reactor start up and onward it tended to be settled and became stable. After 40 d operation, the influent ammonium and nitrite concentrations increased to 208 and 250 mg/L, respectively, and simultaneously the HRT was shortened to 2.38 h (Table 1). It could be observed in Figure 1, effluent nitrite and ammonium decline gradually and nitrate increase progressively. Along the course of time the anammox granules turned to red and had shown the tendency to be settled at the bottom of reactor column. anammox activity appeared to be stable and remarkable accordingly (days 35-40), marking a successful start-up of anammox process. Ultimately, the NH4+-N and NO2--N were almost entirely consumed with ammonium and nitrite removal efficiency of 98.6% and 99.6%, respectively. The obtained NLR and NRR of 4.62 and 4.58 kg/(m3·d), respectively were higher as compared to previous researches [28-31], thus the experimented UASB reactor was believed to be started up and stabilized successfully.
Table 2 Kinetic models and their mathematical expressions
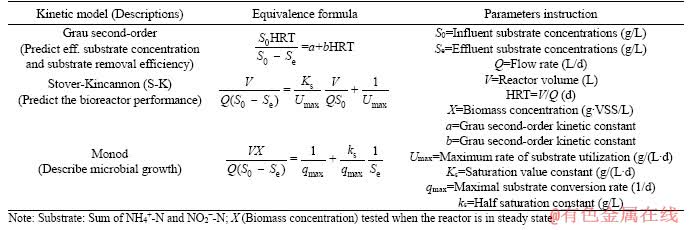
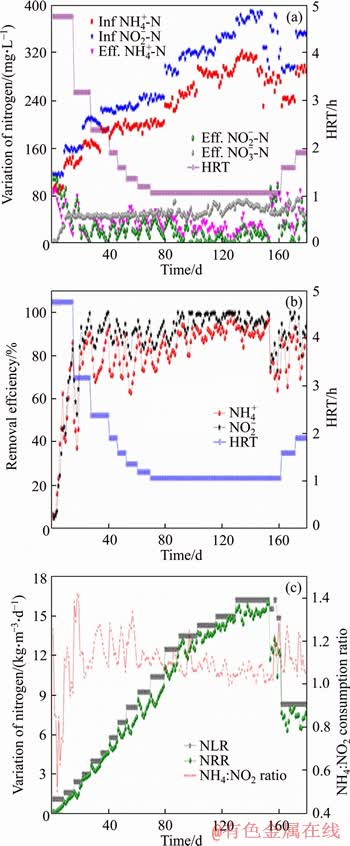
Figure 1 Reactor performance showing variation of nitrogen (a), removal efficiency of ammonium and nitrite (b), and volumetric nitrogen consumption with stoichiometry (c)
3.1.2 Stepwise increase of NLR under steady state (Phase-2: 41-153 days)
The NLR in this phase was increased in two steps. Initially (days 41-80), the HRT of the reactor was shortened from 2.38 to 1.06 h maintaining the influent NH4+-N and NO2--N at 208 and 250 mg/L, respectively. Hence, the NLR was incremented from 4.62 to 10.37 kg/(m3·d). In subsequent step (days 81-153), the enhancement of NH4+-N (208-325 mg/L) and NO2--N (250-390 mg/L) concentration in influent at an HRT of 1.06 h accelerated the NLR to 16.22 kg/(m3·d).During this phase, the UASB reactor exhibited a stable performance with the average removal of ammonium and nitrite of 89.87% and 96.35%, respectively which resulted in increasing the NRR to 15.77 kg/(m3·d) (Figure 1). As compared to start-up phase, the volume of anammox granules inside the reactor was found to be elevated and the sludge became redder. The average stoichiometric ratio of depleted NH4+-N, consumed NO2--N and produced NO3--N was 1:1.17:0.19, which is close to the theoretical value of 1:1.32:0.26.
Anammox reaction is known as an acidity consumption reaction which ultimately increase the reactor pH. The increase of effluent pH indicated the effective anammox reactor performance [32]. During the stable operation, the influent pH was maintained at the range of 7.25-7.65. Due to high anammox activity, the effluent pH surpassed the influent pH and thus the variation of pH value (△pH) during stable operation (with good anammox performance) was obviously higher than that of the initial experimental period (with relatively low anammox activity (Figure 2). In addition, the increase of effluent pH of this study had shown a good linear correlation between total influent nitrogen removal rate (TINRR) and the value of △pH×Q (Figure 2(b)) during stable operation.
