文章编号:1004-0609(2011)08-1974-06
金修饰纳米多孔Pd催化剂对甲酸氧化的电催化活性
牛凤娟1,易清风1,刘云清2
(1. 湖南科技大学 化学化工学院,湘潭 411201;
2. 中南大学 化学化工学院,长沙 410083)
摘 要:
以水热法制备出纳米多孔网状钯催化剂(nanoPd),采用电位扫描在其上沉积金,制成金修饰纳米钯电极(Au/nanoPd),运用循环伏安法(CV)、线性扫描(LSV)和交流阻抗谱(EIS)比较nanoPd和Au/nanoPd电极对甲酸氧化反应的电催化活性。CV和LSV结果表明:金在nanoPd表面的沉积促进钯对甲酸氧化的电催化活性,起始电位提前,电流密度更高。EIS研究结果也表明:在Au/nanoPd电极上,甲酸氧化反应的电荷传递电阻更低。结果表明:金修饰纳米钯电极(Au/nanoPd)对甲酸氧化具有较高的电催化活性。
关键词 纳米钯;金修饰;甲酸氧化;燃料电池
中图分类号:O646 文献标志码:A
Electrocatalytic activity of Au modified nanoporous palladium electrode for formic acid oxidation
NIU Feng-juan1, YI Qing-feng1, LIU Yun-qing2
(1. School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: A novel nanoporous network palladium electrode (nanoPd) was successfully prepared by a hydrothermal method, then a gold-modified nanoparticle palladium electrode (Au/nanoPd) was subsequently fabricated through electrodepositing gold on the nanoPd electrode using a potential scan process. The electrocatalytic activities of the nanoPd and Au/nanoPd towards formic acid in alkaline solution were evaluated by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). CV results reveal that Au/nanoPd present a low onset potential and high anodic peak densities, which shows that the deposited gold on nanoPd electrode can enhance the palladium catalyst for formic acid electrooxidation activity. Also Nyquist plots indicate that the formic acid electrooxidation on the Au/nanoPd exhibits low impedance values. The results show that the prepared Au/nanoPd electrode is an effective electrocatalyst towards formic acid oxidation in alkaline media.
Key words: nanoporous palladium; gold-modified; formic acid oxidation; fuel cell
甲酸具有无毒、不易燃、储运方便等优点,且电化学活性、能量密度、质子导电率较高,因此,是一种较好的燃料电池液态燃料。甲酸氧化有直接氧化和间接氧化两种途径,即[1-4]
HCOOH→CO2+2H++2e (1)
HCOOH→COads+H2O→CO2+2H++2e (2)
甲酸在金属铂上的电氧化过程是间接氧化途径,脱水反应形成CO中间体,导致催化剂中毒,而甲酸在钯催化剂上的氧化过程主要是直接氧化途径,无CO中间体,避免了催化剂中毒而失活[5-8],且呈现出很高的电催化活性。HA等[9]研究发现,纳米钯催化剂在直接甲酸燃料电池中比钯黑具有更高的效率。ZHANG等[10]将Pd颗粒沉积附于碳纳米管,研究它对甲酸的电氧化活性。本文作者采用水热法一步制备出新型纳米多孔钯催化剂电极(nanoPd),然后以nanoPd催化剂做基底,电位扫描沉积金得到金修饰纳米钯电极(Au/nanoPd),通过循环伏安法、线性扫描和交流阻抗谱等研究碱性溶液中nanoPd和Au/nanoPd电极对甲酸氧化的电催化活性。
1 实验
JSM26380LV扫描电子显微镜(SEM),AutoLab PGSTA T30/FRA 电化学分析仪,钛片(纯度99.2%),氯化钯,氢氧化纳,甲酸均为分析纯,高纯氮气(纯度99.99%),实验所用水均为三次水。
钛片置于质量分数为18%的盐酸中,加热微沸10 min,然后超声清洗15 min,冲洗后置于水热反应釜中,依次加入10 mL,5 mmol/L PdCl2和1 mL 10%甲醛(HCHO),然后在红外干燥箱中于180 ℃下反应10 h。反应完成后待冷却至室温,取出钛基体,在100 ℃下烘干0.5 h,制得nanoPd电极。
将上述nanoPd 电极置于N2饱和的25 mmol/L AuCl3溶液中,在-0.5~0.5 V范围内以50 mV/s的扫速进行循环伏安电位扫描,沉积完毕的电极在N2下吹干,即制得Au/nanoPd 电极。
电化学测试在三电极玻璃电解槽中进行,工作电极为商用多晶钯电极(polyPd)、nanoPd 和Au/nanoPd 电极,参比电极为饱和甘汞(SCE),对电极是大面积铂电极。测试前向电解槽内通氮气至少15 min以除去溶解氧,测试过程中,始终保持氮气通过液面上方,所有测试均在室温(22±2 ℃)下进行。
polyPd电极(d=1 mm)经50 nm的Al2O3抛光膏抛光至镜面亮度,经3次水冲洗后超声处理15 min,之后在0.5 mol/L H2SO4中活化30 min,冲洗干净于3次水中浸泡备用。
2 结果与讨论
采用扫描电子显微技术(SEM)对nanoPd电极表面的颗粒形态进行了表征。图1(a)所示为nanoPd电极的SEM像。从图1(a)可以看出,钯颗粒在钛基表面紧密结合。颗粒之间相互牢固地连接在一起形成蜂窝状多孔结构,使得电极实际表面积大大增加,且孔穴的形成为金颗粒的附着提供大量位点,保障Au/nanoPd 催化剂颗粒的高度稳定性。图1(b)中的能谱分析结果表明,nanoPd电极表面有金的沉积。在2.9 keV的能量峰是钯的特征峰,2.1 keV和9.7 keV的能量峰是金的特征峰。
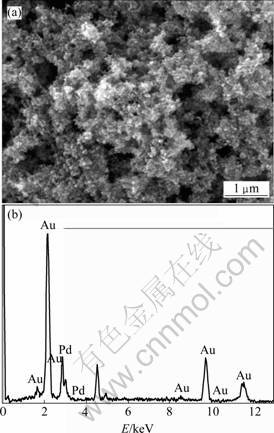
图1 nanoPd 电极的SEM像(a)及能谱分析(b)
Fig.1 SEM image(a) and EDS spectrum(b) of nanoPd electrode