3.1.3 Inhibition and recovery (Phase-3: 154-180 d)
NO2--N is an essential electron acceptor for anammox process, but the long-term exposure to high concentration of NO2- may inhibit the bacterial activity. Under low pH condition, the toxicity of NO2- is mainly because the increased free nitric acid (FNA) [12, 33]. However, under high pH (>pH 7.0), the toxicity of NO2- has shown direct inhibition effects on anammox activity as opposed to the FNA. A wide range of NO2--N concentrations (100 to 350 mg/L) was reported to be an inhibitory threshold. After stable operation of the UASB reactor at 325 mg/L NH4+-N and 390 mg/L NO2--N under the high hydraulic conditions, inhibition did notice on day 154 and within four days a progressive accumulation of ammonium (165.33 mg/L) and nitrite (162.69 mg/L) was observed in the UASB reactor which deteriorated the removal efficiency to 44.52% and 57.69%, respectively. Simultaneously, the NRR decreased to 8.78 kg/(m3·d). During this inhibition, the sludge became pale red.

Figure 2 pH variation (a), relationship between the TINRR (total influent nitrogen removal rate) and △pH×Q (△pH=Eff.pH-Inf.pH, Q is influent flow (L/d)) (b), and stoichiometric ratio of NO2--N and NO3--N to NH4+-N consumption (c)
In order to prevent the inhibition, tap water was immediately flushed to wash out the residual ammonium and nitrite. To enhance the recovery of anammox activity, the influent ammonium and nitrite concentrations were decreased to 250 mg/L and 300 mg/L by prolonging the HRT to 15.9 h. Nonetheless, anammox activity was not recovered which compelled us to further decrease the concentration of NH4+-N and NO2--N to 150 and 180 mg/L, respectively. Along the course of time, the biomass tended to consume influent substrates and after one week the substrates were entirely consumed. Then, the substrate concentration was gradually increased by 50 mg/L in each step based on the effluent properties. Finally, we were able to increase the influent ammonium and nitrite to 250 and 300 mg/L, respectively and thus the NLR was regained to 11.64 kg/(m3·d), demonstrating 72% performance recovery to the highest value of the UASB reactor. During this recovery period, the effluent NO3--N was comparatively lower than the observed value in previous phases.
The influent pH was strictly maintained at 7.0-7.2 during the recovery period. In addition, the effluent NH4+-N and NO2--N were maintained below 50 mg/L to avoid repetition of inhibition in anammox activity. The stoichiometric ratio of NO2--N consumption to NH4+-N depletion also recovered to 1.15: 1 (Figure 2(c)), but the NO3--N production to NH4+-N depletion ratio was only 0.06, unfortunately much lower that standard value (0.26). It may be attributed to the coexistence of heterotrophic bacteria in this recovery period, which could use soluble microbial products (SMP)/EPS or organic matter released during biomass decay as electron donor to denitrify NO3--N/NO2--N.
3.2 High-rate anammox granules properties
The anammox granules prevailed in UASB (day 149) were reddish brown (Figure S1), implying high nitrogen removal activities [7]. However, some sludge became pale red and black in the upper part of the UASB which might be due to the decrease of Heme c content, and long-term starvation [27]. The short HRT condition created massive circulation of influent and biomass in the reactor which resulted in increased shear stress among the whole reactor, under these circumstances, the biomass may secrete extra-extracellular polymeric substances (EPS) which could help the small subunits to be attached together [17]. As a result, the anammox granules became larger from time to time and thus the diameter of anammox granule in this study was 2.5-7.0 mm (average 3.95 mm) (Table S1) which was higher than the reported sludge diameter of 1.75-4.0 mm [34].
As reported, the settleability of granules is proportional to the diameter of sludge [34]. Hence, the high settling velocity of 36.6-77.1 m/h (average 60.5 m/h) (Table S1) was obtained which was very close to the reported highest value (73 to 88 m/h) by LU et al [21] and at least two times greater than those of the flocculated anammox sludge [16]. However, some reports stated that the increase of sludge diameter could deteriorate the reactor performance because of the reduced mass transfer. Other reports differently postulated that the sludge diameter increases due to the excessive gas production inside the sludge granule. NI et al [35] demonstrated that the nitrogen gas production is directly related to the volumetric substrate loading or substrate removal rate. Thus, the excessive gas production of this study confirmed the high substrate removal rate. The SVI5 and SVI30 values of the anammox granules were 22.95 and 20.15 mL/g with SVI30/SVI5 ratio of 0.88 suggesting a magnificent sedimentation property [7]. As shown in Table S1, the concentration of anammox sludge improved to 35.7 and 24.7 g/L, respectively in terms of SS and VSS.
The morphology and structure of anammox granules were monitored via SEM observation. The sludge was presenting in a rounded shape, and the surface showed lightly smooth (Figure S2). Usually the presence of EPS on anammox sludge increase the permeability of sludge which helped to relieve gas from it and thus increasing the sludge settleability. Gas cavities with well-developed opening have been observed in SEM microscopy (Figure S2(b)). The endogenously produced N2 gas could be released easily through those cavities [36] and thus helped to settle the sludge at the bottom of the reactor, which eventually enhanced the substrate removal rate.