图2(a)和(b)所示为nanoPd和晶体Pd(polyPd)电极在1.0 mol/L NaOH溶液中的循环伏安图。图2(a)中A1、C1峰对应于插入图2(b)中a1、c1峰,是氢的吸附和脱附。A2、C2峰对应于a2、c2峰,是钯氧化物的形成和特征还原峰。从图2(a)和(b)中可以看出,nanoPd电极上氢的吸附脱附峰明显正移,且钯的特征还原峰电流密度远远大于polyPd电极,二者分别为58.26 mA/cm2和1.53 mA/cm2。图2(c)所示为nanoPd电极在AuCl3溶液中扫描1圈、2圈和3圈的循环伏安曲线。与nanoPd电极的CV图相比较,Au/nanoPd曲线在0.07V处出现了新的还原峰,该峰随循环次数的增加而增大,这是金的特征还原峰[11-12],金氧化物的形成电位与钯氧化物的形成相似,所以在正向扫描过程中没有出现新的峰。在-0.23 V附近的还原峰电流随着扫描次数的增加,电流减小,这是由于随着金颗粒的沉积,裸露的钯逐渐减少,还原峰由原来的-0.38 V正移到-0.23 V,原来的钯氧化物的形成和还原峰均降低,这也说明发生金的电沉积。
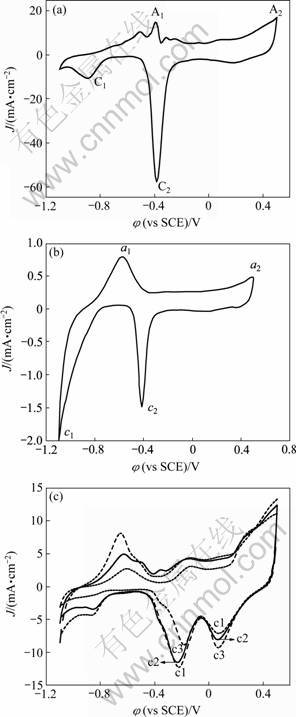
图2 nanoPd、polyPd和Au/nanoPd(2(b))电极在1.0 mol/L NaOH溶液中的循环伏安曲线
Fig.2 CV of nanoPd (a), polyPd (b) and Au/nanoPd (c) electrodes in 1.0 mol/L NaOH solution at scan rate of 50 mV/s
图3所示为nanoPd 和polyPd电极在1.0 mol/L NaOH+0.5 mol/L HCOOH溶液中的循环伏安图。与polyPd电极相比,甲酸在polyPd电极上的起始氧化电位为-0.6 V,氧化峰电流密度为7.5 mA/cm2,而在nanoPd电极上的起始氧化电位负移至-0.81 V,氧化峰电流密度为148.6 mA/cm2。结果表明:nanoPd电极的起始氧化电位提前190 mV左右,氧化峰电流密度增大约20倍。一般认为,甲酸在Pd催化剂表面上的电化学氧化过程表示如下[13-15]:
Pd+HCOOH→Pd-COOH(ads)+H+ + e
Pd-COOH(ads)→CO2 + H++e
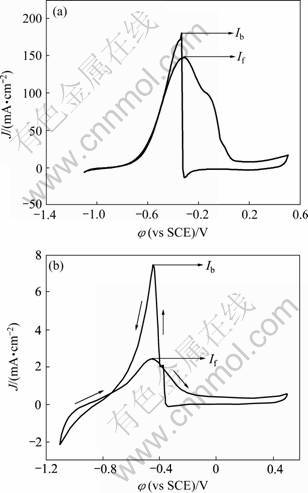
图3 nanoPd和polyPd电极在1.0 mol/L NaOH+0.5 mol/L HCOOH溶液中的循环伏安曲线
Fig.3 CV of nanoPd (a) and polyPd (b) electrodes in 1.0 mol/L NaOH+0.5 mol/L HCOOH solution at scan rate of 50 mV/s
由此看出,甲酸氧化是一个不经CO中间体直接氧化为CO2的过程。从图3看出,nanoPd电极正向扫描氧化峰的峰值If(148.84)与逆向扫描氧化峰峰值Ib(181.02)之比If/Ib为0.83,而polyPd电极If/Ib为0.33,说明nanoPd催化剂抗CO中毒能力有很大改善[16]。
采用线性扫描技术进一步研究nanoPd和Au/nanoPd 电极对甲酸氧化的电催化活性(见图4)。插入图表示在-0.65 V时,不同电极上甲酸氧化的电流密度柱形图。从图4中观察到,甲酸在Au/nanoPd电极上的起始氧化电位约为-0.90 V,比nanoPd电极 (-0.81 V)提前了90 mV。在燃料电池的实际应用中,100 m V的电位负移将会使电池的输出电压提高10%~30%。从柱形图中可直观地看出,在-0.65 V时,Au/nanoPd电极上的电流密度明显大于nanoPd电极的,甲酸在nanoPd、Au/nanoPd(c1)、Au/nanoPd(c2)和Au/nanoPd(c3)电极上电流密度分别为16.47、43.97、38.33和36.36 mA/cm2。Au/nanoPd(c1)氧化峰的电流略高于Pd电极的,但Au/nanoPd(c2)和Au/nanoPd(c3)的氧化峰电流均低于nanoPd电极的,且峰电位较nanoPd电极正移。甲酸在Au/nanoPd电极上电氧化活性的增强可能是由Au与Pd原子之间的协同双功能作用引起的[17]。
通过比较发现,Au/nanoPd电极对甲酸氧化的起始电位比nanoPd电极更负且具有较高的电流密度,但只有Au/nanoPd(c1)电极的氧化峰峰电流密度略高于nanoPd电极的,峰电位没有明显正移;Au/nanoPd(c2)和Au/nanoPd(c3)的电流密度都有所降低且电位正移。由此可见,在nanoPd催化剂中沉积少量的Au能提高Pd对甲酸的电催化活性。金沉积的量不同,对甲酸氧化的活性也会有差异,其原因可能是由于,当Au含量较低时,合金表面的Au可以忽略,此时晶格收缩效应占优,有利于Pd-COOH(ads)的减弱,从而增强Pd-Au电催化甲酸的活性;高含量Au合金表面的Au增加以致表面配体效应占优,从而增强Pd-COOH(ads)作用而不利于甲酸氧化进行[18]。
图5所示为nanoPd和Au/nanoPd催化剂上甲酸氧化的交流阻抗谱(扰动信号为5 mV)。从图5中可以看出,4个阻抗谱图具有相似的双半圆形态,半圆弧Ⅰ大小几乎一致,半圆弧Ⅱ直径由大到小依次为nanoPd、Au/nanoPd(c3)、Au/nanoPd(c2)和Au/nanoPd(c1)。半圆弧Ⅰ的高频端与实轴的交点对应于溶液电阻,半圆弧Ⅰ与Ⅱ分别是OH的吸附过程和甲酸在电极上的氧化过程,OH的吸附过程中溶液组成没有改变(1.0 mol/L NaOH+0.5 mol/L HCOOH),所以4个谱图中的半圆弧Ⅰ直径大小接近。阻抗图中未发现Warburg阻抗[19],因此,在本实验条件下不存在甲酸的浓差极化。交流阻抗谱中半圆弧直径的大小反映电化学体系动力学过程的相对速度快慢,直径越小,电荷传递越容易,表明该体系动力学过程的相对速度越快。图5中半圆弧Ⅱ可以看作是甲酸的电氧化过程,其中Au/nanoPd电极上的甲酸氧化的直径明显小于nanoPd的,说明金的修饰能够提高甲酸在nanoPd电极上的电催化氧化活性,而Au/nanoPd(c1)电极对甲酸电氧化的催化效果最为突出,说明少量Au的存在可以大大提高Pd对甲酸的电氧化活性,这与循环伏安图分析结果一致。
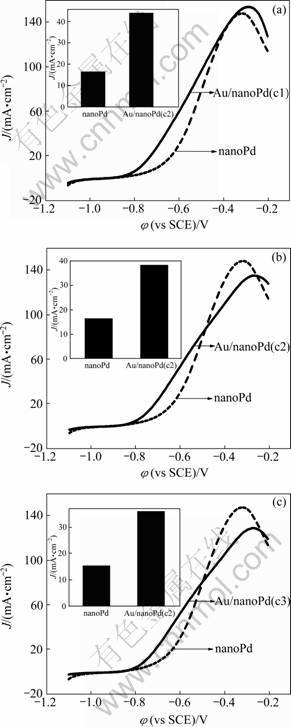
图4 nanoPd电极和Au/nanoPd 电极对甲酸氧化的线性扫描图
Fig.4 LSV curves of nanoPd and Au/nanoPd electrodes towards formic acid oxidation (Insert figure: current density column of formic acid oxidation at -0.65 V)
图6所示为等效电路图能够较好地模拟工作电极的交流阻抗谱,拟合参数见表1。等效电路图中R1、R2、C1和Q分别代表溶液电阻、电荷传递电阻、等效电容和常相位角元件,可以很好地模拟OH的吸附过程。从表1看出,R1值基本保持不变,这与电解液组成没有改变有关,R2值也接近,这是因为溶液中OH-浓度没有改变。R3和C2模拟甲酸氧化部分,其中Au/nanoPd(c1)的电荷传递电阻(R3)最小,接着依次为Au/nanoPd(c2)、Au/nanoPd(c3)和nanoPd,这与图5相一致。
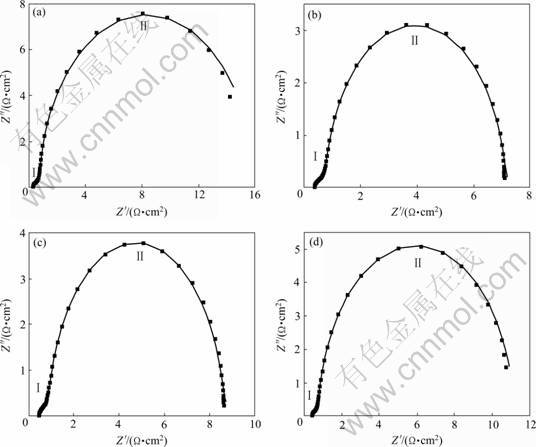
图5 nanoPd和Au/nanoPd 电极在1.0 mol/L NaOH+0.5 mol/L HCOOH溶液中的Nyquist曲线以及相应的模拟曲线