3.3 Process kinetic evaluation
It has been confirmed that the Grau second- order kinetic model was popular models for anammox bioreactor to determine kinetic constant[23]. The S-K model is one of the most widely used in continuously operated bioreactors[24]. Monod model has been widely used in both aerobic and anaerobic reactors to interpret the substrate concentration effects on substrate consumption rate[37]. The steady state operational data were applied to the linear regression arithmetic (Table S2).
Figure 3 and Table S3 showed the fitting results of three kinetics models and the obtained kinetic constants. It was clearly that Grau second- order model and S-K model possess a pretty high R2 value of 0.9976 and 0.9997, respectively. From the regression in S-K model simulation in Figure 3(c), the constant Ks and Umax were obtained as high as 55.12 and 55.68 kg/(m3·d), respectively. From Grau second order model, the parameters of Figures 3(a) and (b) were calculated as 0.0103 d and 0.9024, respectively. Both two models achieved a high R2 in substrate removal. Thus, the effluent nitrogen (both ammonia and nitrite) concentrations and substrate removal efficiency can be predicted by substituting the obtained parameters into the expression. Notably, the predicted Umax (55.68 kg/(m3·d)) from S-K model is significantly higher than the NLR of 16.22 kg/(m3·d) obtained in this study, which means that the granular based anammox UASB reactor have a great potential to deal with high strength nitrogen containing wastewater. The linearized Monod equation was plotted in Figure 3(a), and the values of the maximum rate of substrate utilization (qmax) and half saturation concentration (ks) were 0.489 per day and 14.47 mg/L, respectively. The relatively low ks value proved the high substrate affinity of anammox bacteria cultivated in the UASB reactor. Besides, a relatively lower R2 of 0.9077 from Monod model indicating that the Grau second-order and S-K models were more suitable to describe the substrate removal in anammox UASB reactor in different HRTs.
3.4 Microbial community structures of UASB
The relative abundances of microbial community were obtained from the anammox UASB, analyzing through high-throughput sequencing. At the phylum level, a total of 39 phyla were detected both in the inoculum and mature anammox granules. In addition, the results of Alpha diversity including OTUs, Chao-1 index and Shannon index (Table S4) indicated that the microbial diversity of anammox UASB remains stable after long-term high-load operation.

Figure 3 Linearized plots of Monod model (a), Grau second-order model (b), and S-K model for substrate removal (c)
As shown in Figure 4(a), the relative abundances of dominant phyla in anammox UASB were Planctomycetes, Proteobacteria, Chloroflexi and Chlorobi, which have been commonly regarded as the dominant phyla in many lab-scale anammox reactors. It is worth noting that the phyla Euryarchaeota had a significant abundance of 22.5% in the inoculum. This may be because of the seed anammox sludge was taken from the reactor which started-up by anaerobic granular sludge [38]. After long-term high-load operation, the relative abundance of Euryarchaeota phyla significantly reduced to 0.05%, which means the anaerobic heterotrophic bacteria have been eliminated in the stable phase of the UASB. It is widely known that Planctomycetes contains all known anammox bacteria and increased its abundance from 23.77% to 40.28% in present work. Moreover, Betaproteobaceria contains many groups of N-cycle related microorganisms (including nitrifying and denitrifying) also increased from 6.09% to 11.47% (Figure 4(b)). This indicated that the anammox has been enhanced in the UASB system which supported the high rate reactor performance.
The microbial community were further analyzed at family and genus level (Figure 5). As shown in Figure 5(a), the relative abundance at family level further confirmed the enrichment of anammox biomass in the UASB, since the anammox bacteria have consistently belonging to the family Brocadiaceae. The relative abundance of Brocadiaceae was predominant in both mature granules and the inoculum. In the inoculum, the relative abundance of Brocadiaceae reached approximately 21%, indicating a high level of anammox bacteria. After long-term high-rate operation and the formation of mature anammox granules in UASB, the relative abundance of Brocadiaceae increased to 38.1%, nearly two times rise in 100 d.