Fig.5 Nyquist impedence (dotts ) of nanoPd and Au/nanoPd electrode in 1.0 mol/L NaOH+0.5 mol/L HCOOH at potential of -300 mV and corresponding fitting curves (solid lines)
表1 交流阻抗模拟结果
Table 1 Simulation results of impedance spectra
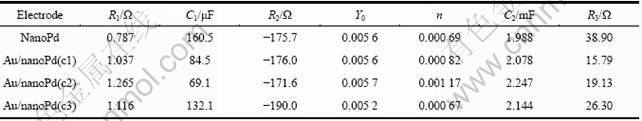
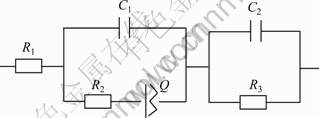
图6 甲酸氧化的等效电路图
Fig.6 Nyquists equivalent electric circuit compatible shown in Fig.5
3 结论
1) 水热法制备的纳米多孔Pd电极(nanoPd)的比表面积大,活性位点多,对甲酸的电催化氧化活性远远高于晶体Pd电极的,起始电位提前190 mV左右。
2) 用电位扫描法在nanoPd电极上沉积Au得到Au/nanoPd,发现少量Au的存在能提高Pd催化剂对甲酸的电催化氧化活性,电流密度增加,起始电位提前90 mV,且电荷传递电阻极低。
REFERENCES
[1] RICE C, HA S, MASEL R I, WASZCZUK P, WIECKOWSKI A, BARNARD T. Direct formic acid fuel cells[J]. J Power Source, 2002, 111(1): 83-89.
[2] MARKOVIC N M, GASTERGER H A, PHILIP N R. Electro-oxidation mechanisms of methanol and formic acid Pt-Ru alloy surface[J]. Electrochim Acta, 1995, 40(1): 91-98.
[3] MROZEK N F, LUO H, WEAVER M J. Formic acid electrooxidation on platinum-group metal: is adsorbed carbon monoxide solely a catalytic poison[J]. Langmuir, 2000, 16(22): 8463-8469.
[4] CHOI J H, JEONG K J, DONG Y J, HAN J, LIM T H, LEE J S, SUNG Y E. Electro-oxidation of methanol and formic acid on PtRu and PtAu for direct liquid fuel cells[J]. J Power Source, 2006, 163(1): 71-75.
[5] ARENZ M, STAMENKOVIC V, SCHMIDT T J, WANDEL T K, ROSS P N, MARKOVIC N M. The electro-oxidation of formic acid on Pt-Pd single crystal bimetallic surfaces[J]. Phys Chem Chem Phys, 2003, 5: 4242-4251.
[6] HOSHI N, KIDA K, NAKAMURA M, NAKADA M, OSADA K. Structural effects of electrochemical oxidation of formic acid on single crystal electrodes of palladium[J]. J Phys Chem B, 2006, 110(25): 12480-12484.
[7] ZHANG L L, TANG Y W, BAO J C, LU T H, LI C A. A carbon-support Pd-P catalyst as the anodic catalyst in a direct formic acid fuel cell[J]. J Power Sources, 2006, 162(1): 177-179.
[8] YI Q F, HUANG W, LIU X P, XU G R, ZHOU Z H, CHEN A C. Electroactivity of titanium-supported nanoporous Pd-Pt catalysts towards formic acid oxidation[J]. J Electroana Chem, 2008, 619/620(15): 197-205.
[9] HA S, LARSEN R, MASEL R I. Performance characterization of Pd/C nanocatalyst for direct formic acid fuel cells[J]. J Power Sources, 2005, 144(1): 28-34.
[10] ZHANG S X, QING M, ZHANG H, TIAN Y. Electrocatalytic oxidation of formic acid on functional MWCNTs supported nanostructured Pd-Au catalyst[J]. Eletrochemi Commun, 2009, 11: 2249-2252.
[11] YI Q F, YU W Q, NIU F J. Novel nanoporous binary Au-Ru electrocatalysts for glucose oxidation[J]. Electroanalysis, 2010, 22(5): 556-563.
[12] 于文强, 易清风. 钛基纳米多孔金电极对甲醛氧化的电催化活性[J]. 无机化学学报, 2010, 26(3): 459-463.
YU Wen-Qiang, YI Qing-Feng. Electrocatalytic oxidation of formaldehyde on anovel titanium-supported nanoporous gold electrode[J]. Chinese Journal of Inorganic Chemistry, 2010, 26(3): 459-463.
[13] ARENZ M, STAMENKOVIC V, SCHMIDT T J, WANDEL T K, ROSS P N, MARKOVIC N M. The electro-oxidation of formic acid on Pt-Pd single crystal bimetallic surfaces[J]. Phys Chem Chem Phys, 2003, 5: 4242-4251.
[14] XU W F, GAO Y, LU T H, TANG Y W, WU B. Kinetic study of formic acid oxidation on highly dispersed carbon supported Pd-TiO2 electrocatalyst[J]. Catal Lett, 2009, 130: 312-317.
[15] ZHOU W P, LEWERA A, LARSEN R, MASEL R I, BAGUS P S, WIECKOWSKI A. Size effects in electronic and catalytic properties of unsupported palladium nanoparticles in electrooxidation of formic acid[J]. J Phys Chem, 2006, 110(27): 13393-13398.
[16] SUN Z P, ZHANG X G, LIU R L, LIANG Y Y, LI H L. A simple approach towards sulfonated multi-walled carbon nanotubes supported by Pd catalysts for methanol electro-oxidation[J]. J Power Sources, 2008, 185(2): 801-806.
[17] 赵杰, 黄思玉, 陈卫祥. PtRu/C和PtNi/C催化剂合成及其对甲醇氧化的电催化性能[J]. 浙江大学学报, 2009, 43(5): 962-967.
ZHAO Jie, HUANG Si-Yu, CHEN Wei-Xiang. Synthesis of RtRu/C and PtNi/C catalysts and their electrocatalytic performance for methanol electrooxidation[J]. Journal of Zhangjiang University: Engineering Science, 2009, 43(5): 962-967.
[18] SUO Y, ZHANG L, LU J. First-principles consideration in the design of Pd-alloy catalysts for oxygen reduction[J]. Angew Chem Int Ed, 2007, 46: 1-4.
[19] 曹楚南, 张鉴清. 电化学阻抗谱导论[M]. 北京: 科学出版社, 2002: 90.
CAO Chu-nan, ZHANG Jian-qing. An introduction to electrochemical impedance spectroscopy[M]. Beijing: Science Press, 2002: 90.
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(20876038);湖南省科技发展项目(2009GK3084);湖南省自然科学省市联合基金资助项目(10JJ9003)
收稿日期:2010-08-19;修订日期:2010-11-29
通信作者:易清风,教授,博士;电话:0731-58290045;E-mail: yqfyy2001@yahoo.com.cn