The variations of microbial community at the genus level give some new information. Firstly, the genus Candidatus Kuenenia and Candidatus Brocadia were the predominant genus in two samples (Figure 5(b)), and Candidatus Jettenia (0.011%-0.014%) was also detected with very small abundances. Then, the relative abundance of Candidatus Kuenenia and Candidatus Brocadia in inoculum were 12.29% and 8.43%, respectively (Figure 5(b)). When the UASB has experienced a high-load operation over 100 d, the relative abundance of Candidatus Kuenenia and Candidatus Brocadia in mature anammox granules were 36.95% and 1.09%, respectively. On one hand, the abundance of anammox genus indeed improved significantly (from 20.72% to 38.04%). On the other hand, a shift from Candidatus Brocadia to Candidatus Kuenenia was observed under present conditions, that is progressively shorter HRT and higher substrate concentrations. Although the niche differentiation between Candidatus Brocadia and Candidatus Kuenenia genera is not clear, such “shift” situation has been reported in some literatures [39, 40]. This mainly because of the different kinetic strategies (i.e., either r- or K-strategist) between Candidatus Brocadia and Candidatus Kuenenia. Considering present work, the synthetic wastewater with high ammonia (250-325 mg /L) and nitrite (300-390 mg/L) concentrations was used, which benefited the growth of Candidatus Kuenenia genera instead of the Brocadia genera. This phenomenon is similar to what has been observed by MIAO et al [41], which a high concentration of substrate was used in their anammox biofilm.
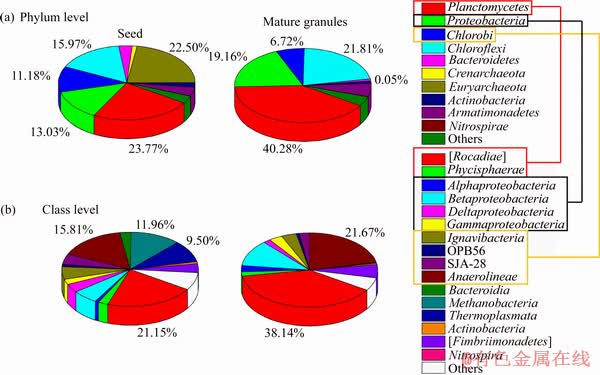
Figure 4 Microbial community structure of UASB at phylum (a) and class (b) levels
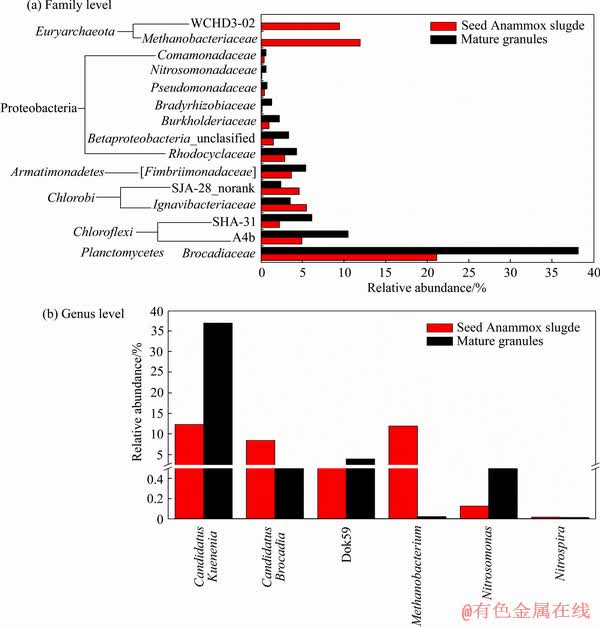
Figure 5 Microbial community structure of UASB at family (a) and genus (b) level (Relative abundance is defined as number of sequences affiliated with taxon divided by total number of sequence (%))
Further, the genera Dok59 a member of Betaproteobacteria was detected to be 3.90% of total sequences in mature anammox granules, and some ammonium-oxidizing bacteria (AOB) genera such as Nitrosomonas, Nitrite-oxidizing bacteria (NOB) genera such as Nitrospira were also detected with relatively low abundance of 0.011% to 0.646% (Figure 5(b)). These nitrogen-related genera are both contributed to the high-rate nitrogen removal performance in the UASB system. Present work also indicated that using tap water as substrate and without strict anaerobic environment, the UASB can realize large amount of enrichment of anammox bacteria and high-rate nitrogen removal performance.
4 Conclusions
1) A high anammox performance of 15.77 kg/(m3·d) of NRR was realized in a UASB reactor with a relative high nitrogen removal efficiency of 93.7% under HRT of 1.06 h. Carmine anammox granules with good settling velocity of 36.6-77.1 m/h were highly enriched in the reactor.
2) It was found that the Grau second order and S-K model would be more appropriate for predicting the nitrogen removal kinetics of the anammox-UASB, because the corresponding high correlation coefficients of 0.9976, 0.9997, respectively. According to the S-K models, the predicted Umax in the tested reactor was as high as 55.68 kg/(m3·d), suggesting that the UASB reactor possessed a great potential to increase the performance through a series of appropriate operations. Additionally, the relatively low value of the half saturation concentration (ks) described by Monod model also proved the high substrate affinity of anammox bacteria in present UASB.
3) The result of microbial community revealed Candidatus Kuenenia as the dominant genus accounting for 36.95% of total sequences in mature anammox granules, which is the clear indication of anammox enrichment in the UASB system and the high substrate removal rate. anammox population shifted from Candidatus Brocadia to Candidatus Kuenenia after long-term HRT shortening and substrate excess.
Appendix
Table S1 Physical characteristics of anammox granules in an UASB reactor

Table S2 Data for kinetics modeling during steady state of anammox UASB

Table S3 Summary of kinetic constants

Table S4 Sequences related parameters of anammox sludge

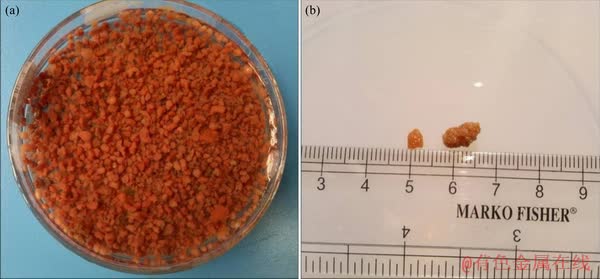
Figure S1 Physical characteristics of granular anammox sludge

Figure S2 SEM images of anammox granular sludge showing morphology (a), surface structure (b) and pattern (c-d) of bacteria communities on sludge surface
References
[1] DU Rui, CAO Shen-bin, LI Bai-kun, NIU Meng, WANG Shu-ying, PENG Yong-zhen. Performance and microbial community analysis of a novel DEAMOX based on partial-denitrification and anammox treating ammonia and nitrate wastewaters [J]. Water Research, 2017, 108: 46-56. DOI: 10.1016/j.watres.2016.10.051
[2] TANG Chong-jian, LIU Zhi-gong, PENG Chong, CHAI Li-yuan, KURODA K, OKIDO M, SONG Yu-xia. New insights into the interaction between heavy metals and struvite: Struvite as platform for heterogeneous nucleation of heavy metal hydroxide [J]. Chemical Engineering Journal, 2019, 365: 60-69. DOI: 10.1016/j.cej.2019.02.034.
[3] FENG Fan, DUAN Cheng-shan, TANG Xi, CHEN Xi, LU Xuan, CHAI Xi-lin, MAHMOOD Q, TANG Chong-jian, CHAI Li-yuan. Performance, microbial community and inhibition kinetics of long-term Cu2+ stress on an air-lift nitritation reactor with self-recirculation [J]. Journal of Environmental Sciences, 2020, 91: 117-127. DOI:10.1016/j.jes.2020.01. 021.
[4] OSHIKI M, SATOH H, OKABE S. Ecology and physiology of anaerobic ammonium oxidizing bacteria [J]. Environmental Microbiology, 2016, 18(9): 2784-2796. DOI: 10.1111/1462-2920.13134.
[5] KUYPERS M M M, MARCHANT H K, KARTAL B. The microbial nitrogen-cycling network [J]. Nature Reviews Microbiology, 2018, 16(5): 263-276. DOI: 10.1038/nrmicro. 2018.9.
[6] YU Cheng, TANG Xi, LI Lu-shan, CHAI Xi-lin, XIAO Rui-yang, WU Di, TANG Chong-jian, CHAI Li-yuan. The long-term effects of hexavalent chromium on anaerobic ammonium oxidation process: Performance inhibition, hexavalent chromium reduction and unexpected nitrite oxidation [J]. Bioresource Technology, 2019, 283: 138-147. DOI: 10.1016/j.biortech.2019.03.081.
[7] XIE Guo-jun, LIU Tao, CAI Chen, HU Shi-hu, YUAN Zhi-guo. Achieving high-level nitrogen removal in mainstream by coupling anammox with denitrifying anaerobic methane oxidation in a membrane biofilm reactor [J]. Water Research, 2017, 131: 196-204. DOI: 10.1016/ j.watres.2017.12.037
[8] CAO Ye-shi, VAN LOOSDRECHT M C M, DAIGGER G T. Mainstream partial nitritation-anammox in municipal wastewater treatment: Status, bottlenecks, and further studies [J]. Applied Microbiology and Biotechnology, 2017, 101(4): 1365-1383. DOI: 10.1007/s00253-016-8058-7.
[9] ZHANG Lei, NARITA Y, GAO Lin, ALI M, OSHIKI M, OKABE S. Maximum specific growth rate of anammox bacteria revisited [J]. Water Research, 2017, 116: 296-303. DOI: 10.1016/j.watres.2017.03.027.
[10] JIN Ren-cun, YANG Guang-feng, YU Jin-jin, ZHENG Ping. The inhibition of the anammox process: A review [J]. Chemical Engineering Journal, 2012, 197: 67-79. DOI: 10.1016/j.cej.2012.05.014.
[11] SONG Yu-xia, CHAI Li-yuan, TANG Chong-jian, XIAO Rui-yang, LI Bing-rong, WU Di, MIN Xiao-bo. Influence of ZnO nanoparticles on anammox granules: The inhibition kinetics and mechanism analysis by batch assays [J]. Biochemical Engineering Journal, 2018, 133: 122-129. DOI:10.1016/j.bej.2018.02.006.
[12] Raudkivi M, Zekker I, Rikmann E, Vabamae P, Kroon K, Tenno T. Nitrite inhibition and limitation-the effect of nitrite spiking on anammox biofilm, suspended and granular biomass [J]. Water Science and Technology, 2017, 75(2): 313-321. DOI: 10.2166/wst.2016.456.
[13] ZHANG Yan-long, MA Hai-yuan, CHEN Rong, NIU Qi-gui, LI Yu-you. Stoichiometric variation and loading capacity of a high-loading anammox attached film expanded bed (AAEEB) reactor [J]. Bioresource Technology, 2018, 253: 130-140. DOI: 10.1016/j.biortech.2018.01.043.
[14] JIN Ren-cun, ZHENG Ping. Kinetics of nitrogen removal in high rate anammox upflow filter [J]. Journal of Hazardous Materials, 2009, 170(2, 3): 652-656. DOI:10.1016/j.jhazmat. 2009.05.016.
[15] YIN Xin, QIAO Sen, ZHOU Ji-ti, TANG Xin. Fast start-up of the anammox process with addition of reduced graphene oxides [J]. Chemical Engineering Journal, 2016, 283: 160-166. DOI: 10.1016/j.cej.2015.07.059.
[16] MIAO Yuan-yuan, PENG Yong-zhen, ZHANG Liang, LI Bai-kun, LI Xi-yao, WU Lei, WANG Si-meng. Partial nitrification-anammox (PNA) treating sewage with intermittent aeration mode: Effect of influent C/N ratios [J]. Chemical Engineering Journal, 2017, 334: 664-672. DOI: 10.1016/j.cej.2017.10.072.
[17] LIU Yu, XU Hai-lou, YANG Shu-fang, TAY J H. Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor [J]. Water Research, 2003, 37(3): 661-673. DOI: 10.1016/S0043-1354(02)00351-2.
[18] MA Bin, PENG Yong-zhen, ZHANG Shun-jun, WANG Jun-min, GAN Yi-ping, JIANG Chang, WANG Shu-ying, WANG Shan-yun, ZHU Gui-bing. Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures [J]. Bioresource Technology, 2013, 129(2): 606-611. DOI: 10.1016/j.biortech.2012.11. 025.
[19] YU Han-qin, TAY J H, FANG H H. The roles of calcium in sludge granulation during UASB reactor start-up [J]. Water Research, 2001, 35(4): 1052-1060. DOI: 10.1016/S0043- 1354(00)00345-6.
[20] TAN Hao, WANG Yun-yan, TANG Xi, LI Lu-shan, FENG Fan, MAHMOOD Q, WU Di, TANG Chong-jian. Quantitative determination of cavitation formation and sludge flotation in anammox granules by using a new diffusion-reaction integrated mathematical model [J]. Water Research, 2020, 174: 115632. DOI: 10.1016/j.watres.2020. 115632.
[21] LU Hui-feng, ZHENG Ping, JI Qi-xing, ZHANG Hong-tao, JI Jun-yuan, WANG Lan, DING Shuang, CHEN Ting-ting, ZHANG Ji-qiang, TANG Chong-jian, CHEN Jian-wei. The structure, density and settlability of anammox granular sludge in high-rate reactors [J]. Bioresource Technology, 2012, 123: 312-317. DOI: 10.1016/j.biortech.2012.07.003.
[22] WU Jing, ZHOU Hong-ming, LI Huai-zhi, ZHANG Peng- cheng, JIANG Jie. Impacts of hydrodynamic shear force on nucleation of flocculent sludge in anaerobic reactor [J]. Water Research, 2009, 43(12): 3029-3036. DOI: 10.1016/ j.watres.2009.04.026.
[23] CHEN Ting-ting, ZHENG Ping, SHEN Li-dong, DING Shuang, MAHMMOD Q. Kinetic characteristics and microbial community of anammox-EGSB reactor [J]. Journal of Hazardous Materials, 2011, 190(1-3): 28-35. DOI: 10.1016/j.jhazmat.2010.12.060.
[24] NI Shou-qing, LEE P H, SUNG S W. The kinetics of nitrogen removal and biogas production in an anammox non-woven membrane reactor [J]. Bioresource Technology, 2010, 101(15): 5767-5773. DOI: 10.1016/j.biortech.2010.02. 074.
[25] XIAO Rui-yang, HE Lei, LUO Zong-hao, SPINNEY R, WEI Zong-su, DIONYSIOU D D, ZHAO Fei-ping. An experimental and theoretical study on the degradation of clonidine by hydroxyl and sulfate radicals [J]. Science of the Total Environment, 2020, 710: 136333. DOI: 10.1016/j. scitotenv.2019.136333.
[26] XIAO Rui-yang, BAI Lu, LIU Kai, SHI Yan, MINAKATA D, HUANG C H, SPINNEY R, SETH R, DIONYSIOU D D, WEI Zong-su, SUN Pei-zhe. Elucidating sulfate radical- mediated disinfection profiles and mechanisms of Escherichia coli and Enterococcus faecalis in municipal wastewater [J]. Water Research, 2020, 173: 115552. DOI: 10.1016/j.watres.2020.115552.
[27] SONG Yu-xia, LIAO Qi, YU Cheng, XIAO Rui-yang, TANG Chong-jian, CHAI Li-yuan, DUAN Cheng-shan. Physicochemical and microbial properties of settled and floating anammox granules in upflow reactor [J]. Biochemical Engineering Journal, 2017, 123: 75-85. DOI: 10.1016/j.bej.2017.04.002.
[28] CHEN Hui, HU Hai-yan, CHEN Qian-qian, SHI Man-ling, JIN Ren-cun. Successful start-up of the anammox process: Influence of the seeding strategy on performance and granule properties [J]. Bioresource Technology, 2016, 211: 594. DOI: 10.1016/j.biortech.2016.03.139.
[29] CHOI M, CHO K, LEE S, CHUNG Y C, PARK J. Effective seeding strategy using flat type poly (vinyl alcohol) cryogel for anammox enrichment [J]. Chemosphere, 2018, 205: 88-97. DOI: 10.1016/j.chemosphere.2018.04.055.
[30] SOBOTKA D, TUSZYNSKA A, KOWAL P, CIESIELSKI S, CZERWIONKA K, MAKINA J. Long-term performance and microbial characteristics of the anammox-enriched granular sludge cultivated in a bench-scale sequencing batch reactor [J]. Biochemical Engineering Journal, 2017, 120: 125-135. DOI: 10.1016/j.bej.2017.01.007.
[31] YANG Wan, HE Shi-long, HAN Ming, WANG Bing-bing, NIU Qi-gui, CHEN Yi, WANG Hai-bo. Nitrogen removal performance and microbial community structure in the start-up and substrate inhibition stages of an anammox reactor [J]. Journal of Bioscience and Bioengineering, 2018, 126(1): 88-95. DOI: 10.1016/j.jbiosc.2018.02.004.
[32] LI Huo-sheng, ZHOU Shao-qi, MA Wei-hao, HUANG Guo-tao, XU Bin. Fast start-up of ANAMMOX reactor: Operational strategy and some characteristics as indicators of reactor performance [J]. Desalination, 2012, 286(1): 436-441. DOI:10.1016/j.desal.2011.11.038.
[33] LALOO A E, WEI J, WANG Dong-bo, NARAYANASAMY S, Vanwonterghem I, Waite D, Steen J, Kaysen A, HEINTZ-BUSCHART A, WANG Qi-lin, SCHULZ B, NOUWENS A, WILMES P, HUGENHOLTZ P, YUAN Zhi-guo, BOND P L. Mechanisms of persistence of the ammonia-oxidizing bacteria nitrosomonas to the biocide free nitrous acid [J]. Environmental Science and Technology, 2018, 52(9): 5386-5397. DOI: 10.1021/acs.est.7b04273.
[34] LU Hui-feng, JI Qi-xing, DING Shuang, ZHENG Ping. The morphological and settling properties of ANAMMOX granular sludge in high-rate reactors [J]. Bioresource Technology, 2013, 143(17): 592-597. DOI: 10.1016/ j.biortech.2013.06.046
[35] NI Bing-jie, HU Bao-lan, FANG Fang, XIE Wen-ming, KARTAL B, LIU Xian-wei, SHENG Guo-ping, JETTEN M, ZHENG Ping, YU Han-qing. Microbial and physicochemical characteristics of compact anaerobic ammonium-oxidizing granules in an upflow anaerobic sludge blanket reactor [J]. Applied and Environmental Microbiology, 2010, 76(8): 2652-2656. DOI:10.1128/AEM.02271-09.
[36] DA Kang, GUO Lei-yan, HU Qian-yi, XU Dong-dong, YU Tao, LI Yi-yu, ZENG Zhuo, LI Wen-ji, SHEN Xiao-jing, ZHENG Ping. Surface convexity of anammox granular sludge: Digital characterization, state indication and formation mechanism [J]. Environment International, 2019, 131: 105017. DOI: 10.1016/j.envint.2019.105017.
[37] PUYOL D, CARVAJAL-ARROYO J M, GARCIA B, SIERRA-ALVAREZ R, FIELD J A. Kinetic characterization of Brocadia spp.-dominated anammox cultures [J]. Bioresource Technology, 2013, 139(7): 94-100. DOI: 10.1016/ j.biortech.2013.04.001.
[38] XIONG Lei, WANG Yun-yan, TANG Chong-jian, CHAI Li-yuan, XU Kang-que, SONG Yu-xia, ALI M, ZHENG Ping. Start-Up characteristics of a granule-based anammox UASB reactor seeded with anaerobic granular sludge [J]. BioMed Research International, 2013, 2013: 1-9. DOI: 10.1155/2013/396487.
[39] ISANTA E, BEZERRA T, FERNANDEZ I, SUAREZ- OJEDA M E, PEREZ J, CARRERA J. Microbial community shifts on an anammox reactor after a temperature shock using 454-pyrosequencing analysis [J]. Bioresource Technology, 2015, 181: 207-213. DOI: 10.1016/j.biortech. 2015.01.064.
[40] DE COCKER P, BESSIERE Y, HERNANDEZ-RAQUET G, DOBOS S, MOZO I, GAVAL G, CALIGARIS M, BARILON B, VLAEMINCK S E, SPERANDIO M. Enrichment and adaptation yield high anammox conversion rates under low temperatures [J]. Bioresource Technology, 2018, 250: 505-512. DOI: 10.1016/j.biortech.2017.11.079
[41] MIAO Lei, ZHANG Qiong, WANG Shu-ying, LI Bai-kun, WANG Zhong, ZHANG Su-jian, ZHANG Man, PENG Yong-zhen. Characterization of EPS Compositions and microbial community in an anammox SBBR system treating landfill leachate [J]. Bioresource Technology, 2017, 249: 108-116. DOI: 10.1016/j.biortech.2017.09.151.
(Edited by HE Yun-bin)
中文导读
高负荷厌氧氨氧化反应器性能、微生物结构和过程动力学特性研究
摘要:本文研究了上流式厌氧污泥床(UASB)中厌氧氨氧化工艺的脱氮性能、污泥性状、微生物群落结构以及过程动力学特性。首先,通过缩短水力停留时间并提高进水基质浓度,研究了负荷提升过程中UASB反应器的脱氮性能,并最终将反应器的总氮去除速率(NRR)提高到15.77 kg/(m3·d),富集了成熟的厌氧氨氧化颗粒污泥。其次,选用3种基质去除动力学模型对反应器稳态运行时期的数据进行了拟合,其中Stover-Kincannon模型的结果表明UASB的最大基质利用速率(Umax)可达55.68 kg/(m3·d)。此外,微生物群落结构分析的结果表明反应器在长期高负荷运行后,占主导地位的脱氮细菌为“Candidatus Kuenenia”属。随着脱氮性能的提高,其相对丰度由12.29%提高到了36.95%,颗粒污泥内的厌氧氨氧化优势菌属的组成也发生了改变。
关键词:厌氧氨氧化;UASB反应器;动力学;颗粒污泥;微生物结构
Foundation item: Project(51878662) supported by the National Natural Science Foundation of China; Project(2017SK2420) supported by the Science and Technology of Hunan Province, China; Project(2019JJ20033) supported by the Distinguished Youth Natural Science Foundation of Hunan Province, China
Received date: 2019-11-24; Accepted date: 2020-03-28
Corresponding author: CHAI Xi-lin, PhD, Senior Engineering; Tel/Fax: +86-731-88710171; E-mail: allenxizi@csu.edu.cn; TANG Chong-jian, PhD, Professor; Tel/Fax: +86-731-88830511; E-mail: chjtang@csu.edu.cn; ORCID: 0000-0003- 3808-6447
Abstract: In this study, a lab-scale upflow anaerobic sludge blanket (UASB) reactor was applied to studying the high-rate nitrogen removal of granule-based anammox process. The nitrogen removal rate (NRR) finally improved to 15.77 kg/m3/d by shortening hydraulic retention time (HRT) to 1.06 h. Well-shaped red anammox granules were extensively enriched inside the reactor. The results of nitrogen removal kinetics indicated that the present bioreactor has great nitrogen removal potential, because the maximum rate of substrate utilization (Umax) predicted by Stover-Kincannon model is suggested as 55.68 kg/(m3·d). Analysis of the microbial community showed that the anammox genus Candidatus Kuenenia dominated the bacterial communities. The relative abundance of Candidatus Kuenenia rose from 12.29% to 36.95% after progressively shorter HRT and higher influent substrate concentrations, illustrating the stability of nitrogen removal performance and biomass enrichment offered by the UASB in carrying out high-rate anammox process.


